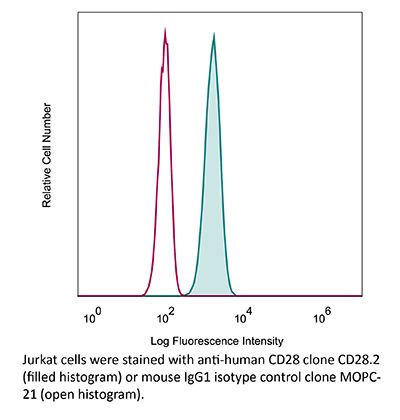
InVivoMAb anti-human/monkey CD28
Product Description
Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Human CD28 transfected cell line |
| Reported Applications |
in vitro T cell stimulation/activation Immunoprecipitation Flow cytometry Immunohistochemistry (frozen) |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687814 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Immunoprecipitation
Zhao, Y., et al (2019). "PD-L1:CD80 Cis-Heterodimer Triggers the Co-stimulatory Receptor CD28 While Repressing the Inhibitory PD-1 and CTLA-4 Pathways" Immunity 51(6): 1059-1073.e1059.
PubMed
Combined immunotherapy targeting the immune checkpoint receptors cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death 1 (PD-1), or CTLA-4 and the PD-1 ligand (PD-L1) exhibits superior anti-tumor responses compared with single-agent therapy. Here, we examined the molecular basis for this synergy. Using reconstitution assays with fluorescence readouts, we found that PD-L1 and the CTLA-4 ligand CD80 heterodimerize in cis but not trans. Quantitative biochemistry and cell biology assays revealed that PD-L1:CD80 cis-heterodimerization inhibited both PD-L1:PD-1 and CD80:CTLA-4 interactions through distinct mechanisms but preserved the ability of CD80 to activate the T cell co-stimulatory receptor CD28. Furthermore, PD-L1 expression on antigen-presenting cells (APCs) prevented CTLA-4-mediated trans-endocytosis of CD80. Atezolizumab (anti-PD-L1), but not anti-PD-1, reduced cell surface expression of CD80 on APCs, and this effect was negated by co-blockade of CTLA-4 with ipilimumab (anti-CTLA-4). Thus, PD-L1 exerts an immunostimulatory effect by repressing the CTLA-4 axis; this has implications to the synergy of anti-PD-L1 and anti-CTLA-4 combination therapy.
in vitro T cell stimulation/activation
Blewett, M. M., et al (2016). "Chemical proteomic map of dimethyl fumarate-sensitive cysteines in primary human T cells" Sci Signal 9(445): rs10.
PubMed
Dimethyl fumarate (DMF) is an electrophilic drug that is used to treat autoimmune conditions, including multiple sclerosis and psoriasis. The mechanism of action of DMF is unclear but may involve the covalent modification of proteins or DMF serving as a prodrug that is converted to monomethyl fumarate (MMF). We found that DMF, but not MMF, blocked the activation of primary human and mouse T cells. Using a quantitative, site-specific chemical proteomic platform, we determined the DMF sensitivity of >2400 cysteine residues in human T cells. Cysteines sensitive to DMF, but not MMF, were identified in several proteins with established biochemical or genetic links to T cell function, including protein kinase Ctheta (PKCtheta). DMF blocked the association of PKCtheta with the costimulatory receptor CD28 by perturbing a CXXC motif in the C2 domain of this kinase. Mutation of these DMF-sensitive cysteines also impaired PKCtheta-CD28 interactions and T cell activation, designating the C2 domain of PKCtheta as a key functional, electrophile-sensing module important for T cell biology.
in vitro T cell stimulation/activation
Edwards, L. J., et al (2015). "Signal transducer and activator of transcription (STAT) 3 inhibition delays the onset of lupus nephritis in MRL/lpr mice" Clin Immunol 158(2): 221-230.
PubMed
The transcription factor STAT3 is overexpressed and hyperactivated in T cells from SLE patients. STAT3 plays a central role in T cell differentiation into Th17 and T follicular helper cells, two subsets that orchestrate autoimmune responses in SLE. Moreover, STAT3 is important in chemokine-mediated T cell migration. To better understand its role in SLE, we inhibited STAT3 in lupus-prone mice using the small molecule Stattic. Stattic-treated mice exhibited delayed onset of proteinuria (3 weeks later than controls), and had lower levels of anti-dsDNA antibodies and inflammatory cytokines. Inhibitor treatment reduced lymphadenopathy, resulted in a 3-fold decrease in total T cell number, and a 4-fold decrease in the numbers of T follicular helper cells. In vitro experiments showed that Stattic-treated T cells exhibited decreased proliferation and a decrease in ability to migrate to CXCL12. We propose that STAT3 inhibition represents a therapeutic target in SLE, particularly lupus nephritis.
in vitro T cell stimulation/activation
Oh, Y. M., et al (2015). "Ndrg1 is a T-cell clonal anergy factor negatively regulated by CD28 costimulation and interleukin-2" Nat Commun 6: 8698.
PubMed
Induction of T-cell clonal anergy involves serial activation of transcription factors, including NFAT and Egr2/3. However, downstream effector mechanisms of these transcription factors are not fully understood yet. Here we identify Ndrg1 as an anergy factor induced by Egr2. Ndrg1 is upregulated by anergic signalling and maintained at high levels in resting anergic T cells. Overexpression of Ndrg1 mimics the anergic state and knockout of the gene prevents anergy induction. Interestingly, Ndrg1 is phosphorylated and degraded by CD28 signalling in a proteasome-dependent manner, explaining the costimulation dependence of anergy prevention. Similarly, IL-2 treatment of anergic T cells, under conditions that lead to the reversal of anergy, also induces Ndrg1 phosphorylation and degradation. Finally, older Ndrg1-deficient mice show T-cell hyperresponsiveness and Ndrg1-deficient T cells aggravate inducible autoimmune inflammation. Thus, Ndrg1 contributes to the maintenance of clonal anergy and inhibition of T-cell-mediated inflammation.
Flow Cytometry
Leonard, J. A., et al (2011). "HIV-1 Nef disrupts intracellular trafficking of major histocompatibility complex class I, CD4, CD8, and CD28 by distinct pathways that share common elements" J Virol 85(14): 6867-6881.
PubMed
The Nef protein is an important HIV virulence factor that promotes the degradation of host proteins to augment virus production and facilitate immune evasion. The best-characterized targets of Nef are major histocompatibility complex class I (MHC-I) and CD4, but Nef also has been reported to target several other proteins, including CD8beta, CD28, CD80, CD86, and CD1d. To compare and contrast the effects of Nef on each protein, we constructed a panel of chimeric proteins in which the extracellular and transmembrane regions of the MHC-I allele HLA-A2 were fused to the cytoplasmic tails of CD4, CD28, CD8beta, CD80, CD86, and CD1d. We found that Nef coprecipitated with and disrupted the expression of molecules with cytoplasmic tails from MHC-I HLA-A2, CD4, CD8beta, and CD28, but Nef did not bind to or alter the expression of molecules with cytoplasmic tails from CD80, CD86, and CD1d. In addition, we used short interfering RNA (siRNA) knockdown and coprecipitation experiments to implicate AP-1 as a cellular cofactor for Nef in the downmodulation of both CD28 and CD8beta. The interaction with AP-1 required for CD28 and CD8beta differed from the AP-1 interaction required for MHC-I downmodulation in that it was mediated through the dileucine motif within Nef (LL(164,165)AA) and did not require the tyrosine binding pocket of the AP-1 mu subunit. In addition, we demonstrate a requirement for beta-COP as a cellular cofactor for Nef that was necessary for the degradation of targeted molecules HLA-A2, CD4, and CD8. These studies provide important new information on the similarities and differences with which Nef affects intracellular trafficking and help focus future research on the best potential pharmaceutical targets.
Flow Cytometry
Rout, N., et al (2010). "Paucity of CD4+ natural killer T (NKT) lymphocytes in sooty mangabeys is associated with lack of NKT cell depletion after SIV infection" PLoS One 5(3): e9787.
PubMed
Lack of chronic immune activation in the presence of persistent viremia is a key feature that distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection in natural hosts from pathogenic SIV and HIV infection. To elucidate novel mechanisms downmodulating immune activation in natural hosts of SIV infection, we investigated natural killer T (NKT) lymphocytes in sooty mangabeys. NKT lymphocytes are a potent immunoregulatory arm of the innate immune system that recognize glycolipid antigens presented on the nonpolymorphic MHC-class I-like CD1d molecules. In a cross-sectional analysis of 50 SIV-negative and 50 naturally SIV-infected sooty mangabeys, ligand alpha-galactosylceramide loaded CD1d tetramers co-staining with Valpha24-positive invariant NKT lymphocytes were detected at frequencies >or=0.002% of circulating T lymphocytes in approximately half of the animals. In contrast to published reports in Asian macaques, sooty mangabey NKT lymphocytes consisted of CD8(+) and CD4/CD8 double-negative T lymphocytes that were CXCR3-positive and CCR5-negative suggesting that they trafficked to sites of inflammation without being susceptible to SIV infection. Consistent with these findings, there was no difference in the frequency or phenotype of NKT lymphocytes between SIV-negative and SIV-infected sooty mangabeys. On stimulation with alpha-galactosylceramide loaded on human CD1d molecules, sooty mangabey NKT lymphocytes underwent degranulation and secreted IFN-gamma, TNF-alpha, IL-2, IL-13, and IL-10, indicating the presence of both effector and immunoregulatory functional capabilities. The unique absence of CD4(+) NKT lymphocytes in sooty mangabeys, combined with their IL-10 cytokine-secreting ability and preservation following SIV infection, raises the possibility that NKT lymphocytes might play a role in downmodulating immune activation in SIV-infected sooty mangabeys.
Immunohistochemistry (frozen)
Tazi, A., et al (1999). "Evidence that Langerhans cells in adult pulmonary Langerhans cell histiocytosis are mature dendritic cells: importance of the cytokine microenvironment" J Immunol 163(6): 3511-3515.
PubMed
Because Langerhans cells (LC) in peripheral tissues are generally “immature” cells with poor lymphostimulatory activity, the contribution of immune responses initiated by LC to the pathogenesis of pulmonary LC histiocytosis (LCH) has been uncertain. In this study we demonstrate that LC accumulating in LCH granulomas are phenotypically similar to mature lymphostimulatory dendritic cells present in lymphoid organs. LC in LCH granulomas intensely expressed B7-1 and B7-2 molecules, whereas normal pulmonary LC and LC accumulating in other pathologic lung disorders did not express these costimulatory molecules. The presence of B7+ LC in LCH granulomas was associated with the expression in these lesions, but not at other sites in the lung, of a unique profile of cytokines (presence of GM-CSF, TNF-alpha, and IL-1beta and the absence of IL-10) that is known to promote the in vitro differentiation of LC into cells expressing a lymphostimulatory phenotype. Finally, LCH granulomas were the only site where CD154-positive T cells could be identified in close contact with LC intensely expressing CD40 Ags. Taken together, these results strongly support the idea that an abnormal immune response initiated by LC may participate in the pathogenesis of pulmonary LCH, and suggest that therapeutic strategies aimed at modifying the lymphostimulatory phenotype of LC may be useful in the treatment of this disorder.
Immunohistochemistry (frozen)
Battifora, M., et al (1998). "B7.1 costimulatory molecule is expressed on thyroid follicular cells in Hashimoto’s thyroiditis, but not in Graves’ disease" J Clin Endocrinol Metab 83(11): 4130-4139.
PubMed
The molecules of the B7 family play a major role in T-lymphocyte costimulation through interaction with their counterreceptors CD28 and CTLA4. In the present study, we analyzed the possible expression of B7 molecules on surgically removed thyroid tissue of patients with autoimmune [Hashimoto’s thyroiditis (HT) or Graves’ disease (GD)] or nonautoimmune [nontoxic goiter (NTG) or papillary cancer (PC)] thyroid diseases. We found clear positivity of thyroid follicular cells for B7.1 in HT but not in GD, nor in nonautoimmune specimens (NTG, PC) using in situ analysis by alkaline phosphatase anti-alkaline phosphatase (APAAP) technique. Double immunostaining experiments in combination with an anti-human thyroglobulin antibody confirmed follicular B7.1 localization. On the contrary, no follicular B7.2 expression was observed in any specimen analyzed. These findings were confirmed by immunofluorescence flow cytometry on isolated follicular cells. The cytokines IL1beta and LPS were able to induce de novo B7.1 expression on cultured thyroid follicular cells. Intrathyroid T cells proved responsive to stimulation via the B7 ligand CD28, even in the absence of IL2. Moreover preliminary evidence was obtained for an inhibitory effect of anti-B7.1 mAb on T-cell proliferation in coculture with isolated thyroid follicular cells. It is conceivable that in HT, expression of B7.1 on follicular cells, together with MHC class II antigens and ICAM1, could provide a local costimulatory signal for T-lymphocyte differentiation toward the type 1 cytokine secretion pattern and maintenance of the autoimmune process.
Product Citations
-
-
Immunology and Microbiology
Inflammatory arthritis immune related adverse events represent a unique autoimmune disease entity primarily driven by T cells, but likely not autoantibodies
In medRxiv on 6 June 2025 by Zhu, X., Yu, Y., et al.
-
-
-
Immunology and Microbiology
Polysialic acid is upregulated on activated immune cells and negatively regulates anticancer immune activity.
In Front Oncol on 4 April 2025 by Drummond-Guy, O., Daly, J., et al.
PubMed
Suppression of anticancer immune function is a key driver of tumorigenesis. Identifying molecular pathways that inhibit anticancer immunity is critical for developing novel immunotherapeutics. One such molecule that has recently been identified is the carbohydrate polysialic acid (polySia), whose expression is dramatically upregulated on both cancer cells and immune cells in breast cancer patient tissues. The role of polySia in the anticancer immune response, however, remains incompletely understood. In this study, we profile polySia expression on both healthy primary immune cells and on infiltrating immune cells in the tumour microenvironment (TME). These studies reveal polySia expression on multiple immune cell subsets in patient breast tumors. We find that stimulation of primary T-cells and macrophages in vitro induces a significant upregulation of polySia expression. We subsequently show that polySia is appended to a range of different carrier proteins within these immune cells. Finally, we find that selective removal of polySia can significantly potentiate killing of breast cancer cells by innate immune cells. These studies implicate polySia as a significant negative regulator of anticancer immunity.
-
-
-
Cancer Research
-
Immunology and Microbiology
Soluble Tim-3 serves as a tumor prognostic marker and therapeutic target for CD8+ T cell exhaustion and anti-PD-1 resistance.
In Cell Rep Med on 20 August 2024 by Chen, C., Zhao, F., et al.
PubMed
Resistance to PD-1 blockade in onco-immunotherapy greatly limits its clinical application. T cell immunoglobulin and mucin domain containing-3 (Tim-3), a promising immune checkpoint target, is cleaved by ADAM10/17 to produce its soluble form (sTim-3) in humans, potentially becoming involved in anti-PD-1 resistance. Herein, serum sTim-3 upregulation was observed in non-small cell lung cancer (NSCLC) and various digestive tumors. Notably, serum sTim-3 is further upregulated in non-responding patients undergoing anti-PD-1 therapy for NSCLC and anti-PD-1-resistant cholangiocarcinoma patients. Furthermore, sTim-3 overexpression facilitates tumor progression and confers anti-PD-1 resistance in multiple tumor mouse models. Mechanistically, sTim-3 induces terminal T cell exhaustion and attenuates CD8+ T cell response to PD-1 blockade through carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM-1). Moreover, the ADAM10 inhibitor GI254023X, which blocks sTim-3 production, reduces tumor progression in Tim-3 humanized mice and reverses anti-PD-1 resistance in human tumor-infiltrating lymphocytes (TILs). Overall, human sTim-3 holds great predictive and therapeutic potential in onco-immunotherapy.
-
-
IL-21-armored B7H3 CAR-iNKT cells exert potent antitumor effects.
In iScience on 19 January 2024 by Liu, Y., Dang, Y., et al.
PubMed
CD1d-restricted invariant NKT (iNKT) cells play a critical role in tumor immunity. However, the scarcity and limited persistence restricts their development and clinical application. Here, we demonstrated that iNKT cells could be efficiently expanded using modified cytokines combination from peripheral blood mononuclear cells. Introduction of IL-21 significantly increased the frequency of CD62L-positive memory-like iNKT cells. iNKT cells armoring with B7H3-targeting second generation CAR and IL-21 showed potent tumor cell killing activity. Moreover, co-expression of IL-21 promoted the activation of Stat3 signaling and reduced the expression of exhaustion markers in CAR-iNKT cells in vitro. Most importantly, IL-21-arming significantly prolonged B7H3 CAR-iNKT cell proliferation and survival in vivo, thus improving their therapeutic efficacy in mouse renal cancer xerograph models without observed cytokine-related adverse events. In summary, these results suggest that B7H3 CAR-iNKT armored with IL-21 is a promising therapeutic strategy for cancer treatment.
-
-
Homo sapiens (Human)
-
Cancer Research
Single-cell protein profiling defines cell populations associated with triple-negative breast cancer aggressiveness.
In Mol Oncol on 1 June 2023 by Kvokačková, B., Fedr, R., et al.
PubMed
Triple-negative breast cancer (TNBC) is an aggressive and complex subtype of breast cancer that lacks targeted therapy. TNBC manifests characteristic, extensive intratumoral heterogeneity that promotes disease progression and influences drug response. Single-cell techniques in combination with next-generation computation provide an unprecedented opportunity to identify molecular events with therapeutic potential. Here, we describe the generation of a comprehensive mass cytometry panel for multiparametric detection of 23 phenotypic markers and 13 signaling molecules. This single-cell proteomic approach allowed us to explore the landscape of TNBC heterogeneity, with particular emphasis on the tumor microenvironment. We prospectively profiled freshly resected tumors from 26 TNBC patients. These tumors contained phenotypically distinct subpopulations of cancer and stromal cells that were associated with the patient's clinical status at the time of surgery. We further classified the epithelial-mesenchymal plasticity of tumor cells, and molecularly defined phenotypically diverse populations of tumor-associated stroma. Furthermore, in a retrospective tissue-microarray TNBC cohort, we showed that the level of CD97 at the time of surgery has prognostic potential.
-
-
-
Homo sapiens (Human)
-
Immunology and Microbiology
PTPN22 R620W gene editing in T cells enhances low-avidity TCR responses.
In Elife on 24 March 2023 by Anderson, W., Barahmand-Pour-Whitman, F., et al.
PubMed
A genetic variant in the gene PTPN22 (R620W, rs2476601) is strongly associated with increased risk for multiple autoimmune diseases and linked to altered TCR regulation and T cell activation. Here, we utilize Crispr/Cas9 gene editing with donor DNA repair templates in human cord blood-derived, naive T cells to generate PTPN22 risk edited (620W), non-risk edited (620R), or knockout T cells from the same donor. PTPN22 risk edited cells exhibited increased activation marker expression following non-specific TCR engagement, findings that mimicked PTPN22 KO cells. Next, using lentiviral delivery of T1D patient-derived TCRs against the pancreatic autoantigen, islet-specific glucose-6 phosphatase catalytic subunit-related protein (IGRP), we demonstrate that loss of PTPN22 function led to enhanced signaling in T cells expressing a lower avidity self-reactive TCR, but not a high-avidity TCR. In this setting, loss of PTPN22 mediated enhanced proliferation and Th1 skewing. Importantly, expression of the risk variant in association with a lower avidity TCR also increased proliferation relative to PTPN22 non-risk T cells. Together, these findings suggest that, in primary human T cells, PTPN22 rs2476601 contributes to autoimmunity risk by permitting increased TCR signaling and activation in mildly self-reactive T cells, thereby potentially expanding the self-reactive T cell pool and skewing this population toward an inflammatory phenotype.
-
-
-
Immunology and Microbiology
Bioinstructive implantable scaffolds for rapid in vivo manufacture and release of CAR-T cells.
In Nat Biotechnol on 1 August 2022 by Agarwalla, P., Ogunnaike, E. A., et al.
PubMed
Despite their clinical success, chimeric antigen receptor (CAR)-T cell therapies for B cell malignancies are limited by lengthy, costly and labor-intensive ex vivo manufacturing procedures that might lead to cell products with heterogeneous composition. Here we describe an implantable Multifunctional Alginate Scaffold for T Cell Engineering and Release (MASTER) that streamlines in vivo CAR-T cell manufacturing and reduces processing time to a single day. When seeded with human peripheral blood mononuclear cells and CD19-encoding retroviral particles, MASTER provides the appropriate interface for viral vector-mediated gene transfer and, after subcutaneous implantation, mediates the release of functional CAR-T cells in mice. We further demonstrate that in vivo-generated CAR-T cells enter the bloodstream and control distal tumor growth in a mouse xenograft model of lymphoma, showing greater persistence than conventional CAR-T cells. MASTER promises to transform CAR-T cell therapy by fast-tracking manufacture and potentially reducing the complexity and resources needed for provision of this type of therapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
Neuropilin-1 cooperates with PD-1 in CD8+ T cells predicting outcomes in melanoma patients treated with anti-PD1.
In iScience on 17 June 2022 by Rossignol, J., Belaid, Z., et al.
PubMed
Targeting immune checkpoints, such as Programmed cell Death 1 (PD1), has improved survival in cancer patients by restoring antitumor immune responses. Most patients, however, relapse or are refractory to immune checkpoint blocking therapies. Neuropilin-1 (NRP1) is a transmembrane glycoprotein required for nervous system and angiogenesis embryonic development, also expressed in immune cells. We hypothesized that NRP1 could be an immune checkpoint co-receptor modulating CD8+ T cells activity in the context of the antitumor immune response. Here, we show that NRP1 is recruited in the cytolytic synapse of PD1+CD8+ T cells, cooperates and enhances PD-1 activity. In mice, CD8+ T cells specific deletion of Nrp1 improves anti-PD1 antibody antitumor immune responses. Likewise, in human metastatic melanoma, the expression of NRP1 in tumor infiltrating CD8+ T cells predicts poor outcome of patients treated with anti-PD1. NRP1 is a promising target to overcome resistance to anti-PD1 therapies.
-
-
-
Cancer Research
-
Immunology and Microbiology
T cells drive negative feedback mechanisms in cancer associated fibroblasts, promoting expression of co-inhibitory ligands, CD73 and IL-27 in non-small cell lung cancer.
In Oncoimmunology on 23 July 2021 by O'Connor, R. A., Chauhan, V., et al.
PubMed
The success of immune checkpoint therapy shows tumor-reactive T cells can eliminate cancer cells but are restrained by immunosuppression within the tumor micro-environment (TME). Cancer associated fibroblasts (CAFs) are the dominant stromal cell in the TME and co-localize with T cells in non-small cell lung cancer. We demonstrate the bidirectional nature of CAF/T cell interactions; T cells promote expression of co-inhibitory ligands, MHC molecules and CD73 on CAFs, increasing their production of IL-6 and eliciting production of IL-27. In turn CAFs upregulate co-inhibitory receptors on T cells including the ectonucleotidase CD39 promoting development of an exhausted but highly cytotoxic phenotype. Our results highlight the bidirectional interaction between T cells and CAFs in promoting components of the immunosuppressive CD39, CD73 adenosine pathway and demonstrate IL-27 production can be induced in CAF by activated T cells.
-
-
-
In vitro experiments
-
Homo sapiens (Human)
-
Immunology and Microbiology
-
Pharmacology
Therapeutic effect of kaempferol on atopic dermatitis by attenuation of T cell activity via interaction with multidrug resistance-associated protein 1.
In Br J Pharmacol on 1 April 2021 by Lee, H. S., Jeong, G. S., et al.
PubMed
Kaempferol is a natural flavonoid widely investigated in various fields due to its antioxidant, anti-cancer, and anti-inflammatory activities, but few studies have shown its inhibitory effect on T cell activation. This study examined the therapeutic potential of kaempferol in atopic dermatitis by modulating T cell activation.
-
-
-
Immunoprecipitation
-
Homo sapiens (Human)
-
Immunology and Microbiology
PD-L1:CD80 Cis-Heterodimer Triggers the Co-stimulatory Receptor CD28 While Repressing the Inhibitory PD-1 and CTLA-4 Pathways.
In Immunity on 17 December 2019 by Zhao, Y., Lee, C. K., et al.
PubMed
Combined immunotherapy targeting the immune checkpoint receptors cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death 1 (PD-1), or CTLA-4 and the PD-1 ligand (PD-L1) exhibits superior anti-tumor responses compared with single-agent therapy. Here, we examined the molecular basis for this synergy. Using reconstitution assays with fluorescence readouts, we found that PD-L1 and the CTLA-4 ligand CD80 heterodimerize in cis but not trans. Quantitative biochemistry and cell biology assays revealed that PD-L1:CD80 cis-heterodimerization inhibited both PD-L1:PD-1 and CD80:CTLA-4 interactions through distinct mechanisms but preserved the ability of CD80 to activate the T cell co-stimulatory receptor CD28. Furthermore, PD-L1 expression on antigen-presenting cells (APCs) prevented CTLA-4-mediated trans-endocytosis of CD80. Atezolizumab (anti-PD-L1), but not anti-PD-1, reduced cell surface expression of CD80 on APCs, and this effect was negated by co-blockade of CTLA-4 with ipilimumab (anti-CTLA-4). Thus, PD-L1 exerts an immunostimulatory effect by repressing the CTLA-4 axis; this has implications to the synergy of anti-PD-L1 and anti-CTLA-4 combination therapy.
-
-
-
Immunology and Microbiology
Glutaminase 1 Inhibition Reduces Glycolysis and Ameliorates Lupus-like Disease in MRL/lpr Mice and Experimental Autoimmune Encephalomyelitis.
In Arthritis Rheumatol on 1 November 2019 by Kono, M., Yoshida, N., et al.
PubMed
Glutaminase 1 (Gls1) is the first enzyme in glutaminolysis. The selective Gls1 inhibitor bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide (BPTES) suppresses Th17 development and ameliorates experimental autoimmune encephalomyelitis (EAE). The present study was undertaken to investigate whether inhibition of glutaminolysis is beneficial for the treatment of systemic lupus erythematosus (SLE), and the involved mechanisms.
-
-
PD-L1:CD80 Heterodimer Triggers CD28 While Repressing Both PD-1 and CTLA4 Pathways
In bioRxiv on 21 April 2019 by Zhao, Y., Lin, C., et al.
-
-
Immunology and Microbiology
-
Neuroscience
Deep-learning based three-dimensional label-free tracking and analysis of immunological synapses of chimeric antigen receptor T cells
In bioRxiv on 4 February 2019 by Lee, M., Lee, Y., et al.
-
-
-
Cancer Research
Human ectonucleotidase-expressing CD25high Th17 cells accumulate in breast cancer tumors and exert immunosuppressive functions.
In Oncoimmunology on 5 March 2016 by Thibaudin, M., Chaix, M., et al.
PubMed
Th17 cells contribute to the development of some autoimmune and allergic diseases by driving tissue inflammation. However, the function of Th17 cells during cancer progression remains controversial. Here, we show that human memory CD25high Th17 cells suppress T cell immunity in breast cancer. Ectonucleotidase-expressing Th17 cells accumulated in breast cancer tumors and suppressed CD4+ and CD8+ T cell activation. These cells expressed both Rorγt and Foxp3 genes and secreted Th17 related cytokines. We further found that CD39 ectonucleotisase expression on tumor-infiltrating Th17 cells was driven by TGF-βand IL-6. Finally, immunohistochemical analysis of localized breast cancer revealed that high-tumor infiltration by IL-17+ cells was associated with a poor clinical outcome and impeded the favorable effect of high CD8+ infiltration. Altogether, these findings suggest that intratumoral Th17 cells compromise anticancer immune responses in breast cancer patients.
-
-
-
In vitro experiments
-
Homo sapiens (Human)
-
Biochemistry and Molecular biology
-
Immunology and Microbiology
Signal transducer and activator of transcription (STAT) 3 inhibition delays the onset of lupus nephritis in MRL/lpr mice.
In Clin Immunol on 1 June 2015 by Edwards, L. J., Mizui, M., et al.
PubMed
The transcription factor STAT3 is overexpressed and hyperactivated in T cells from SLE patients. STAT3 plays a central role in T cell differentiation into Th17 and T follicular helper cells, two subsets that orchestrate autoimmune responses in SLE. Moreover, STAT3 is important in chemokine-mediated T cell migration. To better understand its role in SLE, we inhibited STAT3 in lupus-prone mice using the small molecule Stattic. Stattic-treated mice exhibited delayed onset of proteinuria (3 weeks later than controls), and had lower levels of anti-dsDNA antibodies and inflammatory cytokines. Inhibitor treatment reduced lymphadenopathy, resulted in a 3-fold decrease in total T cell number, and a 4-fold decrease in the numbers of T follicular helper cells. In vitro experiments showed that Stattic-treated T cells exhibited decreased proliferation and a decrease in ability to migrate to CXCL12. We propose that STAT3 inhibition represents a therapeutic target in SLE, particularly lupus nephritis.
-