InVivoMAb anti-mouse CD28
Product Description
Specifications
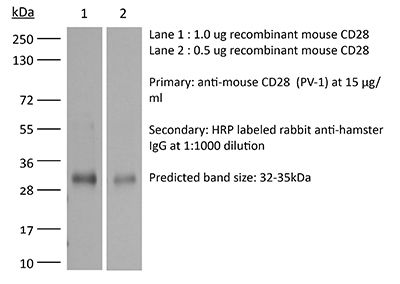
| Isotype | Armenian Hamster IgG, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb polyclonal Armenian hamster IgG |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | C57BL/6 mouse T cell lymphoma EL-4 cells |
| Reported Applications | in vitro T cell stimulation/activation |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107628 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vitro T cell stimulation/activation
Huang, Y., et al (2015). "CRK proteins selectively regulate T cell migration into inflamed tissues" J Clin Invest 125(3): 1019-1032.
PubMed
Effector T cell migration into inflamed sites greatly exacerbates tissue destruction and disease severity in inflammatory diseases, including graft-versus-host disease (GVHD). T cell migration into such sites depends heavily on regulated adhesion and migration, but the signaling pathways that coordinate these functions downstream of chemokine receptors are largely unknown. Using conditional knockout mice, we found that T cells lacking the adaptor proteins CRK and CRK-like (CRKL) exhibit reduced integrin-dependent adhesion, chemotaxis, and diapedesis. Moreover, these two closely related proteins exhibited substantial functional redundancy, as ectopic expression of either protein rescued defects in T cells lacking both CRK and CRKL. We determined that CRK proteins coordinate with the RAP guanine nucleotide exchange factor C3G and the adhesion docking molecule CASL to activate the integrin regulatory GTPase RAP1. CRK proteins were required for effector T cell trafficking into sites of inflammation, but not for migration to lymphoid organs. In a murine bone marrow transplantation model, the differential migration of CRK/CRKL-deficient T cells resulted in efficient graft-versus-leukemia responses with minimal GVHD. Together, the results from our studies show that CRK family proteins selectively regulate T cell adhesion and migration at effector sites and suggest that these proteins have potential as therapeutic targets for preventing GVHD.
in vitro T cell stimulation/activation
Bertin, S., et al (2015). "Dual-specificity phosphatase 6 regulates CD4+ T-cell functions and restrains spontaneous colitis in IL-10-deficient mice" Mucosal Immunol 8(3): 505-515.
PubMed
Mitogen-activated protein kinase (MAPK) phosphatases are dual-specificity phosphatases (DUSPs) that dephosphorylate phosphothreonine and phosphotyrosine residues within MAPKs. DUSP6 preferentially dephosphorylates extracellular signal-regulated kinases 1 and 2 (ERK1/2) rendering them inactive. Here, we study the role of DUSP6 in CD4(+) T-cell function, differentiation, and inflammatory profile in the colon. Upon T-cell receptor (TCR) stimulation, DUSP6 knockout (Dusp6(-/-)) CD4(+) T cells showed increased ERK1/2 activation, proliferation, T helper 1 differentiation, and interferon-gamma production, as well as a marked decrease in survival, interleukin- 17A (IL-17A) secretion, and regulatory T-cell function. To analyze the role of DUSP6 in vivo, we employed the Il10(-/-) model of colitis and generated Il10(-/-)/Dusp6(-/-) double-knockout mice. Il10(-/-)/Dusp6(-/-) mice suffered from accelerated and exacerbated spontaneous colitis, which was prevented by ERK1/2 inhibition. ERK1/2 inhibition also augmented regulatory T-cell differentiation in vitro and in vivo in both C57Bl/6 and Dusp6(-/-) mice. In summary, DUSP6 regulates CD4(+) T-cell activation and differentiation by inhibiting the TCR-dependent ERK1/2 activation. DUSP6 might therefore be a potential intervention target for limiting aberrant T-cell responses in T-cell-mediated diseases, such as inflammatory bowel disease.
in vitro T cell stimulation/activation
Klimatcheva, E., et al (2015). "CXCL13 antibody for the treatment of autoimmune disorders" BMC Immunol 16: 6.
PubMed
BACKGROUND: Homeostatic B Cell-Attracting chemokine 1 (BCA-1) otherwise known as CXCL13 is constitutively expressed in secondary lymphoid organs by follicular dendritic cells (FDC) and macrophages. It is the only known ligand for the CXCR5 receptor, which is expressed on mature B cells, follicular helper T cells (Tfh), Th17 cells and regulatory T (Treg) cells. Aberrant expression of CXCL13 within ectopic germinal centers has been linked to the development of autoimmune disorders (e.g. Rheumatoid Arthritis, Multiple Sclerosis, Systemic Lupus Erythematosis). We, therefore, hypothesized that antibody-mediated disruption of the CXCL13 signaling pathway would interfere with the formation of ectopic lymphoid follicles in the target organs and inhibit autoimmune disease progression. This work describes pre-clinical development of human anti-CXCL13 antibody MAb 5261 and includes therapeutic efficacy data of its mouse counterpart in murine models of autoimmunity. RESULTS: We developed a human IgG1 monoclonal antibody, MAb 5261 that specifically binds to human, rodent and primate CXCL13 with an affinity of approximately 5 nM and is capable of neutralizing the activity of CXCL13 from these various species in in vitro functional assays. For in vivo studies we have engineered a chimeric antibody to contain the same human heavy and light chain variable genes along with mouse constant regions. Treatment with this antibody led to a reduction in the number of germinal centers in mice immunized with 4-Hydroxy-3-nitrophenylacetyl hapten conjugated to Keyhole Limpet Hemocyanin (NP-KLH) and, in adoptive transfer studies, interfered with the trafficking of B cells to the B cell areas of mouse spleen. Furthermore, this mouse anti-CXCL13 antibody demonstrated efficacy in a mouse model of Rheumatoid arthritis (Collagen-Induced Arthritis (CIA)) and Th17-mediated murine model of Multiple Sclerosis (passively-induced Experimental Autoimmune Encephalomyelitis (EAE)). CONCLUSIONS: We developed a novel therapeutic antibody targeting CXCL13-mediated signaling pathway for the treatment of autoimmune disorders.
in vitro T cell stimulation/activation
Pallandre, J. R., et al (2015). "Novel aminotetrazole derivatives as selective STAT3 non-peptide inhibitors" Eur J Med Chem 103: 163-174.
PubMed
The development of inhibitors blocking STAT3 transcriptional activity is a promising therapeutic approach against cancer and inflammatory diseases. In this context, the selectivity of inhibitors against the STAT1 transcription factor is crucial as STAT3 and STAT1 play opposite roles in the apoptosis of tumor cells and polarization of the immune response. A structure-based virtual screening followed by a luciferase-containing promoter assay on STAT3 and STAT1 signaling were used to identify a selective STAT3 inhibitor. An important role of the aminotetrazole group in modulating STAT3 and STAT1 inhibitory activities has been established. Optimization of the hit compound leads to 23. This compound inhibits growth and survival of cells with STAT3 signaling pathway while displaying a minimal effect on STAT1 signaling. Moreover, it prevents lymphocyte T polarization into Th17 and Treg without affecting their differentiation into Th1 lymphocyte.
in vitro T cell stimulation/activation
Bertin, S., et al (2014). "The ion channel TRPV1 regulates the activation and proinflammatory properties of CD4(+) T cells" Nat Immunol 15(11): 1055-1063.
PubMed
TRPV1 is a Ca(2+)-permeable channel studied mostly as a pain receptor in sensory neurons. However, its role in other cell types is poorly understood. Here we found that TRPV1 was functionally expressed in CD4(+) T cells, where it acted as a non-store-operated Ca(2+) channel and contributed to T cell antigen receptor (TCR)-induced Ca(2+) influx, TCR signaling and T cell activation. In models of T cell-mediated colitis, TRPV1 promoted colitogenic T cell responses and intestinal inflammation. Furthermore, genetic and pharmacological inhibition of TRPV1 in human CD4(+) T cells recapitulated the phenotype of mouse Trpv1(-/-) CD4(+) T cells. Our findings suggest that inhibition of TRPV1 could represent a new therapeutic strategy for restraining proinflammatory T cell responses.
in vitro T cell stimulation/activation
Vegran, F., et al (2014). "The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells" Nat Immunol 15(8): 758-766.
PubMed
The TH9 subset of helper T cells was initially shown to contribute to the induction of autoimmune and allergic diseases, but subsequent evidence has suggested that these cells also exert antitumor activities. However, the molecular events that account for their effector properties are elusive. Here we found that the transcription factor IRF1 enhanced the effector function of TH9 cells and dictated their anticancer properties. Under TH9-skewing conditions, interleukin 1beta (IL-1beta) induced phosphorylation of the transcription factor STAT1 and subsequent expression of IRF1, which bound to the promoters of Il9 and Il21 and enhanced secretion of the cytokines IL-9 and IL-21 from TH9 cells. Furthermore, IL-1beta-induced TH9 cells exerted potent anticancer functions in an IRF1- and IL-21-dependent manner. Our findings thus identify IRF1 as a target for controlling the function of TH9 cells.
in vitro T cell stimulation/activation
Heinemann, C., et al (2014). "IL-27 and IL-12 oppose pro-inflammatory IL-23 in CD4+ T cells by inducing Blimp1" Nat Commun 5: 3770.
PubMed
Central nervous system (CNS) autoimmunity is regulated by the balance of pro-inflammatory cytokines and IL-10. Here we identify the transcriptional regulator Blimp1 as crucial to induce IL-10 in inflammatory T helper cells. Pre-committed Th17 cells respond to IL-27 and IL-12 by upregulating Blimp1 and adopt a Tr-1-like phenotype characterized by IL-10 and IFN-gamma production. Accordingly, Blimp1-deficient effector T cells fail to produce IL-10, and deficiency in Tr-1 cell function leads to uncontrolled Th17 cell-driven CNS pathology without the need to stabilize the Th17 phenotype with IL-23. IL-23 counteracts IL-27 and IL-12-mediated effects on Tr-1-development reinforcing the pro-inflammatory phenotype of Th17 cells. Thus, the balance of IL-23 vs IL-12/IL-27 signals into CD4(+) effector T cells determines whether tissue inflammation is perpetuated or resolves.
in vitro T cell stimulation/activation
Berger, H., et al (2013). "SOCS3 transactivation by PPARgamma prevents IL-17-driven cancer growth" Cancer Res 73(12): 3578-3590.
PubMed
Activation of the transcription factor PPARgamma by the n-3 fatty acid docosahexaenoic acid (DHA) is implicated in controlling proinflammatory cytokine secretion, but the intracellular signaling pathways engaged by PPARgamma are incompletely characterized. Here, we identify the adapter-encoding gene SOCS3 as a critical transcriptional target of PPARgamma. SOCS3 promoter binding and gene transactivation by PPARgamma was associated with a repression in differentiation of proinflammatory T-helper (TH)17 cells. Accordingly, TH17 cells induced in vitro displayed increased SOCS3 expression and diminished capacity to produce interleukin (IL)-17 following activation of PPARgamma by DHA. Furthermore, naive CD4 T cells derived from mice fed a DHA-enriched diet displayed less capability to differentiate into TH17 cells. In two different mouse models of cancer, DHA prevented tumor outgrowth and angiogenesis in an IL-17-dependent manner. Altogether, our results uncover a novel molecular pathway by which PPARgamma-induced SOCS3 expression prevents IL-17-mediated cancer growth.
in vitro T cell stimulation/activation
Chen, E. J., et al (2013). "Ezrin and moesin are required for efficient T cell adhesion and homing to lymphoid organs" PLoS One 8(2): e52368.
PubMed
T cell trafficking between the blood and lymphoid organs is a complex, multistep process that requires several highly dynamic and coordinated changes in cyto-architecture. Members of the ezrin, radixin and moesin (ERM) family of actin-binding proteins have been implicated in several aspects of this process, but studies have yielded conflicting results. Using mice with a conditional deletion of ezrin in CD4+ cells and moesin-specific siRNA, we generated T cells lacking ERM proteins, and investigated the effect on specific events required for T cell trafficking. ERM-deficient T cells migrated normally in multiple in vitro and in vivo assays, and could undergo efficient diapedesis in vitro. However, these cells were impaired in their ability to adhere to the beta1 integrin ligand fibronectin, and to polarize appropriately in response to fibronectin and VCAM-1 binding. This defect was specific for beta1 integrins, as adhesion and polarization in response to ICAM-1 were normal. In vivo, ERM-deficient T cells showed defects in homing to lymphoid organs. Taken together, these results show that ERM proteins are largely dispensable for T cell chemotaxis, but are important for beta1 integrin function and homing to lymphoid organs.
in vitro T cell stimulation/activation
Nowak, E. C., et al (2009). "IL-9 as a mediator of Th17-driven inflammatory disease" J Exp Med 206(8): 1653-1660.
PubMed
We report that like other T cells cultured in the presence of transforming growth factor (TGF) beta, Th17 cells also produce interleukin (IL) 9. Th17 cells generated in vitro with IL-6 and TGF-beta as well as purified ex vivo Th17 cells both produced IL-9. To determine if IL-9 has functional consequences in Th17-mediated inflammatory disease, we evaluated the role of IL-9 in the development and progression of experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis. The data show that IL-9 neutralization and IL-9 receptor deficiency attenuates disease, and this correlates with decreases in Th17 cells and IL-6-producing macrophages in the central nervous system, as well as mast cell numbers in the regional lymph nodes. Collectively, these data implicate IL-9 as a Th17-derived cytokine that can contribute to inflammatory disease.
Product Citations
-
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Interleukin-16 enhances anti-tumor immune responses by establishing a Th1 cell-macrophage crosstalk through reprogramming glutamine metabolism in mice.
In Nat Commun on 10 March 2025 by Wen, Z., Liu, T., et al.
PubMed
Overcoming immunosuppression in the tumor microenvironment (TME) is crucial for developing novel cancer immunotherapies. Here, we report that IL-16 administration enhances the polarization of T helper 1 (Th1) cells by inhibiting glutamine catabolism through the downregulation of glutaminase in CD4+ T cells and increases the production of Th1 effector cytokine IFN-γ, thus improving anti-tumor immune responses. Moreover, we find that establishing an IL-16-dependent, Th1-dominant TME relies on mast cell-produced histamine and results in the increased expression of the CXCR3 ligands in tumor-associated macrophages (TAM), thereby improving the therapeutic effectiveness of immune checkpoint blockade (ICB). Cancer patients exhibit impaired production of IL-16, which correlates with poorer prognosis. Additionally, low IL-16 production is associated with unresponsiveness to immunotherapy in cancer patients. Collectively, our findings provided new insights into the biological function of IL-16, emphasizing its potential clinical significance as a therapeutic approach to augment anti-tumor immunity and sensitize ICB-based cancer immunotherapy.
-
-
MFGE8 induces anti-PD-1 therapy resistance by promoting extracellular vesicle sorting of PD-L1.
In Cell Rep Med on 18 February 2025 by Wang, W., Chen, J., et al.
PubMed
Anti-PD-1 therapy, effective in patients with various advanced tumors, still encounters the challenge of insensitivity in most patients. Here, we demonstrate that PD-L1 on tumor cell-derived extracellular vesicles (TEVs) is critical for anti-PD-1 therapy resistance. Reducing endogenous and transferring exogenous TEVs abrogates and induces anti-PD-1 therapy resistance, respectively. Notably, PD-L1 is sorted onto TEVs via the endosomal sorting complex required for transport after ubiquitination by UBE4A and gradually upregulated on TEVs with tumor progression. During progression, increased MFGE8 from tumor cells promotes self αv integrin signaling activation, enabling themselves to upregulate UBE4A, thereby increasing PD-L1 on TEVs and enhancing their immunosuppressive abilities. Translationally, anti-MFGE8-neutralizing antibodies effectively downregulate UBE4A and TEV PD-L1, thereby negating anti-PD-1 therapy resistance. Furthermore, serum MFGE8 and PD-L1+ EV levels of tumor patients correlate positively, and high levels of both indicate poor prognosis after anti-PD-1 therapy. Thus, MFGE8 is a promising target for overcoming resistance and predicting responsiveness to anti-PD-1 therapy.
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
FMNL1 and mDia1 promote efficient T cell migration through complex environments via distinct mechanisms.
In Front Immunol on 21 October 2024 by Sigler, A. L., Thompson, S. B., et al.
PubMed
Lymphocyte trafficking and migration through tissues is critical for adaptive immune function and, to perform their roles, T cells must be able to navigate through diverse tissue environments that present a range of mechanical challenges. T cells predominantly express two members of the formin family of actin effectors, Formin-like 1 (FMNL1) and mammalian diaphanous-related formin 1 (mDia1). While both FMNL1 and mDia1 have been studied individually, they have not been directly compared to determine functional differences in promoting T cell migration. Through in vivo analysis and the use of in vitro 2D and 3D model environments, we demonstrate that FMNL1 and mDia1 are both required for effective T cell migration, but they have different localization and roles in T cells, with specific environment-dependent functions. We found that mDia1 promotes general motility in 3D environments in conjunction with Myosin-II activity. We also show that, while mDia1 is almost entirely in the cytoplasmic compartment, a portion of FMNL1 physically associates with the nucleus. Furthermore, FMNL1 localizes to the rear of migrating T cells and contributes to efficient migration by promoting deformation of the rigid T cell nucleus in confined environments. Overall, our data indicates that while FMNL1 and mDia1 have similar mechanisms of actin polymerization, they have distinct roles in promoting T cell migration. This suggests that differential modulation of FMNL1 and mDia1 can be an attractive therapeutic route to fine-tune T cell migration behavior.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
The type 2 cytokine Fc-IL-4 revitalizes exhausted CD8+ T cells against cancer.
In Nature on 1 October 2024 by Feng, B., Bai, Z., et al.
PubMed
Current cancer immunotherapy predominately focuses on eliciting type 1 immune responses fighting cancer; however, long-term complete remission remains uncommon1,2. A pivotal question arises as to whether type 2 immunity can be orchestrated alongside type 1-centric immunotherapy to achieve enduring response against cancer3,4. Here we show that an interleukin-4 fusion protein (Fc-IL-4), a typical type 2 cytokine, directly acts on CD8+ T cells and enriches functional terminally exhausted CD8+ T (CD8+ TTE) cells in the tumour. Consequently, Fc-IL-4 enhances antitumour efficacy of type 1 immunity-centric adoptive T cell transfer or immune checkpoint blockade therapies and induces durable remission across several syngeneic and xenograft tumour models. Mechanistically, we discovered that Fc-IL-4 signals through both signal transducer and activator of transcription 6 (STAT6) and mammalian target of rapamycin (mTOR) pathways, augmenting the glycolytic metabolism and the nicotinamide adenine dinucleotide (NAD) concentration of CD8+ TTE cells in a lactate dehydrogenase A-dependent manner. The metabolic modulation mediated by Fc-IL-4 is indispensable for reinvigorating intratumoural CD8+ TTE cells. These findings underscore Fc-IL-4 as a potent type 2 cytokine-based immunotherapy that synergizes effectively with type 1 immunity to elicit long-lasting responses against cancer. Our study not only sheds light on the synergy between these two types of immune responses, but also unveils an innovative strategy for advancing next-generation cancer immunotherapy by integrating type 2 immune factors.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Soluble Tim-3 serves as a tumor prognostic marker and therapeutic target for CD8+ T cell exhaustion and anti-PD-1 resistance.
In Cell Rep Med on 20 August 2024 by Chen, C., Zhao, F., et al.
PubMed
Resistance to PD-1 blockade in onco-immunotherapy greatly limits its clinical application. T cell immunoglobulin and mucin domain containing-3 (Tim-3), a promising immune checkpoint target, is cleaved by ADAM10/17 to produce its soluble form (sTim-3) in humans, potentially becoming involved in anti-PD-1 resistance. Herein, serum sTim-3 upregulation was observed in non-small cell lung cancer (NSCLC) and various digestive tumors. Notably, serum sTim-3 is further upregulated in non-responding patients undergoing anti-PD-1 therapy for NSCLC and anti-PD-1-resistant cholangiocarcinoma patients. Furthermore, sTim-3 overexpression facilitates tumor progression and confers anti-PD-1 resistance in multiple tumor mouse models. Mechanistically, sTim-3 induces terminal T cell exhaustion and attenuates CD8+ T cell response to PD-1 blockade through carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM-1). Moreover, the ADAM10 inhibitor GI254023X, which blocks sTim-3 production, reduces tumor progression in Tim-3 humanized mice and reverses anti-PD-1 resistance in human tumor-infiltrating lymphocytes (TILs). Overall, human sTim-3 holds great predictive and therapeutic potential in onco-immunotherapy.
-
-
-
Mus musculus (Mouse)
-
Genetics
-
Immunology and Microbiology
Single-Molecule DNA Methylation Reveals Unique Epigenetic Identity Profiles of T Helper Cells.
In J Immunol on 15 March 2024 by Goldsmith, C., Thevin, V., et al.
PubMed
Both identity and plasticity of CD4 T helper (Th) cells are regulated in part by epigenetic mechanisms. However, a method that reliably and readily profiles DNA base modifications is still needed to finely study Th cell differentiation. Cytosine methylation in CpG context (5mCpG) and cytosine hydroxymethylation (5hmCpG) are DNA modifications that identify stable cell phenotypes, but their potential to characterize intermediate cell transitions has not yet been evaluated. To assess transition states in Th cells, we developed a method to profile Th cell identity using Cas9-targeted single-molecule nanopore sequencing. Targeting as few as 10 selected genomic loci, we were able to distinguish major in vitro polarized murine T cell subtypes, as well as intermediate phenotypes, by their native DNA 5mCpG patterns. Moreover, by using off-target sequences, we were able to infer transcription factor activities relevant to each cell subtype. Detection of 5mCpG and 5hmCpG was validated on intestinal Th17 cells escaping transforming growth factor β control, using single-molecule adaptive sampling. A total of 21 differentially methylated regions mapping to the 10-gene panel were identified in pathogenic Th17 cells relative to their nonpathogenic counterpart. Hence, our data highlight the potential to exploit native DNA methylation profiling to study physiological and pathological transition states of Th cells.
-
-
-
Cancer Research
-
Immunology and Microbiology
Tumor immunogenicity dictates reliance on TCF1 in CD8+ T cells for response to immunotherapy.
In Cancer Cell on 11 September 2023 by Escobar, G., Tooley, K., et al.
PubMed
Stem-like CD8+ T cells are regulated by T cell factor 1 (TCF1) and are considered requisite for immune checkpoint blockade (ICB) response. However, recent findings indicate that reliance on TCF1+CD8+ T cells for ICB efficacy may differ across tumor contexts. We find that TCF1 is essential for optimal priming of tumor antigen-specific CD8+ T cells and ICB response in poorly immunogenic tumors that accumulate TOX+ dysfunctional T cells, but is dispensable for T cell priming and therapy response in highly immunogenic tumors that efficiently expand transitory effectors. Importantly, improving T cell priming by vaccination or by enhancing antigen presentation on tumors rescues the defective responses of TCF1-deficient CD8+ T cells upon ICB in poorly immunogenic tumors. Our study highlights TCF1's role during the early stages of anti-tumor CD8+ T cell responses with important implications for guiding optimal therapeutic interventions in cancers with low TCF1+CD8+ T cells and low-neo-antigen expression.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Restraint of IFN-γ expression through a distal silencer CNS-28 for tissue homeostasis.
In Immunity on 9 May 2023 by Cui, K., Chen, Z., et al.
PubMed
Interferon-γ (IFN-γ) is a key cytokine in response to viral or intracellular bacterial infection in mammals. While a number of enhancers are described to promote IFN-γ responses, to the best of our knowledge, no silencers for the Ifng gene have been identified. By examining H3K4me1 histone modification in naive CD4+ T cells within Ifng locus, we identified a silencer (CNS-28) that restrains Ifng expression. Mechanistically, CNS-28 maintains Ifng silence by diminishing enhancer-promoter interactions within Ifng locus in a GATA3-dependent but T-bet-independent manner. Functionally, CNS-28 restrains Ifng transcription in NK cells, CD4+ cells, and CD8+ T cells during both innate and adaptive immune responses. Moreover, CNS-28 deficiency resulted in repressed type 2 responses due to elevated IFN-γ expression, shifting Th1 and Th2 paradigm. Thus, CNS-28 activity ensures immune cell quiescence by cooperating with other regulatory cis elements within the Ifng gene locus to minimize autoimmunity.
-
-
-
Mus musculus (Mouse)
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Molecular, metabolic and functional CD4 T cell paralysis impedes tumor control
In bioRxiv on 17 April 2023 by Guo, M., Abd-Rabbo, D., et al.
-
-
-
Immunology and Microbiology
-
Neuroscience
-
In vitro experiments
-
Mus musculus (Mouse)
Small intestine and colon tissue-resident memory CD8+ T cells exhibit molecular heterogeneity and differential dependence on Eomes.
In Immunity on 10 January 2023 by Lin, Y. H., Duong, H. G., et al.
PubMed
Tissue-resident memory CD8+ T (TRM) cells are a subset of memory T cells that play a critical role in limiting early pathogen spread and controlling infection. TRM cells exhibit differences across tissues, but their potential heterogeneity among distinct anatomic compartments within the small intestine and colon has not been well recognized. Here, by analyzing TRM cells from the lamina propria and epithelial compartments of the small intestine and colon, we showed that intestinal TRM cells exhibited distinctive patterns of cytokine and granzyme expression along with substantial transcriptional, epigenetic, and functional heterogeneity. The T-box transcription factor Eomes, which represses TRM cell formation in some tissues, exhibited unexpected context-specific regulatory roles in supporting the maintenance of established TRM cells in the small intestine, but not in the colon. Taken together, these data provide previously unappreciated insights into the heterogeneity and differential requirements for the formation vs. maintenance of intestinal TRM cells.
-
-
-
Genetics
-
Immunology and Microbiology
Forced expression of the non-coding RNA miR-17∼92 restores activation and function in CD28-deficient CD4+ T cells.
In iScience on 18 November 2022 by Dölz, M., Hasiuk, M., et al.
PubMed
CD28 provides the prototypical costimulatory signal required for productive T-cell activation. Known molecular consequences of CD28 costimulation are mostly based on studies of protein signaling molecules. The microRNA cluster miR-17∼92 is induced by T cell receptor stimulation and further enhanced by combined CD28 costimulation. We demonstrate that transgenic miR-17∼92 cell-intrinsically largely overcomes defects caused by CD28 deficiency. Combining genetics, transcriptomics, bioinformatics, and biochemical miRNA:mRNA interaction maps we empirically validate miR-17∼92 target genes that include several negative regulators of T cell activation. CD28-deficient T cells exhibit derepressed miR-17∼92 target genes during activation. CRISPR/Cas9-mediated ablation of the miR-17∼92 targets Pten and Nrbp1 in naive CD28-/- CD4+ T cells differentially increases proliferation and expression of the activation markers CD25 and CD44, respectively. Thus, we propose that miR-17∼92 constitutes a central mediator for T cell activation, integrating signals by the TCR and CD28 costimulation by dampening multiple brakes that prevent T cell activation.
-
-
-
Mus musculus (Mouse)
-
Biochemistry and Molecular biology
-
Cancer Research
-
Immunology and Microbiology
Tumor cells dictate anti-tumor immune responses by altering pyruvate utilization and succinate signaling in CD8+ T cells.
In Cell Metab on 2 August 2022 by Elia, I., Rowe, J. H., et al.
PubMed
The tumor microenvironment (TME) is a unique metabolic niche that can inhibit T cell metabolism and cytotoxicity. To dissect the metabolic interplay between tumors and T cells, we establish an in vitro system that recapitulates the metabolic niche of the TME and allows us to define cell-specific metabolism. We identify tumor-derived lactate as an inhibitor of CD8+ T cell cytotoxicity, revealing an unexpected metabolic shunt in the TCA cycle. Metabolically fit cytotoxic T cells shunt succinate out of the TCA cycle to promote autocrine signaling via the succinate receptor (SUCNR1). Cytotoxic T cells are reliant on pyruvate carboxylase (PC) to replenish TCA cycle intermediates. By contrast, lactate reduces PC-mediated anaplerosis. The inhibition of pyruvate dehydrogenase (PDH) is sufficient to restore PC activity, succinate secretion, and the activation of SUCNR1. These studies identify PDH as a potential drug target to allow CD8+ T cells to retain cytotoxicity and overcome a lactate-enriched TME.
-
-
-
Biochemistry and Molecular biology
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
-
Mus musculus (Mouse)
Localization of a TORC1-eIF4F translation complex during CD8+ T cell activation drives divergent cell fate.
In Mol Cell on 7 July 2022 by Liedmann, S., Liu, X., et al.
PubMed
Activated CD8+ T lymphocytes differentiate into heterogeneous subsets. Using super-resolution imaging, we found that prior to the first division, dynein-dependent vesicular transport polarized active TORC1 toward the microtubule-organizing center (MTOC) at the proximal pole. This active TORC1 was physically associated with active eIF4F, required for the translation of c-myc mRNA. As a consequence, c-myc-translating polysomes polarized toward the cellular pole proximal to the immune synapse, resulting in localized c-myc translation. Upon division, the TORC1-eIF4A complex preferentially sorted to the proximal daughter cell, facilitating asymmetric c-Myc synthesis. Transient disruption of eIF4A activity at first division skewed long-term cell fate trajectories to memory-like function. Using a genetic barcoding approach, we found that first-division sister cells often displayed differences in transcriptional profiles that largely correlated with c-Myc and TORC1 target genes. Our findings provide mechanistic insights as to how distinct T cell fate trajectories can be established during the first division.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
STAT1 signaling protects self-reactive T cells from control by innate cells during neuroinflammation.
In JCI Insight on 22 June 2022 by Arbelaez, C. A., Palle, P., et al.
PubMed
The transcription factor STAT1 plays a critical role in modulating the differentiation of CD4+ T cells producing IL-17 and GM-CSF, which promote the development of experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS). The protective role of STAT1 in MS and EAE has been largely attributed to its ability to limit pathogenic Th cells and promote Tregs. Using mice with selective deletion of STAT1 in T cells (STAT1CD4-Cre), we identified a potentially novel mechanism by which STAT1 regulates neuroinflammation independently of Foxp3+ Tregs. STAT1-deficient effector T cells became the target of NK cell-mediated killing, limiting their capacity to induce EAE. STAT1-deficient T cells promoted their own killing by producing more IL-2 that, in return, activated NK cells. Elimination of NK cells restored EAE susceptibility in STAT1CD4-Cre mice. Therefore, our study suggests that the STAT1 pathway can be manipulated to limit autoreactive T cells during autoimmunity directed against the CNS.
-
-
-
Flow cytometry/Cell sorting
-
Mus musculus (Mouse)
Ursodeoxycholic acid reduces antitumor immunosuppression by inducing CHIP-mediated TGF-β degradation.
In Nat Commun on 14 June 2022 by Shen, Y., Lu, C., et al.
PubMed
TGF-β is essential for inducing systemic tumor immunosuppression; thus, blocking TGF-β can greatly enhance antitumor immunity. However, there are still no effective TGF-β inhibitors in clinical use. Here, we show that the clinically approved compound ursodeoxycholic acid (UDCA), by degrading TGF-β, enhances antitumor immunity through restraining Treg cell differentiation and activation in tumor-bearing mice. Furthermore, UDCA synergizes with anti-PD-1 to enhance antitumor immunity and tumor-specific immune memory in tumor-bearing mice. UDCA phosphorylates TGF-β at T282 site via TGR5-cAMP-PKA axis, causing increased binding of TGF-β to carboxyl terminus of Hsc70-interacting protein (CHIP). Then, CHIP ubiquitinates TGF-β at the K315 site, initiating p62-dependent autophagic sorting and subsequent degradation of TGF-β. Notably, results of retrospective analysis shows that combination therapy with anti-PD-1 or anti-PD-L1 and UDCA has better efficacy in tumor patients than anti-PD-1 or anti-PD-L1 alone. Thus, our results show a mechanism for TGF-β regulation and implicate UDCA as a potential TGF-β inhibitor to enhance antitumor immunity.
-
-
-
Genetics
-
Immunology and Microbiology
-
Mus musculus (Mouse)
The RNA-binding protein IMP2 drives a stromal-Th17 cell circuit in autoimmune neuroinflammation.
In JCI Insight on 8 February 2022 by Bechara, R., Amatya, N., et al.
PubMed
Stromal cells are emerging as key drivers of autoimmunity, partially because they produce inflammatory chemokines that orchestrate inflammation. Chemokine expression is regulated transcriptionally but also through posttranscriptional mechanisms, the specific drivers of which are still incompletely defined. CCL2 (MCP1) is a multifunctional chemokine that drives myeloid cell recruitment. During experimental autoimmune encephalomyelitis (EAE), an IL-17-driven model of multiple sclerosis, CCL2 produced by lymph node (LN) stromal cells was essential for immunopathology. Here, we showed that Ccl2 mRNA upregulation in human stromal fibroblasts in response to IL-17 required the RNA-binding protein IGF-2 mRNA-binding protein 2 (IGF2BP2, IMP2), which is expressed almost exclusively in nonhematopoietic cells. IMP2 binds directly to CCL2 mRNA, markedly extending its transcript half-life, and is thus required for efficient CCL2 secretion. Consistent with this, Imp2-/- mice showed reduced CCL2 production in LNs during EAE, causing impairments in monocyte recruitment and Th17 cell polarization. Imp2-/- mice were fully protected from CNS inflammation. Moreover, deletion of IMP2 after EAE onset was sufficient to mitigate disease severity. These data showed that posttranscriptional control of Ccl2 in stromal cells by IMP2 was required to permit IL-17-driven progression of EAE pathogenesis.
-
-
-
In vitro experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
-
Neuroscience
Astrocyte-Derived Pleiotrophin Mitigates Late-Stage Autoimmune CNS Inflammation.
In Front Immunol on 21 January 2022 by Linnerbauer, M., Lößlein, L., et al.
PubMed
Astrocytes are the most abundant glial cells in the central nervous system (CNS) with the capacity to sense and react to injury and inflammatory events. While it has been widely documented that astrocytes can exert tissue-degenerative functions, less is known about their protective and disease-limiting roles. Here, we report the upregulation of pleiotrophin (PTN) by mouse and human astrocytes in multiple sclerosis (MS) and its preclinical model experimental autoimmune encephalomyelitis (EAE). Using CRISPR-Cas9-based genetic perturbation systems, we demonstrate in vivo that astrocyte-derived PTN is critical for the recovery phase of EAE and limits chronic CNS inflammation. PTN reduces pro-inflammatory signaling in astrocytes and microglia and promotes neuronal survival following inflammatory challenge. Finally, we show that intranasal administration of PTN during the late phase of EAE successfully reduces disease severity, making it a potential therapeutic candidate for the treatment of progressive MS, for which existing therapies are limited.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Mus musculus (Mouse)
-
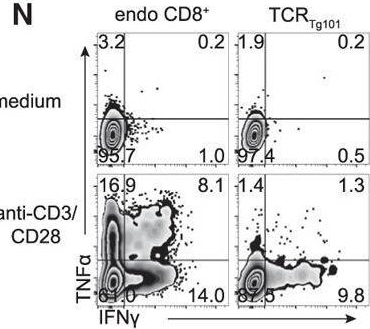
Divergent fates of antigen-specific CD8+ T cell clones in mice with acute leukemia.
In Cell Rep on 9 November 2021 by Chen, X., MacNabb, B. W., et al.
PubMed
The existence of a dysfunctional CD8+ T cell state in cancer is well established. However, the degree to which CD8+ T cell fates are influenced by the context in which they encounter cognate tumor antigen is less clear. We previously demonstrated that CD8+ T cells reactive to a model leukemia antigen were deleted by antigen cross-presenting type 1 conventional dendritic cells (cDC1s). Here, through a study of T cell receptor (TCR) transgenic CD8+ T cells (TCRTg101) reactive to a native C1498 leukemia cell antigen, we uncover a different mode of T cell tolerance in which TCRTg101 undergo progressive expansion and differentiation into an exhausted state. Antigen encounter by TCRTg101 requires leukemia cell major histocompatibility complex (MHC)-I expression and is independent of DCs, implying that leukemia cells directly mediate the exhausted TCRTg101 phenotype. Collectively, our data reveal that leukemia antigens are presented to CD8+ T cells via discrete pathways, leading to distinct tolerant states.
-
-
-
Cell Culture
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Urolithin A ameliorates experimental autoimmune encephalomyelitis by targeting aryl hydrocarbon receptor.
In EBioMedicine on 1 February 2021 by Shen, P. X., Li, X., et al.
PubMed
Urolithin A (URA) is an intestinal microbiota metabolic product from ellagitannin-containing foods with multiple biological activities. However, its role in autoimmune diseases is largely unknown. Here, for first time, we demonstrate the therapeutic effect of URA in an experimental autoimmune encephalomyelitis (EAE) animal model.
-
-
-
Mus musculus (Mouse)
-
Biochemistry and Molecular biology
An IL-27-Driven Transcriptional Network Identifies Regulators of IL-10 Expression across T Helper Cell Subsets.
In Cell Rep on 24 November 2020 by Zhang, H., Madi, A., et al.
PubMed
Interleukin-27 (IL-27) is an immunoregulatory cytokine that suppresses inflammation through multiple mechanisms, including induction of IL-10, but the transcriptional network mediating its diverse functions remains unclear. Combining temporal RNA profiling with computational algorithms, we predict 79 transcription factors induced by IL-27 in T cells. We validate 11 known and discover 5 positive (Cebpb, Fosl2, Tbx21, Hlx, and Atf3) and 2 negative (Irf9 and Irf8) Il10 regulators, generating an experimentally refined regulatory network for Il10. We report two central regulators, Prdm1 and Maf, that cooperatively drive the expression of signature genes induced by IL-27 in type 1 regulatory T cells, mediate IL-10 expression in all T helper cells, and determine the regulatory phenotype of colonic Foxp3+ regulatory T cells. Prdm1/Maf double-knockout mice develop spontaneous colitis, phenocopying ll10-deficient mice. Our work provides insights into IL-27-driven transcriptional networks and identifies two shared Il10 regulators that orchestrate immunoregulatory programs across T helper cell subsets.
-