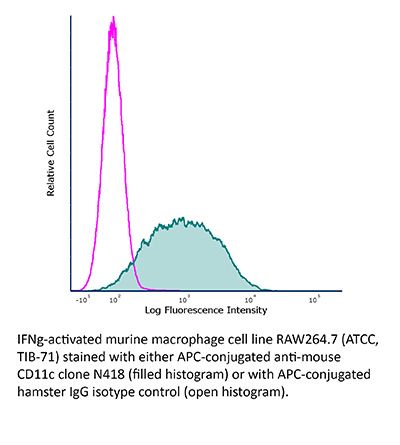
FlowMAb APC anti-mouse CD11c
Product Description
Specifications
| Isotype | Armenian Hamster IgG2 |
|---|---|
| Conjugation | APC |
| Excitation Source | Red 627-640 nm |
| Excitation Max | 651 nm |
| Emission Max | 660 nm |
| Immunogen | Mouse spleen dendritic cells |
| Reported Applications |
Flow cytometry Immunohistochemistry (frozen) Immunohistochemistry (paraffin) Immunofluorescence |
| Protocol Information | It is recommended that the reagent be carefully titrated for optimal performance in the assay of interest. |
| Concentration | 0.2 mg/ml |
| Formulation |
PBS, pH 7.0 Contains 0.09% Sodium Azide |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A. Conjugated with allophycocyanin under optimal conditions. |
| Storage | The antibody solution should be stored at the stock concentration at 4°C and protected from prolonged exposure to light. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Immunohistochemistry (frozen)
Castro FV, Tutt AL, White AL, Teeling JL, James S, French RR, Glennie MJ (2008). "CD11c provides an effective immunotarget for the generation of both CD4 and CD8 T cell responses" Eur J Immunol 38(8):2263-73.
PubMed
The magnitude and quality of T cell responses generated when Ag is targeted to receptors on DC is influenced by both the specific receptor targeted and its distribution among DC subsets. Here we examine the targeting of the model Ag OVA to potential DC targets, including CD11c, CD205, MHC class II, CD40, TLR2 and FcgammaRII/III, using a panel of (Fab' x OVA) conjugates. In vitro studies identified CD11c, CD205 and MHC class II as superior and comparably effective immunotargets for the delivery of OVA to APC for presentation to T cells. In vivo studies, however, showed a marked advantage of targeting Ag to CD11c for both CD4 (OT-II) and CD8 (OT-I) responses, with robust stimulation after a single, low dose (equivalent to 0.5 microg OVA); in contrast, (anti-CD205 x OVA) and (anti-MHC class II x OVA) resulted in markedly less proliferation of both OT-I and OT-II cells. Biodistribution and immunohistochemical studies suggest that the exceptional ability of CD11c to capture Ag in lymphoid tissues may, at least partially, explain its ability to promote T cell responses. These results suggest that targeting antigen via CD11c offers a previously unappreciated strategy for vaccine development which, unlike most targets, delivers robust responses of both CD4 and CD8 T cells.
Flow Cytometry
Crowley M, Inaba K, Steinman RM (1990). "Dendritic cells are the principal cells in mouse spleen bearing immunogenic fragments of foreign proteins" J Exp Med 172(1):383-6.
PubMed
We monitored the APC function of cells taken from the spleen and peritoneal cavity of mice that had been given protein antigens via the intravenous or intraperitoneal routes. Using the mAb 33D1 and N418 to negatively and positively select dendritic cells, we obtained evidence that dendritic cells are the main cell type in spleen that carries the protein in a form that is immunogenic for antigen-specific T cells. In vivo pulsed macrophages were not immunogenic and did not appear capable of transferring peptide fragments to dendritic cells.
Flow Cytometry
Immunohistochemistry (frozen)
Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM (1990). "The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies" J Exp Med 171(5):1753-71.
PubMed
Hybridoma fusions with hamster hosts were undertaken to generate mAbs to mouse spleen dendritic cells. Two mAb were obtained and used to uncover the distinct integrins of these APC. One, 2E6, bound a determinant common to all members of the CD11/CD18 family, most likely the shared 90 kD CD18 beta chain. 2E6 immunoprecipitated the characteristic beta 2 integrin heterodimers from lymphocytes (p180, 90; CD11a) and macrophages (p170,90; CD11b), but from dendritic cells, a p150,90 (presumably CD11c) integrin was the predominant species. 2E6 inhibited the binding function of the CD11a and CD11b integrins on B cells and macrophages in appropriate assays, but 2E6 exerted little or no inhibition on the clustering of dendritic cells to T cells early in primary MLR, suggesting a CD11/CD18-independent mechanism for this binding. The second mAb, N418, precipitated a 150, 90 kD heterodimer that shared the 2E6 CD18 epitope. This N418 epitope may be the murine homologue of the previously characterized human CD11c molecule, but the epitope was only detected on dendritic cells. N418 did not react with peritoneal macrophages, anti-Ig-induced spleen B blasts, or bulk lymph node cells. When used to stain sections of spleen, N418 stained dendritic cells in the T-dependent areas, much like anti-class II mAbs that were also generated in these fusions. In addition, N418 revealed nests of dendritic cells that punctuated the rim of marginal zone macrophages between red and white pulp. This localization positioned most dendritic cells at regions where arterial vessels and T cells enter the white pulp. We conclude that the p150, 90 heterodimer is the major beta 2 integrin of spleen dendritic cells, and we speculate that it may function to localize these APC at sites that permit access to the recirculating pool of resting T cells.
Immunohistochemistry (paraffin)
Flow Cytometry
Chaussabel D, Pajak B, Vercruysse V, Bisseyé C, Garzé V, Habib M, Goldman M, Moser M, Vray B (2003). "Alteration of migration and maturation of dendritic cells and T-cell depletion in the course of experimental Trypanosoma cruzi infection" Lab Invest
PubMed
Trypanosoma cruzi, the etiologic agent of Chagas disease, induces infection that affects most immunocompetent cells. However, its effect on dendritic cells (DC) is still unknown in vivo. In this report, we show, by immunohistochemical staining, that T. cruzi infection triggers a huge increase in the number of CD11c(+) DC in the spleen of infected mice at Days 14 and 21 post-inoculation (pi). In mice reaching the chronic phase (starting on Day 35 pi), the number of splenic DC (sDC) returned progressively to normal (ending on Day 98 pi). In the spleens of noninfected mice, most of the CD8alpha(+)CD11c(+) and CD8alpha(-)CD11c(+) DC were found in the red pulp and the marginal and T-cell zones. However, starting on Day 14 pi, a progressive decline of CD8alpha(+)CD11c(+) was observed. In addition, sDC expressed low levels of the costimulatory molecule B7.2 at Days 14 and 21 pi, suggesting that they remained immature in the course of the infection. As expected, in lipopolysaccharide-treated and noninfected mice, the expression of B7.2 molecules was sharply up-regulated on sDC that migrated toward the T-cell zone. In contrast, upon lipopolysaccharide stimulation, sDC from T. cruzi-infected mice did not migrate toward the T-cell zone nor did they undergo maturation. Finally, white pulp was severely depleted in both CD4(+) and CD8(+) T cells at the peak of infection. Taken together, these results indicate that profound alterations of migration and maturation of sDC and depletion/redistribution of T cells occur during the acute phase of T. cruzi infection and could be part of another strategy to escape immune surveillance and to persist in the host.
Immunohistochemistry (frozen)
Probst HC, Tschannen K, Odermatt B, Schwendener R, Zinkernagel RM, Van Den Broek M (2005). "Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells" Clin Exp Immunol 141(3):398-404.
PubMed
To investigate the dependence of individual immunological processes on DC, a transgenic mouse system (CD11c-DTR/GFP mice) has been developed that allows conditional depletion of CD11c+ DC in vivo through administration of diphtheria toxin. We have performed careful histological analysis of CD11c-DTR/GFP mice at different time points after diphtheria toxin injection and confirmed the transient depletion of CD11c+ cells from lymph nodes and spleen. Unexpectedly, the injection of diphtheria toxin completely depleted marginal zone and metallophilic M(Phi) from the spleen and their sinusoidal counterparts from the lymph nodes. This finding limits the use of CD11c-DTR/GFP mice for the analysis of the role of DC to models and read outs that are proven to be independent of marginal zone and sinusoidal M(Phi).
Immunohistochemistry (frozen)
Reis e Sousa C, Germain RN (1999). "Analysis of adjuvant function by direct visualization of antigen presentation in vivo: endotoxin promotes accumulation of antigen-bearing dendritic cells in the T cell areas of lymphoid tissue" J Immunol 162(11):65
PubMed
T cell activation requires exposure to processed Ag and signaling by cytokines and costimulatory ligands. Adjuvants are thought to enhance immunity primarily through up-regulation of the latter signals. Here, we explore the effect of the bacterial adjuvant, endotoxin, on Ag presentation by B cells and dendritic cells (DC). Using an mAb (C4H3) specific for the hen egg lysozyme (HEL) 46-61 determinant bound to I-Ak, we analyze processed Ag expression and the tissue distribution of presenting cells following systemic administration of soluble HEL to mice. In both LPS-responsive and -hyporesponsive mice given endotoxin-containing HEL, B cells rapidly display surface 46-61/I-Ak complexes. In marked contrast, in LPS-hyporesponsive mice, splenic DC show little gain in C4H3 staining. In LPS-responsive animals, interdigitating DC in T cell areas show no staining above background at early times after HEL administration, but C4H3+ DC rapidly accumulate in the outer periarteriolar lymphoid sheaths (PALS) and in follicular areas. Within a few hours, C4H3+ DC appear in the T cell areas, concomitant with a decline in C4H3+ cells in the outer PALS, suggesting migration between these two sites. Endotoxin enhancement of C4H3 staining is seen for both CD8alpha- and CD8alpha+ DC subsets. These data suggest that a major effect of adjuvants is to promote mobilization of Ag-bearing DC to the T areas of lymphoid tissue, and possibly also to enhance Ag processing by these DC. Thus, microbial products promote T cell immunity not only through DC activation for cosignaling, but through improvement in signal 1 delivery.
Flow Cytometry
Immunohistochemistry (frozen)
Witmer-Pack MD, Crowley MT, Inaba K, Steinman RM (1993). "Macrophages, but not dendritic cells, accumulate colloidal carbon following administration in situ" J Cell Sci .
PubMed
The dendritic cell system operates in situ to capture and present antigens in a form that is immunogenic to T cells. It is likely that dendritic cells require endocytic activity in order to process antigens. On the other hand, macrophages are considered to be the principal cells that internalize substrates in situ. We therefore investigated the phenotype of cells that scavenge the indigestible endocytic tracer, colloidal carbon, by phenotyping the endocytic cells with monoclonal antibodies that help distinguish macrophages from dendritic cells. Of some importance was the monoclonal N418, an antibody to the p150/90 leukocyte beta 2 integrin. FACS analyses on isolates from blood, spleen and peritoneal cavity showed that N418 reacts primarily with dendritic cells. N418 also stained dendritic profiles strongly in tissue sections of liver and spleen, but most of the cells that actively endocytosed carbon in both organs showed little or no N418 staining. Likewise, carbon could not be identified in cells that react with M342, which stains intracellular granules of dendritic cells. In contrast, the carbon-labeled cells in both liver and spleen were labeled with antibodies (SER-4, F4/80, FA11) that bind primarily to isolated macrophages. Therefore the clearance of colloidal carbon in situ reflects the scavenging activity of macrophages and not the endocytic activity that underlies the antigen presenting function of dendritic cells.
Flow Cytometry
Crowley MT, Inaba K, Witmer-Pack MD, Gezelter S, Steinman RM (1990). "Use of the fluorescence activated cell sorter to enrich dendritic cells from mouse spleen" J Immunol Methods 133(1):55-66.
PubMed
Dendritic cells are a specialized but trace population of antigen presenting cells that always have been enriched by multi-step procedures over a period of 1 or more days in tissue culture. Here we describe the isolation of dendritic cells from fresh mouse spleen suspensions using the FACS and a monoclonal antibody, N418, to the p150/90 member of the leukocyte integrin family (Metlay et al., 1990). By two color fluorescence activated cell sorter (FACS) analyses, the trace N418+ subset expressed most of the surface markers, including the 33D1 antigen, that are characteristic of dendritic cells isolated by other methods. An exception was that small amounts of Fc receptors, CD4 and F4/80 antigen were detected initially, but these diminished upon culture. In functional assays, sorted N418+ cells from fresh spleen were at least 30 times more active than N418- cells in presenting antigen to T cells. The assays were stimulation of the primary mixed leukocyte reaction and presentation of exogenous protein antigens to sensitized populations of lymph node T cells. The viability and MLR stimulating function of the sorted populations both were increased upon exposure to the cytokine, granulocyte-macrophage colony stimulating factor (GM-CSF). These results indicate that dendritic cells can be enriched from fresh isolates of mouse spleen using the FACS, and that when this is done, many of the distinctive features of dendritic cells - phenotype, APC function, and sensitivity to appropriate cytokines - are apparent.
Immunohistochemistry (paraffin)
Henderson WR, Chi EY, Albert RK, Chu SJ, Lamm WJ, Rochon Y, Jonas M, Christie PE, Harlan JM (1997). "Blockade of CD49d (alpha4 integrin) on intrapulmonary but not circulating leukocytes inhibits airway inflammation and hyperresponsiveness in a mouse
PubMed
Immunized mice after inhalation of specific antigen have the following characteristic features of human asthma: airway eosinophilia, mucus and Th2 cytokine release, and hyperresponsiveness to methacholine. A model of late-phase allergic pulmonary inflammation in ovalbumin-sensitized mice was used to address the role of the alpha4 integrin (CD49d) in mediating the airway inflammation and hyperresponsiveness. Local, intrapulmonary blockade of CD49d by intranasal administration of CD49d mAb inhibited all signs of lung inflammation, IL-4 and IL-5 release, and hyperresponsiveness to methacholine. In contrast, CD49d blockade on circulating leukocytes by intraperitoneal CD49d mAb treatment only prevented the airway eosinophilia. In this asthma model, a CD49d-positive intrapulmonary leukocyte distinct from the eosinophil is the key effector cell of allergen-induced pulmonary inflammation and hyperresponsiveness.
Immunohistochemistry (frozen)
Karrer U, Althage A, Odermatt B, Roberts CW, Korsmeyer SJ, Miyawaki S, Hengartner H, Zinkernagel RM (1997). "On the key role of secondary lymphoid organs in antiviral immune responses studied in alymphoplastic (aly/aly) and spleenless (Hox11(-)/-) mu
PubMed
The role of the spleen and of other organized secondary lymphoid organs for the induction of protective antiviral immune responses was evaluated in orphan homeobox gene 11 knockout mice (Hox11(-/-)) lacking the spleen, and in homozygous alymphoplastic mutant mice (aly/aly) possessing a structurally altered spleen but lacking lymph nodes and Peyer's patches. Absence of the spleen had no major effects on the immune response, other than delaying the antibody response by 1-2 d. In aly/aly mice, the thymus-independent IgM response against vesicular stomatitis virus (VSV) was delayed and reduced, whereas the T-dependent switch to the protective IgG was absent. Therefore, aly/aly mice were highly susceptible to VSV infection. Since aly/aly spleen cells yielded neutralizing IgM and IgG after adoptive transfer into recipients with normally structured secondary lymphoid organs, these data suggest that the structural defect was mainly responsible for inefficient T-B cooperation. Although aly/aly mice generated detectable, but reduced, CTL responses after infection with vaccinia virus (VV) and lymphocytic choriomeningitis virus (LCMV), the elimination of these viruses was either delayed (VV) or virtually impossible (LCMV); irrespective of the dose or the route of infection, aly/aly mice developed life-long LCMV persistence. These results document the critical role of organized secondary lymphoid organs in the induction of naive T and B cells. These structures also provide the basis for cooperative interactions between antigen-presenting cells, T cells, and B cells, which are a prerequisite for recovery from primary virus infections via skin or via blood.
Flow Cytometry
Paczesny S, Beranger S, Salzmann JL, Klatzmann D, Colombo BM (2001). "Protection of mice against leukemia after vaccination with bone marrow-derived dendritic cells loaded with apoptotic leukemia cells" Cancer Res 61(6):2386-9.
PubMed
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) that need to be activated before they can function to initiate primary and secondary immune responses in vivo. DCs are also specialized to maintain peripheral tolerance to self after uptake of apoptotic material, likely corresponding to both apoptotic bodies and whole apoptotic cells. Here, we report that murine bone marrow-derived DCs can be activated in vitro by exogenous signals received from apoptotic leukemia cells expressing on the cell surface a model tumor-associated antigen. Injected in vivo, these exogenously activated DCs can function as adjuvants to protect mice against leukemia by stimulating an antigen-specific cellular-mediated cytotoxic immune response. To our knowledge, this is the first report indicating that DCs loaded with apoptotic leukemia cells protect mice against leukemia development.
Immunohistochemistry (frozen)
Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD (2008). "Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii" Immunity 29(2):306-17.
PubMed
The enteric pathogen Toxoplasma gondii is controlled by a vigorous innate T helper 1 (Th1) cell response in the murine model. We demonstrated that after oral infection, the parasite rapidly recruited inflammatory monocytes [Gr1(+) (Ly6C(+), Ly6G(-)) F4/80(+)CD11b(+)CD11c(-)], which established a vital defensive perimeter within the villi of the ileum in the small intestine. Mice deficient of the chemokine receptor CCR2 or the ligand CCL2 failed to recruit Gr1(+) inflammatory monocytes, whereas dendritic cells and resident tissue macrophages remained unaltered. The selective lack of Gr1(+) inflammatory monocytes resulted in an inability of mice to control replication of the parasite, high influx of neutrophils, extensive intestinal necrosis, and rapid death. Adoptive transfer of sorted Gr1(+) inflammatory monocytes demonstrated their ability to home to the ileum in infected animals and protect Ccr2(-/-) mice, which were otherwise highly susceptible to oral toxoplasmosis. Collectively, these findings illustrate the critical importance of inflammatory monocytes as a first line of defense in controlling intestinal pathogens.
Immunohistochemistry (frozen)
Ingulli E, Mondino A, Khoruts A, Jenkins MK (1997). "In vivo detection of dendritic cell antigen presentation to CD4(+) T cells" J Exp Med 185(12):2133-41.
PubMed
Although lymphoid dendritic cells (DC) are thought to play an essential role in T cell activation, the initial physical interaction between antigen-bearing DC and antigen-specific T cells has never been directly observed in vivo under conditions where the specificity of the responding T cells for the relevant antigen could be unambiguously assessed. We used confocal microscopy to track the in vivo location of fluorescent dye-labeled DC and naive TCR transgenic CD4(+) T cells specific for an OVA peptide-I-Ad complex after adoptive transfer into syngeneic recipients. DC that were not exposed to the OVA peptide, homed to the paracortical regions of the lymph nodes but did not interact with the OVA peptide-specific T cells. In contrast, the OVA peptide-specific T cells formed large clusters around paracortical DC that were pulsed in vitro with the OVA peptide before injection. Interactions were also observed between paracortical DC of the recipient and OVA peptide-specific T cells after administration of intact OVA. Injection of OVA peptide-pulsed DC caused the specific T cells to produce IL-2 in vivo, proliferate, and differentiate into effector cells capable of causing a delayed-type hypersensitivity reaction. Surprisingly, by 48 h after injection, OVA peptide-pulsed, but not unpulsed DC disappeared from the lymph nodes of mice that contained the transferred TCR transgenic population. These results demonstrate that antigen-bearing DC directly interact with naive antigen-specific T cells within the T cell-rich regions of lymph nodes. This interaction results in T cell activation and disappearance of the DC.
Immunofluorescence
Flow Cytometry
Gu P, Gao JF, D', Souza CA, Kowalczyk A, Chou KY, Zhang L (2012). "Trogocytosis of CD80 and CD86 by induced regulatory T cells" Cell Mol Immunol 9(2):136-46.
PubMed
Trogocytosis is a process which involves the transfer of membrane fragments and cell surface proteins between cells. Various types of T cells have been shown to be able to acquire membrane-bound proteins from antigen-presenting cells and their functions can be modulated following trogocytosis. However, it is not known whether induced regulatory T cells (iTregs) can undergo trogocytosis, and if so, what the functional consequences of this process might entail. In this study, we show that iTregs can be generated from CD80(-/-)CD86(-/-) double knockout (DKO) mice. Using flow cytometry and confocal fluorescence microscopy, we demonstrate that iTregs generated from DKO mice are able to acquire both CD80 and CD86 from mature dendritic cells (mDCs) and that the acquisition of CD86 occurs to a higher extent than that of CD80. Furthermore, we found that after co-incubation with iTregs, dendritic cells (DCs) downregulate their surface expression of CD80 and CD86. The trogocytosis of both CD80 and CD86 occurs in a cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), CD28 and programmed death ligand-1 (PDL1)-independent manner. Importantly, we showed that iTregs that acquired CD86 from mDCs expressed higher activation markers and their ability to suppress naive CD4(+) T-cell proliferation was enhanced, compared to iTregs that did not acquire CD86. These data demonstrate, for the first time, that iTregs can acquire CD80 and CD86 from mDCs, and the acquisition of CD86 may enhance their suppressive function. These findings provide novel understanding of the interaction between iTregs and DCs, suggesting that trogocytosis may play a significant role in iTreg-mediated immune suppression.
Immunofluorescence
Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL (2002). "Identification of a novel macrophage population in the normal mouse corneal stroma" Invest Ophthalmol Vis Sci 43(7):2264-71.
PubMed
Purpose: To examine the normal murine corneal stroma for the presence of bone marrow-derived leukocytes. Methods: Wholemounts of paraformaldehyde-fixed corneal stroma from normal mice at 5 to 16 weeks of age were examined in single- and double-color immunomorphologic studies performed with confocal microscopy. The phenotype, morphology, distribution, and density of immunopositive cells were determined. Results: Numerous CD45(+) cells with pleomorphic and dendriform morphology were found within the pericentral and central region of the corneal stroma (200-300 cells/mm(2)). Dual-color immunostaining demonstrated that 100% of the CD45(+) cells coexpressed CD11b and 50% coexpressed F4/80. Approximately 30% of the total cells and 50% of the F4/80(+) cells coexpressed major histocompatibility complex (MHC) class II antigens. Very small to negligible numbers of cells expressed markers of dendritic cells (CD11c) or granulocytes (Ly6G). Markers for T-cells and NK cells were absent from the corneal stroma, indicating that all the cells identified in the stroma were of the myeloid lineage. Conclusions: The normal murine corneal stroma contains a significant number of CD45(+) leukocytes. Most these cells express the CD11b marker, but not other dendrite, granulocyte, T-cell, or NK markers, placing them in the monocyte/macrophage lineage.