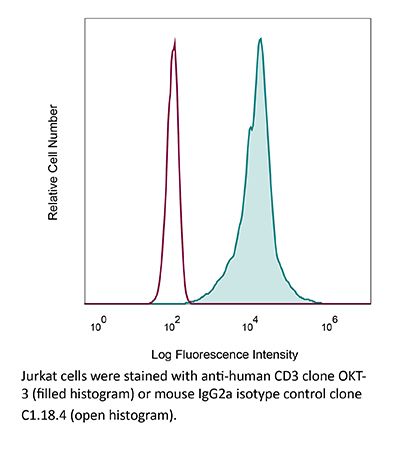
InVivoMAb anti-human CD3
Product Description
Specifications
| Isotype | Mouse IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG2a isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Not available or unknown |
| Reported Applications |
in vitro T cell stimulation/activation in vivo T cell depletion in humanized mice ex vivo T cell inhibition for xenografts Flow cytometry in vitro Organoids/Organ-on-Chip |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107632 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vitro Organoids/Organ-on-Chip
Vella N, Fenech AG, Petroni Magri V (2024). "3D cell culture models in research: applications to lung cancer pharmacology" Front Pharmacol .
PubMed
Lung cancer remains one of the leading causes of cancer-related mortality worldwide, necessitating innovative research methodologies to improve treatment outcomes and develop novel strategies. The advent of three-dimensional (3D) cell cultures has marked a significant advancement in lung cancer research, offering a more physiologically relevant model compared to traditional two-dimensional (2D) cultures. This review elucidates the various types of 3D cell culture models currently used in lung cancer pharmacology, including spheroids, organoids and engineered tissue models, having pivotal roles in enhancing our understanding of lung cancer biology, facilitating drug development, and advancing precision medicine. 3D cell culture systems mimic the complex spatial architecture and microenvironment of lung tumours, providing critical insights into the cellular and molecular mechanisms of tumour progression, metastasis and drug responses. Spheroids, derived from commercialized cell lines, effectively model the tumour microenvironment (TME), including the formation of hypoxic and nutrient gradients, crucial for evaluating the penetration and efficacy of anti-cancer therapeutics. Organoids and tumouroids, derived from primary tissues, recapitulate the heterogeneity of lung cancers and are instrumental in personalized medicine approaches, supporting the simulation of in vivo pharmacological responses in a patient-specific context. Moreover, these models have been co-cultured with various cell types and biomimicry extracellular matrix (ECM) components to further recapitulate the heterotypic cell-cell and cell-ECM interactions present within the lung TME. 3D cultures have been significantly contributing to the identification of novel therapeutic targets and the understanding of resistance mechanisms against conventional therapies. Therefore, this review summarizes the latest findings in drug research involving lung cancer 3D models, together with the common laboratory-based assays used to study drug effects. Additionally, the integration of 3D cell cultures into lung cancer drug development workflows and precision medicine is discussed. This integration is pivotal in accelerating the translation of laboratory findings into clinical applications, thereby advancing the landscape of lung cancer treatment. By closely mirroring human lung tumours, these models not only enhance our understanding of the disease but also pave the way for the development of more effective and personalized therapeutic strategies.
in vitro T cell stimulation/activation
Liu, H., et al (2015). "The Immune Adaptor SLP-76 Binds to SUMO-RANGAP1 at Nuclear Pore Complex Filaments to Regulate Nuclear Import of Transcription Factors in T Cells" Mol Cell 59(5): 840-849.
PubMed
While immune cell adaptors regulate proximal T cell signaling, direct regulation of the nuclear pore complex (NPC) has not been reported. NPC has cytoplasmic filaments composed of RanGAP1 and RanBP2 with the potential to interact with cytoplasmic mediators. Here, we show that the immune cell adaptor SLP-76 binds directly to SUMO-RanGAP1 of cytoplasmic fibrils of the NPC, and that this interaction is needed for optimal NFATc1 and NF-kappaB p65 nuclear entry in T cells. Transmission electron microscopy showed anti-SLP-76 cytoplasmic labeling of the majority of NPCs in anti-CD3 activated T cells. Further, SUMO-RanGAP1 bound to the N-terminal lysine 56 of SLP-76 where the interaction was needed for optimal RanGAP1-NPC localization and GAP exchange activity. While the SLP-76-RanGAP1 (K56E) mutant had no effect on proximal signaling, it impaired NF-ATc1 and p65/RelA nuclear entry and in vivo responses to OVA peptide. Overall, we have identified SLP-76 as a direct regulator of nuclear pore function in T cells.
in vitro T cell stimulation/activation
Hill, E. V., et al (2015). "Glycogen synthase kinase-3 controls IL-10 expression in CD4(+) effector T-cell subsets through epigenetic modification of the IL-10 promoter" Eur J Immunol 45(4): 1103-1115.
PubMed
The serine/threonine kinase glycogen synthase kinase-3 (GSK3) plays an important role in balancing pro- and anti-inflammatory cytokines. We have examined the role of GSK3 in production of IL-10 by subsets of CD4(+) T helper cells. Treatment of naive murine CD4(+) T cells with GSK3 inhibitors did not affect their production of IL-10. However, treatment of Th1 and Th2 cells with GSK3 inhibitors dramatically increased production of IL-10. GSK3 inhibition also led to upregulation of IL-10 among Th1, Th2, and Th17 subsets isolated from human blood. The encephalitogenic potential of GSK3 inhibitor treated murine Th1 cells was significantly reduced in adoptive transfer experiments by an IL-10-dependent mechanism. Analysis of the murine IL-10 promoter in response to inhibition of GSK3 in Th1 cells showed modification to a transcriptionally active state indicated by changes in histone H3 acetylation and methylation. Additionally, GSK3 inhibition increased expression of the transcription factors c-Maf, Nfil3, and GATA3, correlating with the increase in IL-10. These findings are important in the context of autoimmune disease since they show that it is possible to reprogram disease-causing cells through GSK3 inhibition.
in vitro T cell stimulation/activation
Rochman, Y., et al (2015). "Functional characterization of human T cell hyporesponsiveness induced by CTLA4-Ig" PLoS One 10(4): e0122198.
PubMed
During activation, T cells integrate multiple signals from APCs and cytokine milieu. The blockade of these signals can have clinical benefits as exemplified by CTLA4-Ig, which blocks interaction of B7 co-stimulatory molecules on APCs with CD28 on T cells. Variants of CTLA4-Ig, abatacept and belatacept are FDA approved as immunosuppressive agents in arthritis and transplantation, yet murine studies suggested that CTLA4-Ig could be beneficial in a number of other diseases. However, detailed analysis of human CD4 cell hyporesponsivness induced by CTLA4-Ig has not been performed. Herein, we established a model to study the effect of CTLA4-Ig on the activation of human naive T cells in a human mixed lymphocytes system. Comparison of human CD4 cells activated in the presence or absence of CTLA4-Ig showed that co-stimulation blockade during TCR activation does not affect NFAT signaling but results in decreased activation of NF-kappaB and AP-1 transcription factors followed by a profound decrease in proliferation and cytokine production. The resulting T cells become hyporesponsive to secondary activation and, although capable of receiving TCR signals, fail to proliferate or produce cytokines, demonstrating properties of anergic cells. However, unlike some models of T cell anergy, these cells did not possess increased levels of the TCR signaling inhibitor CBLB. Rather, the CTLA4-Ig-induced hyporesponsiveness was associated with an elevated level of p27kip1 cyclin-dependent kinase inhibitor.
in vivo T cell depletion in humanized mice
ex vivo T cell inhibtion for xenografts
Wunderlich, M., et al (2014). "OKT3 prevents xenogeneic GVHD and allows reliable xenograft initiation from unfractionated human hematopoietic tissues" Blood 123(24): e134-144.
PubMed
Immunodeficient mice are now readily engrafted with human hematopoietic cells. However, these mice are susceptible to graft-versus-host disease (GVHD) induced by the engraftment and rapid expansion of coinjected human T cells. Therefore, highly purified sample populations must be used, adding significant time, expense, and effort. Here, we have explored in vivo and in vitro methods utilizing anti-T-cell antibodies to circumvent this problem. Intraperitoneal injection of the antibody within 48 hours prevented GVHD. Alternatively, short-term in vitro incubation of cells with antibody immediately before transplant was equally effective. Although in vitro antithymocyte globulin treatment resulted in a dramatic loss of SCID-repopulating cells (SRCs), treatment with OKT3 or UCHT1 abrogated GVHD risk and preserved engraftment potential. Leukemia samples that presented with substantial human T-cell contamination were effectively rescued from GVHD. In addition, OKT3 treatment of unfractionated cord blood resulted in robust engraftment of primary and secondary mice that was indistinguishable from grafts obtained using purified CD34(+) cells. Limiting dilution analysis of unfractionated blood demonstrated a SRC frequency of 1 in 300 to 500 CD34(+) cells, similar to that of purified hematopoietic stem and progenitor cells. This protocol streamlines xenograft studies while significantly reducing the cost and time of the procedure.
in vitro T cell stimulation/activation
Esposito, L., et al (2014). "Investigation of soluble and transmembrane CTLA-4 isoforms in serum and microvesicles" J Immunol 193(2): 889-900.
PubMed
Expression of the CTLA-4 gene is absolutely required for immune homeostasis, but aspects of its molecular nature remain undefined. In particular, the characterization of the soluble CTLA-4 (sCTLA-4) protein isoform generated by an alternatively spliced mRNA of CTLA4 lacking transmembrane-encoding exon 3 has been hindered by the difficulty in distinguishing it from the transmembrane isoform of CTLA-4, Tm-CTLA-4. In the current study, sCTLA-4 has been analyzed using novel mAbs and polyclonal Abs specific for its unique C-terminal amino acid sequence. We demonstrate that the sCTLA-4 protein is secreted at low levels following the activation of primary human CD4(+) T cells and is increased only rarely in the serum of autoimmune patients. Unexpectedly, during our studies aimed to define the kinetics of sCTLA-4 produced by activated human CD4(+) T cells, we discovered that Tm-CTLA-4 is associated with microvesicles produced by the activated cells. The functional roles of sCTLA-4 and microvesicle-associated Tm-CTLA-4 warrant further investigation, especially as they relate to the multiple mechanisms of action described for the more commonly studied cell-associated Tm-CTLA-4.
in vitro T cell stimulation/activation
Lines, J. L., et al (2014). "VISTA is an immune checkpoint molecule for human T cells" Cancer Res 74(7): 1924-1932.
PubMed
V-domain Ig suppressor of T cell activation (VISTA) is a potent negative regulator of T-cell function that is expressed on hematopoietic cells. VISTA levels are heightened within the tumor microenvironment, in which its blockade can enhance antitumor immune responses in mice. In humans, blockade of the related programmed cell death 1 (PD-1) pathway has shown great potential in clinical immunotherapy trials. Here, we report the structure of human VISTA and examine its function in lymphocyte negative regulation in cancer. VISTA is expressed predominantly within the hematopoietic compartment with highest expression within the myeloid lineage. VISTA-Ig suppressed proliferation of T cells but not B cells and blunted the production of T-cell cytokines and activation markers. Our results establish VISTA as a negative checkpoint regulator that suppresses T-cell activation, induces Foxp3 expression, and is highly expressed within the tumor microenvironment. By analogy to PD-1 and PD-L1 blockade, VISTA blockade may offer an immunotherapeutic strategy for human cancer.
in vitro T cell stimulation/activation
Sturner, K. H., et al (2014). "A multiple sclerosis-associated variant of CBLB links genetic risk with type I IFN function" J Immunol 193(9): 4439-4447.
PubMed
Multiple sclerosis (MS) is an autoimmune disease of the CNS, and autoreactive CD4(+) T cells are considered important for its pathogenesis. The etiology of MS involves a complex genetic trait and environmental triggers that include viral infections, particularly the EBV. Among the risk alleles that have repeatedly been identified by genome-wide association studies, three are located near the Casitas B-lineage lymphoma proto-oncogene b gene (CBLB). The CBLB protein (CBL-B) is a key regulator of peripheral immune tolerance by limiting T cell activation and expansion and hence T cell-mediated autoimmunity through its ubiquitin E3-ligase activity. In this study, we show that CBL-B expression is reduced in CD4(+) T cells from relapsing-remitting MS (RR-MS) patients during relapse. The MS risk-related single nucleotide polymorphism of CBLB rs12487066 is associated with diminished CBL-B expression levels and alters the effects of type I IFNs on human CD4(+) T cell proliferation. Mechanistically, the CBLB rs12487066 risk allele mediates increased binding of the transcription factor C/EBPbeta and reduced CBL-B expression in human CD4(+) T cells. Our data suggest a role of the CBLB rs12487066 variant in the interactions of a genetic risk factor and IFN function during viral infections in MS.
in vitro T cell stimulation/activation
Flow Cytometry
Willing, A., et al (2014). "CD8(+) MAIT cells infiltrate into the CNS and alterations in their blood frequencies correlate with IL-18 serum levels in multiple sclerosis" Eur J Immunol 44(10): 3119-3128.
PubMed
Recent findings indicate a pathogenic involvement of IL-17-producing CD8(+) T cells in multiple sclerosis (MS). IL-17 production has been attributed to a subset of CD8(+) T cells that belong to the mucosal-associated invariant T (MAIT) cell population. Here, we report a reduction of CD8(+) MAIT cells in the blood of MS patients compared with healthy individuals, which significantly correlated with IL-18 serum levels in MS patients. In vitro stimulation of peripheral blood mononuclear cells from healthy individuals and MS patients with IL-18 specifically activated CD8(+) MAIT cells. Moreover, IL-18 together with T-cell receptor stimulation induced, specifically on CD8(+) MAIT cells, an upregulation of the integrin very late antigen-4 that is essential for the infiltration of CD8(+) T cells into the CNS. Notably, we were able to identify CD8(+) MAIT cells in MS brain lesions by immunohistochemistry while they were almost absent in the cerebrospinal fluid (CSF). In summary, our findings indicate that an IL-18-driven activation of CD8(+) MAIT cells contributes to their CNS infiltration in MS, in turn leading to reduced CD8(+) MAIT-cell frequencies in the blood. Therefore, CD8(+) MAIT cells seem to play a role in the innate arm of immunopathology in MS.
Product Citations
-
Rapid and Uniform NHS-Ester-Based Membrane Protein Labeling of Live Mammalian Cells.
In Bio Protoc on 5 October 2025 by Burgess, A., Gunasekara, H., et al.
PubMed
Rapid and uniform labeling of plasma membrane proteins is essential for high-resolution imaging of dynamic membrane topologies and intercellular communication in live mammalian cells. Existing strategies for labeling live cell membranes, such as fluorescent fusion proteins, enzyme-mediated tags, metabolic bioorthogonal labeling, and lipophilic dyes, face trade-offs in the requirement of genetic manipulation, the presence of non-uniform labeling, the need for extensive preparation times, and limited choices of fluorophores. Here, we present a streamlined protocol that leverages N-hydroxysuccinimide (NHS)-ester chemistry to achieve rapid (≤5 min), covalent conjugation of synthetic small-molecule dyes to surface-exposed primary amines, enabling pan-membrane-protein labeling. This workflow covers dye stock preparation, labeling for suspension and adherent cells, multiplex live-cell imaging, fusion protein co-staining (including insulin-triggered receptor endocytosis), 3D membrane visualization, and in vivo assays for visualizing membrane-derived material transfers between donor and recipient cells using a lymphoma T-cell mouse model. This high-density labeling approach is compatible with various cell types across diverse imaging platforms. Its speed, versatility, and stability make it a broadly applicable tool for studying plasma membrane dynamics and intercellular membrane trafficking. Key features • Rapid high-density membrane labeling with small-molecule fluorescent dyes. • Enables live-cell multiplexed imaging, amenable to primary cells and cells expressing fluorescent fusion proteins, and supports in vivo studies of membrane-associated cell-cell communications. • Compatible with various fluorescence imaging modalities.
-
A20's linear ubiquitin-binding motif restrains pathogenic activation of Th17 cells and IL-22-driven enteritis.
In J Clin Invest on 2 September 2025 by Bowman, C. J., Stibor, D. M., et al.
PubMed
A20, encoded by the TNFAIP3 gene, is a protein linked to Crohn's disease and celiac disease in humans. We now find that mice expressing point mutations in A20's M1-ubiquitin-binding zinc finger 7 (ZF7) motif spontaneously develop proximal enteritis that requires both luminal microbes and T cells. Cellular and transcriptomic profiling reveals expansion of Th17 cells and exuberant expression of IL-17A and IL-22 in intestinal lamina propria of A20ZF7 mice. While deletion of IL-17A from A20ZF7/ZF7 mice exacerbates enteritis, deletion of IL-22 abrogates intestinal epithelial cell hyperproliferation, barrier dysfunction, and alarmin expression. Colonization of adult germ-free mice with microbiota from adult WT specific pathogen-free mice drives duodenal IL-22 expression and duodenitis. A20ZF7/ZF7 Th17 cells autonomously express more RORγt and IL-22 after differentiation in vitro. ATAC sequencing identified an enhancer region upstream of the Il22 gene, and this enhancer demonstrated increased activating histone acetylation coupled with exaggerated Il22 transcription in A20ZF7/ZF7 T cells. Acute inhibition of RORγt normalized histone acetylation at this enhancer. Finally, CRISPR/Cas9-mediated ablation of A20ZF7 in human T cells increases RORγt expression and IL22 transcription. These studies link A20's M1-ubiquitin binding function with RORγt expression, expansion of Th17 cells, and epigenetic activation of IL-22-driven enteritis.
-
-
Immunology and Microbiology
-
COVID-19
Immune signatures of SARS-CoV-2 infection resolution in human lung tissues.
In PLoS Pathog on 1 September 2025 by Kenney, D., O'Connell, A. K., et al.
PubMed
While human autopsy samples have provided insights into pulmonary immune mechanisms associated with severe viral respiratory diseases, the mechanisms that contribute to a clinically favorable resolution of viral respiratory infections remain unclear due to the lack of proper experimental systems. Using mice co-engrafted with a genetically matched human immune system and fetal lung xenograft (fLX), we mapped the immunological events defining successful resolution of SARS-CoV-2 infection in human lung tissues. Viral infection is rapidly cleared from fLX following a peak of viral replication, histopathological manifestations of lung disease and loss of AT2 program, as reported in human COVID-19 patients. Infection resolution is associated with the activation of a limited number of hematopoietic subsets, including inflammatory monocytes and CD3-expressing macrophage-like cells, which are highly enriched in viral RNA and dissipate upon infection resolution. Specific human fibroblast and endothelial subsets also elicit robust antiviral and monocyte chemotaxis signatures, respectively. Notably, systemic depletion of human CD4 + cells, but not CD3 + cells, significantly abrogates infection resolution in fLX and induces persistent infection, supporting the dominant role of peripheral CD4 + monocytes over T-cells in the resolution of acute SARS-CoV-2 infection. Collectively, our findings unravel a comprehensive picture of the immunological events defining effective resolution of SARS-CoV-2 infection in human lung tissues, revealing markedly divergent immunological trajectories between resolving and fatal COVID-19 cases.
-
-
-
Neuroscience
-
Immunology and Microbiology
CD99-mediated immunological synapse formation potentiates CAR-T cell function.
In Nat Commun on 27 August 2025 by Nam, G., Yeon, H. R., et al.
PubMed
Despite the efficacy of chimeric antigen receptor (CAR)-T cells in selected hematological malignancies, further improvement on CAR-T designs is still desirable. We hypothesize that modifying the CAR structure to enhance immunological synapse (IS) stabilization and CAR target-binding may be a feasible strategy. Here we show that the membrane protein, CD99, is critical for IS formation in T cells by mediating actin-microtubule interaction. CD99 deficiency abolishes IS formation and prevents effective in vivo T cell immunity. Mechanistically, CD99 interacts with microtubules and actins through the transmembrane and cytoplasmic domains, respectively, with which myosin and IQGAP1 interact. As such, incorporating the transmembrane and juxtamembrane domains of CD99 into the CAR structure enhances IS formation and improves the therapeutic efficacy of human CAR-T cells against lymphoma in immune-deficient mice. Our data thus suggest that CD99-mediated IS stabilization may help improve CAR design and efficacy.
-
-
-
Immunology and Microbiology
BLIMP1 negatively regulates IL-2 signaling in T cells.
In Sci Adv on 18 July 2025 by Roy, S., Ren, M., et al.
PubMed
Interleukin-2 (IL-2) regulates immune homeostasis by fine-tuning the balance between effector and regulatory T (Treg) cells. To identify regulators of IL-2 signaling, we performed genome-wide CRISPR-knockout screening in IL-2-dependent cells derived from a patient with adult T cell leukemia (ATL) and found enrichment of single guide RNAs targeting PRDM1, which encodes B lymphocyte-induced maturation protein 1 (BLIMP1). BLIMP1 inhibits IL-2 production by T cells; however, its role in IL-2 signaling remains unknown. Here, we show that overexpressing Prdm1 down-regulated IL-2 signaling, whereas Prdm1-deficiency enhanced IL-2 signaling in mouse CD4+ T cells and Treg cells with augmented IL-2 signaling in T cells from influenza-infected mice and during adoptive T cell transfer-induced colitis. Deleting PRDM1 in human CD4+ T cells and Treg cells also increased IL-2 signaling. Furthermore, CD4+ T cells from patients with ATL expressed less BLIMP1 and had enhanced IL-2 signaling, whereas overexpressing PRDM1 in ATL cells suppressed IL-2 signaling. Thus, BLIMP1 inhibits IL-2 signaling during normal and pathophysiological responses, suggesting that manipulating BLIMP1 could have therapeutic potential.
-
-
-
Cell Biology
-
Cardiovascular biology
-
Cancer Research
The STAT3-VDAC1 axis modulates mitochondrial function and plays a critical role in the survival of acute myeloid leukemia cells.
In Haematologica on 19 June 2025 by Gil, K. B., Borg, J., et al.
PubMed
Signal transducer and activator of transcription 3 (STAT3) is a well-described transcription factor that mediates oxidative phosphorylation and glutamine uptake in bulk acute myeloid leukemia (AML) cells and leukemic stem cells (LSCs). STAT3 has also been shown to translocate to the mitochondria in AML cells, and phosphorylation at the serine 727 (pSTAT3 S727) residue has been shown to be especially important for STAT3's mitochondrial functions. We demonstrate that inhibition of STAT3 results in impaired mitochondrial function and decreased leukemia cell viability. We discovered a novel interaction of STAT3 with voltage-dependent anion channel 1 (VDAC1) in the mitochondria which provides a mechanism through which STAT3 modulates mitochondrial function and cell survival. Through VDAC1, STAT3 regulates calcium and oxidative phosphorylation in the mitochondria. STAT3 and VDAC1 inhibition also result in significantly reduced engraftment potential of LSCs, including primary samples resistant to venetoclax. These results implicate STAT3 as a therapeutic target in AML.
-
-
-
Immunology and Microbiology
Inflammatory arthritis immune related adverse events represent a unique autoimmune disease entity primarily driven by T cells, but likely not autoantibodies
In medRxiv on 6 June 2025 by Zhu, X., Yu, Y., et al.
-
-
FcRn-silencing of IL-12Fc prevents toxicity of local IL-12 therapy and prolongs survival in experimental glioblastoma.
In Nat Commun on 22 May 2025 by Beffinger, M. M., Schellhammer, L., et al.
PubMed
Glioblastoma remains a challenging indication for immunotherapy: the blood-brain barrier hampers accessibility for systemic treatments and the immunosuppressive microenvironment impedes immune attack. Intratumoral therapy with the proinflammatory cytokine interleukin-12 (IL-12) can revert immunosuppression but leakage into the circulation causes treatment-limiting toxicity. Here we engineer an IL-12Fc fusion cytokine with reduced binding to the neonatal Fc receptor FcRn. FcRn-silenced IL-12Fc avoids FcRn-mediated brain export, thus exhibits prolonged brain retention and reduced blood levels, which prevents toxicity. In murine glioblastoma, FcRn-silenced IL-12Fc induces more durable responses with negligible systemic cytokine exposure and boosts the efficacy of radio- and chemotherapy. It triggers anti-tumor responses independently of peripheral T cell influx or lymphopenia and leads to inflammatory polarization of the tumor microenvironment in patient-derived glioblastoma explants. FcRn-silencing of IL-12Fc may unlock the full potential of IL-12 for brain cancer therapy and could be further applied to containing the activity of other therapeutics targeting neurological diseases.
-
Unveiling cellular communications through rapid pan-membrane-protein labeling.
In Nat Commun on 15 April 2025 by Gunasekara, H., Cheng, Y. S., et al.
PubMed
Dynamic protein distribution within and across the plasma membrane is pivotal in regulating cell communication. However, rapid, high-density labeling methods for multiplexed live imaging across diverse cell types remain scarce. Here, we demonstrate N-hydroxysuccinimide (NHS)-ester-based amine crosslinking of fluorescent dyes to uniformly label live mammalian cell surface proteins. Using model cell systems, we capture previously elusive membrane topology and cell-cell interactions. Live imaging shows transient membrane protein accumulation at cell-cell contacts and bidirectional migration patterns guided by membrane fibers in DC2.4 dendritic cells. Multiplexed superresolution imaging reveals the biogenesis of membrane tunneling nanotubes that facilitate intercellular transfer in DC2.4 cells, and caveolin 1-dependent endocytosis of insulin receptors in HEK293T cells. 3D superresolution imaging reveals membrane topology remodeling in response to stimulation, generation of microvesicles, and phagocytic activities in Jurkat T cells. Furthermore, NHS-labeling remains stable in vivo, enabling visualization of intercellular transfer among splenocytes using a T cell lymphoma mouse model.
-
-
Immunology and Microbiology
Polysialic acid is upregulated on activated immune cells and negatively regulates anticancer immune activity.
In Front Oncol on 4 April 2025 by Drummond-Guy, O., Daly, J., et al.
PubMed
Suppression of anticancer immune function is a key driver of tumorigenesis. Identifying molecular pathways that inhibit anticancer immunity is critical for developing novel immunotherapeutics. One such molecule that has recently been identified is the carbohydrate polysialic acid (polySia), whose expression is dramatically upregulated on both cancer cells and immune cells in breast cancer patient tissues. The role of polySia in the anticancer immune response, however, remains incompletely understood. In this study, we profile polySia expression on both healthy primary immune cells and on infiltrating immune cells in the tumour microenvironment (TME). These studies reveal polySia expression on multiple immune cell subsets in patient breast tumors. We find that stimulation of primary T-cells and macrophages in vitro induces a significant upregulation of polySia expression. We subsequently show that polySia is appended to a range of different carrier proteins within these immune cells. Finally, we find that selective removal of polySia can significantly potentiate killing of breast cancer cells by innate immune cells. These studies implicate polySia as a significant negative regulator of anticancer immunity.
-
-
-
Immunology and Microbiology
Preclinical characterization of MTX-101: a novel bispecific CD8 Treg modulator that restores CD8 Treg functions to suppress pathogenic T cells in autoimmune diseases.
In Front Immunol on 19 November 2024 by Gardell, J. L., Maurer, M. E., et al.
PubMed
Regulatory CD8 T cells (CD8 Treg) are responsible for the selective killing of self-reactive and pathogenic CD4 T cells. In autoimmune disease, CD8 Treg may accumulate in the peripheral blood but fail to control the expansion of pathogenic CD4 T cells that subsequently cause tissue destruction. This CD8 Treg dysfunction is due in part to the expression of inhibitory killer immunoglobulin-like receptors (KIR; KIR2DL isoforms [KIR2DL1, KIR2DL2, and KIR2DL3]); these molecules serve as autoimmune checkpoints and limit CD8 Treg activation.
-
-
-
Immunology and Microbiology
Progranulin protects against Clostridioides difficile infection by enhancing IL-22 production.
In Gut Microbes on 1 October 2024 by Huang, J., Liu, B., et al.
PubMed
Enhanced mortality, relapse rates, and increased prevalence of Clostridioides difficile infection (CDI) emphasize the need for better therapies and management approaches. Modulating host immune response to ameliorate CDI-associated immunopathology may provide new advantages to currently inadequate antibiotic therapies. Here, we identified progranulin (PGRN) as an important immune target upregulated in response to CDI. PGRN-deficient mice displayed dramatically higher mortality and aggravated epithelial barrier disruption compared with wild type (WT) mice after CDI despite equivalent levels of bacterial burden or toxin in the large intestine. Mechanistically, PGRN protection was mediated by IL-22 production from CD4+ T helper cells, as demonstrated by a decrease in colonic IL-22-producing CD4+ T helper cells in the intestine of PGRN-deficient mice upon CDI and a boost of IL-22-producing CD4+ T helper cells activated by PGRN ex vivo. Clinical evidence suggests that CDI patients had significantly higher serum levels of PGRN compared with healthy controls, which was significantly and positively correlated with IL-22. Our findings thus indicate a critical role for PGRN-promoted CD4+ T cell IL-22 production in shaping gut immunity and reestablishing the intestinal barrier during CDI. As an alternative to pathogen-targeted therapy, this study may provide a new host-directed therapeutic strategy to attenuate severe, refractory CDI.
-
-
-
Immunology and Microbiology
CD38 in SLE CD4 T cells promotes Ca2+ flux and suppresses interleukin-2 production by enhancing the expression of GM2 on the surface membrane.
In Nat Commun on 27 September 2024 by Katsuyama, E., Humbel, M., et al.
PubMed
CD38 has emerged as a potential therapeutic target for patients with systemic lupus erythematosus (SLE) but it is not known whether CD38 alters CD4+ T cell function. Using primary human T cells and CD38-sufficient and CD38-deficient Jurkat T cells, we demonstrate that CD38 shifts the T cell lipid profile of gangliosides from GM3 to GM2 by upregulating B4GALNT1 in a Sirtuin 1-dependent manner. Enhanced expression of GM2 causes ER stress by enhancing Ca2+ flux through the PLCγ1-IP3 pathway. Interestingly, correction of the calcium overload by an IP3 receptor inhibitor, but not by a store-operated calcium entry (SOCE) inhibitor, improves IL-2 production by CD4+ T cells in SLE. This study demonstrates that CD38 affects calcium homeostasis in CD4+ T cells by controlling cell membrane lipid composition that results in suppressed IL-2 production. CD38 inhibition with biologics or small drugs should be expected to benefit patients with SLE.
-
-
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
Spermidine metabolism regulates leukemia stem and progenitor cell function through KAT7 expression in patient-derived mouse models.
In Sci Transl Med on 25 September 2024 by Rondeau, V., Berman, J., et al.
PubMed
Acute myeloid leukemia (AML) is a devastating disease initiated and maintained by a rare subset of cells called leukemia stem cells (LSCs). LSCs are responsible for driving disease relapse, making the development of new therapeutic strategies to target LSCs urgently needed. The use of mass spectrometry-based metabolomics profiling has enabled the discovery of unique and targetable metabolic properties in LSCs. However, we do not have a comprehensive understanding of metabolite differences between LSCs and their normal counterparts, hematopoietic stem and progenitor cells (HSPCs). In this study, we used an unbiased mass spectrometry-based metabolomics analysis to define differences in metabolites between primary human LSCs and HSPCs, which revealed that LSCs have a distinct metabolome. Spermidine was the most enriched metabolite in LSCs compared with HSPCs. Pharmacological reduction of spermidine concentrations decreased LSC function but spared normal HSPCs. Polyamine depletion also decreased leukemic burden in patient-derived xenografts. Mechanistically, spermidine depletion induced LSC myeloid differentiation by decreasing eIF5A-dependent protein synthesis, resulting in reduced expression of a select subset of proteins. KAT7, a histone acetyltransferase, was one of the top candidates identified to be down-regulated by spermidine depletion. Overexpression of KAT7 partially rescued polyamine depletion-induced decreased colony-forming ability, demonstrating that loss of KAT7 is an essential part of the mechanism by which spermidine depletion targets AML clonogenic potential. Together, we identified and mechanistically dissected a metabolic vulnerability of LSCs that has the potential to be rapidly translated into clinical trials to improve outcomes for patients with AML.
-
-
-
Cancer Research
-
Immunology and Microbiology
Soluble Tim-3 serves as a tumor prognostic marker and therapeutic target for CD8+ T cell exhaustion and anti-PD-1 resistance.
In Cell Rep Med on 20 August 2024 by Chen, C., Zhao, F., et al.
PubMed
Resistance to PD-1 blockade in onco-immunotherapy greatly limits its clinical application. T cell immunoglobulin and mucin domain containing-3 (Tim-3), a promising immune checkpoint target, is cleaved by ADAM10/17 to produce its soluble form (sTim-3) in humans, potentially becoming involved in anti-PD-1 resistance. Herein, serum sTim-3 upregulation was observed in non-small cell lung cancer (NSCLC) and various digestive tumors. Notably, serum sTim-3 is further upregulated in non-responding patients undergoing anti-PD-1 therapy for NSCLC and anti-PD-1-resistant cholangiocarcinoma patients. Furthermore, sTim-3 overexpression facilitates tumor progression and confers anti-PD-1 resistance in multiple tumor mouse models. Mechanistically, sTim-3 induces terminal T cell exhaustion and attenuates CD8+ T cell response to PD-1 blockade through carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM-1). Moreover, the ADAM10 inhibitor GI254023X, which blocks sTim-3 production, reduces tumor progression in Tim-3 humanized mice and reverses anti-PD-1 resistance in human tumor-infiltrating lymphocytes (TILs). Overall, human sTim-3 holds great predictive and therapeutic potential in onco-immunotherapy.
-
-
-
Cancer Research
Improvement of Tumor Neoantigen Detection by High-Field Asymmetric Waveform Ion Mobility Mass Spectrometry.
In Cancer Immunol Res on 1 August 2024 by Meng, W., Takeuchi, Y., et al.
PubMed
Cancer neoantigens have been shown to elicit cancer-specific T-cell responses and have garnered much attention for their roles in both spontaneous and therapeutically induced antitumor responses. Mass spectrometry (MS) profiling of tumor immunopeptidomes has been used, in part, to identify MHC-bound mutant neoantigen ligands. However, under standard conditions, MS-based detection of such rare but clinically relevant neoantigens is relatively insensitive, requiring 300 million cells or more. Here, to quantitatively define the minimum detectable amounts of therapeutically relevant MHC-I and MHC-II neoantigen peptides, we analyzed different dilutions of immunopeptidomes isolated from the well-characterized T3 mouse methylcholanthrene (MCA)-induced cell line by MS. Using either data-dependent acquisition or parallel reaction monitoring (PRM), we established the minimum amount of material required to detect the major T3 neoantigens in the presence or absence of high field asymmetric waveform ion mobility spectrometry (FAIMS). This analysis yielded a 14-fold enhancement of sensitivity in detecting the major T3 MHC-I neoantigen (mLama4) with FAIMS-PRM compared with PRM without FAIMS, allowing ex vivo detection of this neoantigen from an individual 100 mg T3 tumor. These findings were then extended to two other independent MCA-sarcoma lines (1956 and F244). This study demonstrates that FAIMS substantially increases the sensitivity of MS-based characterization of validated neoantigens from tumors.
-
-
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
Antigen Presenting Cell Mimetic Lipid Nanoparticles for Rapid mRNA CAR T Cell Cancer Immunotherapy.
In Adv Mater on 1 June 2024 by Metzloff, A. E., Padilla, M. S., et al.
PubMed
Chimeric antigen receptor (CAR) T cell therapy has achieved remarkable clinical success in the treatment of hematological malignancies. However, producing these bespoke cancer-killing cells is a complicated ex vivo process involving leukapheresis, artificial T cell activation, and CAR construct introduction. The activation step requires the engagement of CD3/TCR and CD28 and is vital for T cell transfection and differentiation. Though antigen-presenting cells (APCs) facilitate activation in vivo, ex vivo activation relies on antibodies against CD3 and CD28 conjugated to magnetic beads. While effective, this artificial activation adds to the complexity of CAR T cell production as the beads must be removed prior to clinical implementation. To overcome this challenge, this work develops activating lipid nanoparticles (aLNPs) that mimic APCs to combine the activation of magnetic beads and the transfection capabilities of LNPs. It is shown that aLNPs enable one-step activation and transfection of primary human T cells with the resulting mRNA CAR T cells reducing tumor burden in a murine xenograft model, validating aLNPs as a promising platform for the rapid production of mRNA CAR T cells.
-
-
-
COVID-19
-
Immunology and Microbiology
Resolution of SARS-CoV-2 infection in human lung tissues is driven by extravascular CD163+ monocytes
In bioRxiv on 8 March 2024 by Kenney, D., O’Connell, A. K., et al.
-
-
-
Immunology and Microbiology
Gut microbiota-derived LCA mediates the protective effect of PEDV infection in piglets.
In Microbiome on 5 February 2024 by Xing, J. H., Niu, T. M., et al.
PubMed
The gut microbiota is a critical factor in the regulation of host health, but the relationship between the differential resistance of hosts to pathogens and the interaction of gut microbes is not yet clear. Herein, we investigated the potential correlation between the gut microbiota of piglets and their disease resistance using single-cell transcriptomics, 16S amplicon sequencing, metagenomics, and untargeted metabolomics.
-
-
IL-21-armored B7H3 CAR-iNKT cells exert potent antitumor effects.
In iScience on 19 January 2024 by Liu, Y., Dang, Y., et al.
PubMed
CD1d-restricted invariant NKT (iNKT) cells play a critical role in tumor immunity. However, the scarcity and limited persistence restricts their development and clinical application. Here, we demonstrated that iNKT cells could be efficiently expanded using modified cytokines combination from peripheral blood mononuclear cells. Introduction of IL-21 significantly increased the frequency of CD62L-positive memory-like iNKT cells. iNKT cells armoring with B7H3-targeting second generation CAR and IL-21 showed potent tumor cell killing activity. Moreover, co-expression of IL-21 promoted the activation of Stat3 signaling and reduced the expression of exhaustion markers in CAR-iNKT cells in vitro. Most importantly, IL-21-arming significantly prolonged B7H3 CAR-iNKT cell proliferation and survival in vivo, thus improving their therapeutic efficacy in mouse renal cancer xerograph models without observed cytokine-related adverse events. In summary, these results suggest that B7H3 CAR-iNKT armored with IL-21 is a promising therapeutic strategy for cancer treatment.