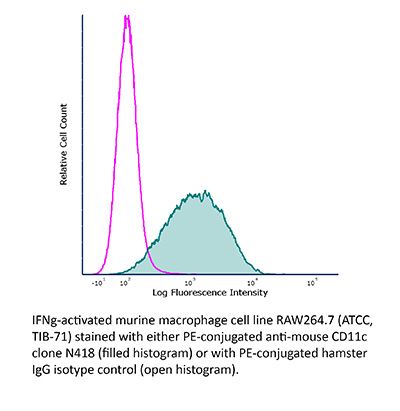
InVivoMAb anti-mouse CD11c
Product Description
Specifications
| Isotype | Armenian Hamster IgG2 |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb polyclonal Armenian hamster IgG |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse spleen dendritic cells |
| Reported Applications |
in vivo targeting of dendritic cells Functional assays Flow cytometry Immunohistochemistry (frozen) Immunohistochemistry (paraffin) Immunofluorescence Immunoprecipitation |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo targeting of dendritic cells
Matsuo H, Somiya M, Iijima M, Arakawa T, Kuroda S (2018). "CD11c-specific bio-nanocapsule enhances vaccine immunogenicity by targeting immune cells" J Nanobiotechnology 16(1):59.
PubMed
Background: Various nanocarriers have been used to deliver subunit vaccines specifically to dendritic cells (DCs) for the improvement of immunogenicity. However, due to their insufficient DC priming ability, these vaccines could not elicit effective innate immunity. We have recently developed a DC-targeting bio-nanocapsule (BNC) by displaying anti-CD11c IgGs via protein A-derived IgG Fc-binding Z domain on the hepatitis B virus envelope L protein particles (α-DC-ZZ-BNC). Results: After the chemical modification with antigens (Ags), the α-DC-ZZ-BNC-Ag complex could deliver Ags to DCs efficiently, leading to effective DC maturation and efficient endosomal escape of Ags, followed by Ag-specific T cell responses and IgG productions. Moreover, the α-DC-ZZ-BNC modified with Japanese encephalitis virus (JEV) envelope-derived D3 Ags could confer protection against 50-fold lethal dose of JEV injection on mice. Conclusion: The α-DC-ZZ-BNC-Ag platform was shown to induce humoral and cellular immunities effectively without any adjuvant.
Immunofluorescence
Flow Cytometry
Gu P, Gao JF, D', Souza CA, Kowalczyk A, Chou KY, Zhang L (2012). "Trogocytosis of CD80 and CD86 by induced regulatory T cells" Cell Mol Immunol 9(2):136-46.
PubMed
Trogocytosis is a process which involves the transfer of membrane fragments and cell surface proteins between cells. Various types of T cells have been shown to be able to acquire membrane-bound proteins from antigen-presenting cells and their functions can be modulated following trogocytosis. However, it is not known whether induced regulatory T cells (iTregs) can undergo trogocytosis, and if so, what the functional consequences of this process might entail. In this study, we show that iTregs can be generated from CD80(-/-)CD86(-/-) double knockout (DKO) mice. Using flow cytometry and confocal fluorescence microscopy, we demonstrate that iTregs generated from DKO mice are able to acquire both CD80 and CD86 from mature dendritic cells (mDCs) and that the acquisition of CD86 occurs to a higher extent than that of CD80. Furthermore, we found that after co-incubation with iTregs, dendritic cells (DCs) downregulate their surface expression of CD80 and CD86. The trogocytosis of both CD80 and CD86 occurs in a cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), CD28 and programmed death ligand-1 (PDL1)-independent manner. Importantly, we showed that iTregs that acquired CD86 from mDCs expressed higher activation markers and their ability to suppress naive CD4(+) T-cell proliferation was enhanced, compared to iTregs that did not acquire CD86. These data demonstrate, for the first time, that iTregs can acquire CD80 and CD86 from mDCs, and the acquisition of CD86 may enhance their suppressive function. These findings provide novel understanding of the interaction between iTregs and DCs, suggesting that trogocytosis may play a significant role in iTreg-mediated immune suppression.
in vivo targeting of dendritic cells
Matsuo H, Yoshimoto N, Iijima M, Niimi T, Jung J, Jeong SY, Choi EK, Sewaki T, Arakawa T, Kuroda S (2012). "Engineered hepatitis B virus surface antigen L protein particles for in vivo active targeting of splenic dendritic cells" Int J Nanomedicine .
PubMed
Dendritic cells (DCs) are key regulators of adaptive T-cell responses. By capturing exogenous antigens and presenting antigen-derived peptides via major histocompatibility complex molecules to naïve T cells, DCs induce antigen-specific immune responses in vivo. In order to induce effective host immune responses, active delivery of exogenous antigens to DCs is considered important for future vaccine development. We recently generated bionanocapsules (BNCs) consisting of hepatitis B virus surface antigens that mediate stringent in vivo cell targeting and efficient endosomal escape, and after the fusion with liposomes (LP) containing therapeutic materials, the BNC-LP complexes deliver them to human liver-derived tissues in vivo. BNCs were further modified to present the immunoglobulin G (IgG) Fc-interacting domain (Z domain) derived from Staphylococcus aureus protein A in tandem. When mixed with IgGs, modified BNCs (ZZ-BNCs) displayed the IgG Fv regions outwardly for efficient binding to antigens in an oriented-immobilization manner. Due to the affinity of the displayed IgGs, the IgG-ZZ-BNC complexes accumulated in specific cells and tissues in vitro and in vivo. After mixing ZZ-BNCs with antibodies against DCs, we used immunocytochemistry to examine which antibodies delivered ZZ-BNCs to mouse splenic DCs following intravenous injection of the ZZ-BNCs. ZZ-BNCs displaying anti-CD11c monoclonal antibodies (α-CD11c-ZZ-BNCs) were found to accumulate with approximately 62% of splenic DCs, and reside within some of them. After the fusion with liposomes containing antigens, the α-CD11c-ZZ-BNCs could elicit the respective antibodies more efficiently than other nontargeting control vaccines, suggesting that this DC-specific nanocarrier is promising for future vaccines.
Functional Assays
Ejaz A, Ammann CG, Werner R, Huber G, Oberhauser V, Hörl S, Schimmer S, Dittmer U, von Laer D, Stoiber H, Bánki Z (2012). "Targeting viral antigens to CD11c on dendritic cells induces retrovirus-specific T cell responses" PLoS One 7(9):e45102.
PubMed
Dendritic cells (DC) represent the most potent antigen presenting cells and induce efficient cytotoxic T lymphocyte (CTL) responses against viral infections. Targeting antigens (Ag) to receptors on DCs is a promising strategy to enhance antitumor and antiviral immune responses induced by DCs. Here, we investigated the potential of CD11c-specific single-chain fragments (scFv) fused to an immunodominant peptide of Friend retrovirus for induction of virus-specific T cell responses by DCs. In vitro CD11c-specific scFv selectively targeted viral antigens to DCs and thereby significantly improved the activation of virus-specific T cells. In vaccination experiments DCs loaded with viral Ag targeted to CD11c provided improved rejection of FV-derived tumors and efficiently primed virus-specific CTL responses after virus challenge. Since the induction of strong virus-specific T cell responses is critical in viral infections, CD11c targeted protein vaccines might provide means to enhance the cellular immune response to prophylactic or therapeutic levels.
in vivo targeting of dendritic cells
Immunohistochemistry (frozen)
Castro FV, Tutt AL, White AL, Teeling JL, James S, French RR, Glennie MJ (2008). "CD11c provides an effective immunotarget for the generation of both CD4 and CD8 T cell responses" Eur J Immunol 38(8):2263-73.
PubMed
The magnitude and quality of T cell responses generated when Ag is targeted to receptors on DC is influenced by both the specific receptor targeted and its distribution among DC subsets. Here we examine the targeting of the model Ag OVA to potential DC targets, including CD11c, CD205, MHC class II, CD40, TLR2 and FcgammaRII/III, using a panel of (Fab' x OVA) conjugates. In vitro studies identified CD11c, CD205 and MHC class II as superior and comparably effective immunotargets for the delivery of OVA to APC for presentation to T cells. In vivo studies, however, showed a marked advantage of targeting Ag to CD11c for both CD4 (OT-II) and CD8 (OT-I) responses, with robust stimulation after a single, low dose (equivalent to 0.5 microg OVA); in contrast, (anti-CD205 x OVA) and (anti-MHC class II x OVA) resulted in markedly less proliferation of both OT-I and OT-II cells. Biodistribution and immunohistochemical studies suggest that the exceptional ability of CD11c to capture Ag in lymphoid tissues may, at least partially, explain its ability to promote T cell responses. These results suggest that targeting antigen via CD11c offers a previously unappreciated strategy for vaccine development which, unlike most targets, delivers robust responses of both CD4 and CD8 T cells.
Immunohistochemistry (frozen)
Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD (2008). "Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii" Immunity 29(2):306-17.
PubMed
The enteric pathogen Toxoplasma gondii is controlled by a vigorous innate T helper 1 (Th1) cell response in the murine model. We demonstrated that after oral infection, the parasite rapidly recruited inflammatory monocytes [Gr1(+) (Ly6C(+), Ly6G(-)) F4/80(+)CD11b(+)CD11c(-)], which established a vital defensive perimeter within the villi of the ileum in the small intestine. Mice deficient of the chemokine receptor CCR2 or the ligand CCL2 failed to recruit Gr1(+) inflammatory monocytes, whereas dendritic cells and resident tissue macrophages remained unaltered. The selective lack of Gr1(+) inflammatory monocytes resulted in an inability of mice to control replication of the parasite, high influx of neutrophils, extensive intestinal necrosis, and rapid death. Adoptive transfer of sorted Gr1(+) inflammatory monocytes demonstrated their ability to home to the ileum in infected animals and protect Ccr2(-/-) mice, which were otherwise highly susceptible to oral toxoplasmosis. Collectively, these findings illustrate the critical importance of inflammatory monocytes as a first line of defense in controlling intestinal pathogens.
Functional Assays
Sadhu C, Ting HJ, Lipsky B, Hensley K, Garcia-Martinez LF, Simon SI, Staunton DE (2007). "CD11c/CD18: novel ligands and a role in delayed-type hypersensitivity" J Leukoc Biol 81(6):1395-403.
PubMed
CD11c, a member of the leukointegrin family, is expressed prominently on tissue macrophages and dendritic cells and binds to complement fragment (iC3b), provisional matrix molecules (fibrinogen), and the Ig superfamily cell adhesion molecule, ICAM-1. CD11c has been proposed to function in phagocytosis, cell migration, and cytokine production by monocytes/macrophages as well as induction of T cell proliferation by Langerhans cells. Using assays to quantify CD11c-mediated cell adhesion, we demonstrate that CD11c recognizes ICAM-2 and VCAM-1. The CD11c-binding site on VCAM-1 appears to be different from that used by the integrin alpha4. CD11c and alpha4beta1 contributed to monocyte capture and transmigration on inflamed human aortic endothelial cells. We discovered that the anti-mouse CD11c mAb N418 blocks CD11c binding to iC3b, ICAM-1, and VCAM-1. Treatment of mice with N418 reduced SRBC-induced delayed-type hypersensitivity significantly. CD11c appeared to contribute predominantly to the sensitization phase and somewhat less to the response to SRBC challenge. This suggests a novel role for CD11c during leukocyte recruitment, antigen uptake, and the survival of APC.
Immunohistochemistry (frozen)
Probst HC, Tschannen K, Odermatt B, Schwendener R, Zinkernagel RM, Van Den Broek M (2005). "Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells" Clin Exp Immunol 141(3):398-404.
PubMed
To investigate the dependence of individual immunological processes on DC, a transgenic mouse system (CD11c-DTR/GFP mice) has been developed that allows conditional depletion of CD11c+ DC in vivo through administration of diphtheria toxin. We have performed careful histological analysis of CD11c-DTR/GFP mice at different time points after diphtheria toxin injection and confirmed the transient depletion of CD11c+ cells from lymph nodes and spleen. Unexpectedly, the injection of diphtheria toxin completely depleted marginal zone and metallophilic M(Phi) from the spleen and their sinusoidal counterparts from the lymph nodes. This finding limits the use of CD11c-DTR/GFP mice for the analysis of the role of DC to models and read outs that are proven to be independent of marginal zone and sinusoidal M(Phi).
in vivo targeting of dendritic cells
van Broekhoven CL, Parish CR, Demangel C, Britton WJ, Altin JG (2004). "Targeting dendritic cells with antigen-containing liposomes: a highly effective procedure for induction of antitumor immunity and for tumor immunotherapy" Cancer Res 64(12):4357-
PubMed
Dendritic cells (DCs) are potent stimulators of immunity, and DCs pulsed with tumor antigen ex vivo have applications in tumor immunotherapy. However, DCs are a small population of cells, and their isolation and pulsing with antigen can be impractical. Here we show that a crude preparation of plasma membrane vesicles (PMV) from the highly metastatic murine melanoma (B16-OVA) and a surrogate tumor antigen (OVA) can be targeted directly to DCs in vivo to elicit functional effects. A novel metal-chelating lipid, 3(nitrilotriacetic acid)-ditetradecylamine, was incorporated into B16-OVA-derived PMV, allowing recombinant hexahistidine-tagged forms of single chain antibody fragments to the DC surface molecules CD11c and DEC-205, to be conveniently "engrafted" onto the vesicle surface by metal-chelating linkage. The modified PMV, or similarly engrafted synthetic stealth liposomes containing OVA or OVA peptide antigen, were found to target DCs in vitro and in vivo, in experiments using flow cytometry and fluorescence confocal microscopy. When used as vaccines in syngeneic mice, the preparations stimulated strong B16-OVA-specific CTL responses in splenic T cells and a marked protection against tumor growth. Protection was dependent on the simultaneous delivery of both antigen and a DC maturation or "danger signal" signal (IFN-gamma or lipopolysaccharide). Administration of the DC-targeting vaccine to mice challenged with B16-OVA cells induced a dramatic immunotherapeutic effect and prolonged disease-free survival. The results show that the targeting of antigen to DCs in this way is highly effective at inducing immunity and protection against the tumor, with protection being at least partially dependent on the eosinophil chemokine eotaxin.
in vivo targeting of dendritic cells
Berry JD, Licea A, Popkov M, Cortez X, Fuller R, Elia M, Kerwin L, Kubitz D, Barbas CF (2003). "Rapid monoclonal antibody generation via dendritic cell targeting in vivo" Hybrid Hybridomics 22(1):23-31.
PubMed
Dendritic cells (DC) are the professional antigen-presenting cells of the immune system. Previous studies have demonstrated that targeting foreign antigens to DC leads to enhanced antigen (Ag)-specific responses in vivo. However, the utility of this strategy for the generation of MAbs has not been investigated. To address this question we immunized mice with IgG-peptide conjugates prepared with the hamster anti-murine CD11c MAb N418. Synthetic peptides corresponding to two different exposed regions of DC-specific ICAM-3 grabbing nonintegrin (DC-SIGN), a human C-type lectin, were conjugated to N418 using thiol-based chemistry. The N418 MAb served as the targeting molecule and synthetic peptides as the Ag (MAb-Ag). A rapid and peptide specific serum IgG response was produced by Day 7 when the synthetic peptides were linked to the N418 MAb, compared to peptide co-delivered with the N418 without linkage. Spleen cells from N418-peptide immunized mice were fused on Day 10, and three IgG1/k monoclonal antibodies (MAbs) were selected to one of the peptide epitopes (MID-peptide). One of the MAbs, Novik 2, bound to two forms of recombinant DC-SIGN protein in enzyme-linked immunosorbent assay (ELISA), and was specifically inhibited by the MID-peptide in solution. Two of these MAbs show specific binding to DC-SIGN expressed by cultured human primary DC. We conclude that in vivo DC targeting enhances the immunogenicity of synthetic peptides and is an effective method for the rapid generation of MAbs to predetermined epitopes.
Functional Assays
Berry JD, Licea A, Popkov M, Cortez X, Fuller R, Elia M, Kerwin L, Kubitz D, Barbas CF (2003). "Rapid monoclonal antibody generation via dendritic cell targeting in vivo" Hybrid Hybridomics 22(1):23-31.
PubMed
Dendritic cells (DC) are the professional antigen-presenting cells of the immune system. Previous studies have demonstrated that targeting foreign antigens to DC leads to enhanced antigen (Ag)-specific responses in vivo. However, the utility of this strategy for the generation of MAbs has not been investigated. To address this question we immunized mice with IgG-peptide conjugates prepared with the hamster anti-murine CD11c MAb N418. Synthetic peptides corresponding to two different exposed regions of DC-specific ICAM-3 grabbing nonintegrin (DC-SIGN), a human C-type lectin, were conjugated to N418 using thiol-based chemistry. The N418 MAb served as the targeting molecule and synthetic peptides as the Ag (MAb-Ag). A rapid and peptide specific serum IgG response was produced by Day 7 when the synthetic peptides were linked to the N418 MAb, compared to peptide co-delivered with the N418 without linkage. Spleen cells from N418-peptide immunized mice were fused on Day 10, and three IgG1/k monoclonal antibodies (MAbs) were selected to one of the peptide epitopes (MID-peptide). One of the MAbs, Novik 2, bound to two forms of recombinant DC-SIGN protein in enzyme-linked immunosorbent assay (ELISA), and was specifically inhibited by the MID-peptide in solution. Two of these MAbs show specific binding to DC-SIGN expressed by cultured human primary DC. We conclude that in vivo DC targeting enhances the immunogenicity of synthetic peptides and is an effective method for the rapid generation of MAbs to predetermined epitopes.
Immunohistochemistry (paraffin)
Flow Cytometry
Chaussabel D, Pajak B, Vercruysse V, Bisseyé C, Garzé V, Habib M, Goldman M, Moser M, Vray B (2003). "Alteration of migration and maturation of dendritic cells and T-cell depletion in the course of experimental Trypanosoma cruzi infection" Lab Invest
PubMed
Trypanosoma cruzi, the etiologic agent of Chagas disease, induces infection that affects most immunocompetent cells. However, its effect on dendritic cells (DC) is still unknown in vivo. In this report, we show, by immunohistochemical staining, that T. cruzi infection triggers a huge increase in the number of CD11c(+) DC in the spleen of infected mice at Days 14 and 21 post-inoculation (pi). In mice reaching the chronic phase (starting on Day 35 pi), the number of splenic DC (sDC) returned progressively to normal (ending on Day 98 pi). In the spleens of noninfected mice, most of the CD8alpha(+)CD11c(+) and CD8alpha(-)CD11c(+) DC were found in the red pulp and the marginal and T-cell zones. However, starting on Day 14 pi, a progressive decline of CD8alpha(+)CD11c(+) was observed. In addition, sDC expressed low levels of the costimulatory molecule B7.2 at Days 14 and 21 pi, suggesting that they remained immature in the course of the infection. As expected, in lipopolysaccharide-treated and noninfected mice, the expression of B7.2 molecules was sharply up-regulated on sDC that migrated toward the T-cell zone. In contrast, upon lipopolysaccharide stimulation, sDC from T. cruzi-infected mice did not migrate toward the T-cell zone nor did they undergo maturation. Finally, white pulp was severely depleted in both CD4(+) and CD8(+) T cells at the peak of infection. Taken together, these results indicate that profound alterations of migration and maturation of sDC and depletion/redistribution of T cells occur during the acute phase of T. cruzi infection and could be part of another strategy to escape immune surveillance and to persist in the host.
Immunofluorescence
Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL (2002). "Identification of a novel macrophage population in the normal mouse corneal stroma" Invest Ophthalmol Vis Sci 43(7):2264-71.
PubMed
Purpose: To examine the normal murine corneal stroma for the presence of bone marrow-derived leukocytes. Methods: Wholemounts of paraformaldehyde-fixed corneal stroma from normal mice at 5 to 16 weeks of age were examined in single- and double-color immunomorphologic studies performed with confocal microscopy. The phenotype, morphology, distribution, and density of immunopositive cells were determined. Results: Numerous CD45(+) cells with pleomorphic and dendriform morphology were found within the pericentral and central region of the corneal stroma (200-300 cells/mm(2)). Dual-color immunostaining demonstrated that 100% of the CD45(+) cells coexpressed CD11b and 50% coexpressed F4/80. Approximately 30% of the total cells and 50% of the F4/80(+) cells coexpressed major histocompatibility complex (MHC) class II antigens. Very small to negligible numbers of cells expressed markers of dendritic cells (CD11c) or granulocytes (Ly6G). Markers for T-cells and NK cells were absent from the corneal stroma, indicating that all the cells identified in the stroma were of the myeloid lineage. Conclusions: The normal murine corneal stroma contains a significant number of CD45(+) leukocytes. Most these cells express the CD11b marker, but not other dendrite, granulocyte, T-cell, or NK markers, placing them in the monocyte/macrophage lineage.
Flow Cytometry
Paczesny S, Beranger S, Salzmann JL, Klatzmann D, Colombo BM (2001). "Protection of mice against leukemia after vaccination with bone marrow-derived dendritic cells loaded with apoptotic leukemia cells" Cancer Res 61(6):2386-9.
PubMed
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) that need to be activated before they can function to initiate primary and secondary immune responses in vivo. DCs are also specialized to maintain peripheral tolerance to self after uptake of apoptotic material, likely corresponding to both apoptotic bodies and whole apoptotic cells. Here, we report that murine bone marrow-derived DCs can be activated in vitro by exogenous signals received from apoptotic leukemia cells expressing on the cell surface a model tumor-associated antigen. Injected in vivo, these exogenously activated DCs can function as adjuvants to protect mice against leukemia by stimulating an antigen-specific cellular-mediated cytotoxic immune response. To our knowledge, this is the first report indicating that DCs loaded with apoptotic leukemia cells protect mice against leukemia development.
in vivo targeting of dendritic cells
Wang H, Griffiths MN, Burton DR, Ghazal P (2000). "Rapid antibody responses by low-dose, single-step, dendritic cell-targeted immunization" Proc Natl Acad Sci U S A 97(2):847-52.
PubMed
We have compared the kinetics of antibody responses in conventional and dendritic cell-targeted immunization by using a model antigen in mice. Targeting was achieved by linking the reporter antigen (polyclonal goat anti-hamster antibody) to N418, a hamster mAb that binds to the CD11c molecule on the surface of murine dendritic cells. Intradermal injection of submicrogram quantities of goat anti-hamster antibody complexed to mAb N418 elicited goat antibody-specific serum IgG in mice. Antigen-specific IgG titers were detectable by day 5, with titers that ranged from 1:1000 to 1:100,000 by day 7. In contrast, when the goat antigen was injected alone or in the presence of a hamster antibody control to form nontargeted complexes, goat-specific serum IgG was undetectable at day 7. Additional control experiments showed that the interaction between the model antigen and mAb N418 is required for amplification of the serum antibody response. These studies demonstrate that a single-step, facilitated-delivery of small amounts of protein antigen to dendritic cells in vivo can give very rapid and high antibody responses. The approach may be particularly useful for vaccination immediately before or just after exposure to a pathogen and may enhance the utility of subunit antigens as immunogens.
Immunohistochemistry (frozen)
Reis e Sousa C, Germain RN (1999). "Analysis of adjuvant function by direct visualization of antigen presentation in vivo: endotoxin promotes accumulation of antigen-bearing dendritic cells in the T cell areas of lymphoid tissue" J Immunol 162(11):65
PubMed
T cell activation requires exposure to processed Ag and signaling by cytokines and costimulatory ligands. Adjuvants are thought to enhance immunity primarily through up-regulation of the latter signals. Here, we explore the effect of the bacterial adjuvant, endotoxin, on Ag presentation by B cells and dendritic cells (DC). Using an mAb (C4H3) specific for the hen egg lysozyme (HEL) 46-61 determinant bound to I-Ak, we analyze processed Ag expression and the tissue distribution of presenting cells following systemic administration of soluble HEL to mice. In both LPS-responsive and -hyporesponsive mice given endotoxin-containing HEL, B cells rapidly display surface 46-61/I-Ak complexes. In marked contrast, in LPS-hyporesponsive mice, splenic DC show little gain in C4H3 staining. In LPS-responsive animals, interdigitating DC in T cell areas show no staining above background at early times after HEL administration, but C4H3+ DC rapidly accumulate in the outer periarteriolar lymphoid sheaths (PALS) and in follicular areas. Within a few hours, C4H3+ DC appear in the T cell areas, concomitant with a decline in C4H3+ cells in the outer PALS, suggesting migration between these two sites. Endotoxin enhancement of C4H3 staining is seen for both CD8alpha- and CD8alpha+ DC subsets. These data suggest that a major effect of adjuvants is to promote mobilization of Ag-bearing DC to the T areas of lymphoid tissue, and possibly also to enhance Ag processing by these DC. Thus, microbial products promote T cell immunity not only through DC activation for cosignaling, but through improvement in signal 1 delivery.
Immunohistochemistry (paraffin)
Henderson WR, Chi EY, Albert RK, Chu SJ, Lamm WJ, Rochon Y, Jonas M, Christie PE, Harlan JM (1997). "Blockade of CD49d (alpha4 integrin) on intrapulmonary but not circulating leukocytes inhibits airway inflammation and hyperresponsiveness in a mouse
PubMed
Immunized mice after inhalation of specific antigen have the following characteristic features of human asthma: airway eosinophilia, mucus and Th2 cytokine release, and hyperresponsiveness to methacholine. A model of late-phase allergic pulmonary inflammation in ovalbumin-sensitized mice was used to address the role of the alpha4 integrin (CD49d) in mediating the airway inflammation and hyperresponsiveness. Local, intrapulmonary blockade of CD49d by intranasal administration of CD49d mAb inhibited all signs of lung inflammation, IL-4 and IL-5 release, and hyperresponsiveness to methacholine. In contrast, CD49d blockade on circulating leukocytes by intraperitoneal CD49d mAb treatment only prevented the airway eosinophilia. In this asthma model, a CD49d-positive intrapulmonary leukocyte distinct from the eosinophil is the key effector cell of allergen-induced pulmonary inflammation and hyperresponsiveness.
Immunohistochemistry (frozen)
Karrer U, Althage A, Odermatt B, Roberts CW, Korsmeyer SJ, Miyawaki S, Hengartner H, Zinkernagel RM (1997). "On the key role of secondary lymphoid organs in antiviral immune responses studied in alymphoplastic (aly/aly) and spleenless (Hox11(-)/-) mu
PubMed
The role of the spleen and of other organized secondary lymphoid organs for the induction of protective antiviral immune responses was evaluated in orphan homeobox gene 11 knockout mice (Hox11(-/-)) lacking the spleen, and in homozygous alymphoplastic mutant mice (aly/aly) possessing a structurally altered spleen but lacking lymph nodes and Peyer's patches. Absence of the spleen had no major effects on the immune response, other than delaying the antibody response by 1-2 d. In aly/aly mice, the thymus-independent IgM response against vesicular stomatitis virus (VSV) was delayed and reduced, whereas the T-dependent switch to the protective IgG was absent. Therefore, aly/aly mice were highly susceptible to VSV infection. Since aly/aly spleen cells yielded neutralizing IgM and IgG after adoptive transfer into recipients with normally structured secondary lymphoid organs, these data suggest that the structural defect was mainly responsible for inefficient T-B cooperation. Although aly/aly mice generated detectable, but reduced, CTL responses after infection with vaccinia virus (VV) and lymphocytic choriomeningitis virus (LCMV), the elimination of these viruses was either delayed (VV) or virtually impossible (LCMV); irrespective of the dose or the route of infection, aly/aly mice developed life-long LCMV persistence. These results document the critical role of organized secondary lymphoid organs in the induction of naive T and B cells. These structures also provide the basis for cooperative interactions between antigen-presenting cells, T cells, and B cells, which are a prerequisite for recovery from primary virus infections via skin or via blood.
Immunohistochemistry (frozen)
Ingulli E, Mondino A, Khoruts A, Jenkins MK (1997). "In vivo detection of dendritic cell antigen presentation to CD4(+) T cells" J Exp Med 185(12):2133-41.
PubMed
Although lymphoid dendritic cells (DC) are thought to play an essential role in T cell activation, the initial physical interaction between antigen-bearing DC and antigen-specific T cells has never been directly observed in vivo under conditions where the specificity of the responding T cells for the relevant antigen could be unambiguously assessed. We used confocal microscopy to track the in vivo location of fluorescent dye-labeled DC and naive TCR transgenic CD4(+) T cells specific for an OVA peptide-I-Ad complex after adoptive transfer into syngeneic recipients. DC that were not exposed to the OVA peptide, homed to the paracortical regions of the lymph nodes but did not interact with the OVA peptide-specific T cells. In contrast, the OVA peptide-specific T cells formed large clusters around paracortical DC that were pulsed in vitro with the OVA peptide before injection. Interactions were also observed between paracortical DC of the recipient and OVA peptide-specific T cells after administration of intact OVA. Injection of OVA peptide-pulsed DC caused the specific T cells to produce IL-2 in vivo, proliferate, and differentiate into effector cells capable of causing a delayed-type hypersensitivity reaction. Surprisingly, by 48 h after injection, OVA peptide-pulsed, but not unpulsed DC disappeared from the lymph nodes of mice that contained the transferred TCR transgenic population. These results demonstrate that antigen-bearing DC directly interact with naive antigen-specific T cells within the T cell-rich regions of lymph nodes. This interaction results in T cell activation and disappearance of the DC.
in vivo targeting of dendritic cells
Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC (1996). "Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion" J Immunol 157(4):1406-14.
PubMed
Dendritic cells (DC) are unmatched among APCs in their ability to bind, process, and present Ag. Presentation by such potent APCs, if always immunogenic and never tolerogenic, might stimulate pathogenic autoimmune responses. To determine whether Ag presentation by DC can induce tolerance, mice were injected with a rat IgG2b anti-splenic DC mAb, 33D1, and challenged 13 to 28 days later with a stimulatory rat IgG2b mAb. Injection of mice with 1 ng/100 micrograms of 33D1 rarely induced an anti-rat IgG2b Ab response and, in most mice, induced rat IgG2b-specific T cell and B cell tolerance. Tolerant mice had decreased ability to secrete Ab and make both type 1 and type 2 cytokine mRNA and protein in response to immunization with rat IgG2b. 33D1 was 100- to 1000-fold more potent as a tolerogen than an isotype-matched control rat IgG2b mAb. Injecting mice with aggregated 33D1, 33D1 plus anti-IgD mAb, or 33D1 plus IL-1 induced an IgG1 anti-rat IgG2b Ab response rather than tolerance. IL-1 injected 3 days after 33D1 still induced an Ab response rather than tolerance. Not all anti-DC mAbs are tolerogenic. Injection of a DC-specific hamster anti-CD11c mAb (N418) stimulates an IgG anti-hamster response, and injection of 33D1 plus N418 stimulates both anti-hamster and anti-rat IgG2b responses. These observations indicate that DCs can present Ag in either a tolerogenic or stimulatory manner and suggest that inflammatory stimuli can convert an otherwise tolerogenic signal to a stimulatory signal.
Immunoprecipitation
Huleatt JW, Lefrançois L (1995). "Antigen-driven induction of CD11c on intestinal intraepithelial lymphocytes and CD8+ T cells in vivo" J Immunol 154(11):5684-93.
PubMed
Intraepithelial lymphocytes (IEL) of the intestinal epithelium represent a phenotypically and functionally distinct subpopulation of peripheral T cells. In this study, we report the production of a mAb, designated HL3, which exhibits reactivity with a subset of IEL. In differential screening assays HL3 reacted with 30 to 50% of IEL, but not with T cells of the thymus, spleen, or lymph nodes. Biochemical characterization revealed that the HL3 mAb recognized p150,95 (CD11c/CD18; CR4), a member of the beta 2-integrin family. Fluorescence flow cytometric analyses showed that p150,95 was expressed by TCR-alpha beta or TCR-gamma delta CD4-8+ IEL but not by CD4+8- IEL. Induction of graft-vs-host (GVH) disease resulted in up-regulation of p150,95 expression on donor-derived CD8+ T cells in the intestinal epithelium, as well as in the spleen and lymph nodes. GVH also induced MAC-1 (CD11b) expression on a subset of CD8+ lymph node T cells, but MAC-1 was not up-regulated on CD8+ IEL in this situation. In contrast, activation of identical T cell responders in vitro resulted in weak induction of p150,95 and MAC-1 expression. This result suggested that activation alone was insufficient for p150,95 up-regulation and that additional factors available in vivo were essential in this process. In the intestine, induction of p150,95 required the presence of intestinal flora as IEL from germfree mice lacked p150,95. Interestingly, gamma delta IEL expressing a non-IEL type transgenic TCR were also p150,95-, but exposure to Ag in vivo, but not in vitro, resulted in p150,95 induction. This result indicated that the constitutive expression of p150,95 on IEL is likely due to Ag stimulation via the TCR and not a bystander phenomenon. Overall, the results demonstrated p150,95 to be a hallmark of T cell activation in vivo and an indicator of ongoing antigen-specific T cell activation in the intestinal epithelium.
Flow Cytometry
Immunohistochemistry (frozen)
Witmer-Pack MD, Crowley MT, Inaba K, Steinman RM (1993). "Macrophages, but not dendritic cells, accumulate colloidal carbon following administration in situ" J Cell Sci .
PubMed
The dendritic cell system operates in situ to capture and present antigens in a form that is immunogenic to T cells. It is likely that dendritic cells require endocytic activity in order to process antigens. On the other hand, macrophages are considered to be the principal cells that internalize substrates in situ. We therefore investigated the phenotype of cells that scavenge the indigestible endocytic tracer, colloidal carbon, by phenotyping the endocytic cells with monoclonal antibodies that help distinguish macrophages from dendritic cells. Of some importance was the monoclonal N418, an antibody to the p150/90 leukocyte beta 2 integrin. FACS analyses on isolates from blood, spleen and peritoneal cavity showed that N418 reacts primarily with dendritic cells. N418 also stained dendritic profiles strongly in tissue sections of liver and spleen, but most of the cells that actively endocytosed carbon in both organs showed little or no N418 staining. Likewise, carbon could not be identified in cells that react with M342, which stains intracellular granules of dendritic cells. In contrast, the carbon-labeled cells in both liver and spleen were labeled with antibodies (SER-4, F4/80, FA11) that bind primarily to isolated macrophages. Therefore the clearance of colloidal carbon in situ reflects the scavenging activity of macrophages and not the endocytic activity that underlies the antigen presenting function of dendritic cells.
Flow Cytometry
Immunohistochemistry (frozen)
Immunoprecipitation
Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM (1990). "The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies" J Exp Med 171(5):1753-71.
PubMed
Hybridoma fusions with hamster hosts were undertaken to generate mAbs to mouse spleen dendritic cells. Two mAb were obtained and used to uncover the distinct integrins of these APC. One, 2E6, bound a determinant common to all members of the CD11/CD18 family, most likely the shared 90 kD CD18 beta chain. 2E6 immunoprecipitated the characteristic beta 2 integrin heterodimers from lymphocytes (p180, 90; CD11a) and macrophages (p170,90; CD11b), but from dendritic cells, a p150,90 (presumably CD11c) integrin was the predominant species. 2E6 inhibited the binding function of the CD11a and CD11b integrins on B cells and macrophages in appropriate assays, but 2E6 exerted little or no inhibition on the clustering of dendritic cells to T cells early in primary MLR, suggesting a CD11/CD18-independent mechanism for this binding. The second mAb, N418, precipitated a 150, 90 kD heterodimer that shared the 2E6 CD18 epitope. This N418 epitope may be the murine homologue of the previously characterized human CD11c molecule, but the epitope was only detected on dendritic cells. N418 did not react with peritoneal macrophages, anti-Ig-induced spleen B blasts, or bulk lymph node cells. When used to stain sections of spleen, N418 stained dendritic cells in the T-dependent areas, much like anti-class II mAbs that were also generated in these fusions. In addition, N418 revealed nests of dendritic cells that punctuated the rim of marginal zone macrophages between red and white pulp. This localization positioned most dendritic cells at regions where arterial vessels and T cells enter the white pulp. We conclude that the p150, 90 heterodimer is the major beta 2 integrin of spleen dendritic cells, and we speculate that it may function to localize these APC at sites that permit access to the recirculating pool of resting T cells.
Flow Cytometry
Functional Assays
Crowley MT, Inaba K, Witmer-Pack MD, Gezelter S, Steinman RM (1990). "Use of the fluorescence activated cell sorter to enrich dendritic cells from mouse spleen" J Immunol Methods 133(1):55-66.
PubMed
Dendritic cells are a specialized but trace population of antigen presenting cells that always have been enriched by multi-step procedures over a period of 1 or more days in tissue culture. Here we describe the isolation of dendritic cells from fresh mouse spleen suspensions using the FACS and a monoclonal antibody, N418, to the p150/90 member of the leukocyte integrin family (Metlay et al., 1990). By two color fluorescence activated cell sorter (FACS) analyses, the trace N418+ subset expressed most of the surface markers, including the 33D1 antigen, that are characteristic of dendritic cells isolated by other methods. An exception was that small amounts of Fc receptors, CD4 and F4/80 antigen were detected initially, but these diminished upon culture. In functional assays, sorted N418+ cells from fresh spleen were at least 30 times more active than N418- cells in presenting antigen to T cells. The assays were stimulation of the primary mixed leukocyte reaction and presentation of exogenous protein antigens to sensitized populations of lymph node T cells. The viability and MLR stimulating function of the sorted populations both were increased upon exposure to the cytokine, granulocyte-macrophage colony stimulating factor (GM-CSF). These results indicate that dendritic cells can be enriched from fresh isolates of mouse spleen using the FACS, and that when this is done, many of the distinctive features of dendritic cells - phenotype, APC function, and sensitivity to appropriate cytokines - are apparent.
Flow Cytometry
Crowley M, Inaba K, Steinman RM (1990). "Dendritic cells are the principal cells in mouse spleen bearing immunogenic fragments of foreign proteins" J Exp Med 172(1):383-6.
PubMed
We monitored the APC function of cells taken from the spleen and peritoneal cavity of mice that had been given protein antigens via the intravenous or intraperitoneal routes. Using the mAb 33D1 and N418 to negatively and positively select dendritic cells, we obtained evidence that dendritic cells are the main cell type in spleen that carries the protein in a form that is immunogenic for antigen-specific T cells. In vivo pulsed macrophages were not immunogenic and did not appear capable of transferring peptide fragments to dendritic cells.
Product Citations
-
-
Cancer Research
Neutrophils drive vascular occlusion, tumour necrosis and metastasis.
In Nature on 1 September 2025 by Adrover, J. M., Han, X., et al.
PubMed
Tumour necrosis is associated with poor prognosis in cancer1,2 and is thought to occur passively when tumour growth outpaces nutrient supply. Here we report, however, that neutrophils actively induce tumour necrosis. In multiple cancer mouse models, we found a tumour-elicited Ly6GHighLy6CLow neutrophil population that was unable to extravasate in response to inflammatory challenges but formed neutrophil extracellular traps (NETs) more efficiently than classical Ly6GHighLy6CHigh neutrophils. The presence of these 'vascular-restricted' neutrophils correlated with the appearance of a 'pleomorphic' necrotic architecture in mice. In tumours with pleomorphic necrosis, we found intravascular aggregates of neutrophils and NETs that caused occlusion of the tumour vasculature, driving hypoxia and necrosis of downstream vascular beds. Furthermore, we found that cancer cells adjacent to these necrotic regions (that is, in 'perinecrotic' areas) underwent epithelial-to-mesenchymal transition, explaining the paradoxical metastasis-enhancing effect of tumour necrosis. Blocking NET formation genetically or pharmacologically reduced the extent of tumour necrosis and lung metastasis. Thus, by showing that NETs drive vascular occlusion, pleomorphic necrosis and metastasis, we demonstrate that tumour necrosis is not necessarily a passive byproduct of tumour growth and that it can be blocked to reduce metastatic spread.
-
-
-
Cell Biology
-
Neuroscience
The gut metabolite indole-3 propionate promotes nerve regeneration and repair.
In Nature on 1 July 2022 by Serger, E., Luengo-Gutierrez, L., et al.
PubMed
The regenerative potential of mammalian peripheral nervous system neurons after injury is critically limited by their slow axonal regenerative rate1. Regenerative ability is influenced by both injury-dependent and injury-independent mechanisms2. Among the latter, environmental factors such as exercise and environmental enrichment have been shown to affect signalling pathways that promote axonal regeneration3. Several of these pathways, including modifications in gene transcription and protein synthesis, mitochondrial metabolism and the release of neurotrophins, can be activated by intermittent fasting (IF)4,5. However, whether IF influences the axonal regenerative ability remains to be investigated. Here we show that IF promotes axonal regeneration after sciatic nerve crush in mice through an unexpected mechanism that relies on the gram-positive gut microbiome and an increase in the gut bacteria-derived metabolite indole-3-propionic acid (IPA) in the serum. IPA production by Clostridium sporogenes is required for efficient axonal regeneration, and delivery of IPA after sciatic injury significantly enhances axonal regeneration, accelerating the recovery of sensory function. Mechanistically, RNA sequencing analysis from sciatic dorsal root ganglia suggested a role for neutrophil chemotaxis in the IPA-dependent regenerative phenotype, which was confirmed by inhibition of neutrophil chemotaxis. Our results demonstrate the ability of a microbiome-derived metabolite, such as IPA, to facilitate regeneration and functional recovery of sensory axons through an immune-mediated mechanism.
-
-
-
Cell Biology
-
Neuroscience
-
Mus musculus (Mouse)
The intermittent fasting-dependent gut microbial metabolite indole-3 propionate promotes nerve regeneration and recovery after injury
In Research Square on 16 December 2020 by Giovanni, S. D., Serger, E., et al.
-