InVivoPlus anti-mouse CD16/CD32
Product Details
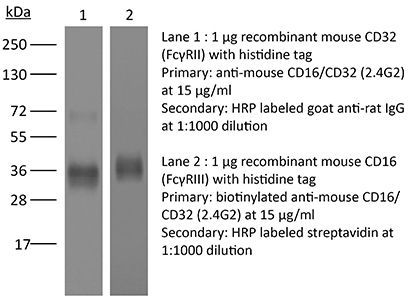
The 2.4G2 monoclonal antibody reacts specifically with mouse CD16 (FcγRIII) and CD32 (FcγRII). It has also been reported to react non-specifically via its Fc domain to FcγRI. CD16 and CD32 are expressed on B cells, monocytes/macrophages, NK cells, granulocytes, mast cells, and dendritic cells. These receptors bind to the Fc portion of antibody-antigen complexes and play a role in adaptive immune responses. The 2.4G2 antibody is commonly used in flow cytometry and immunofluorescence staining experiments to prevent non-specific binding of the Fc portion of IgG to the FcγIII and FcγII, and possibly FcγI, receptors prior to staining with antigen specific primary antibodies. The complete antibody and Fab fragments of the 2.4G2 antibody have also been used to block Fc receptors in vivo. Note that when 2.4G2 is used for Fc blocking in immunoassays and an anti-IgG secondary-step is necessary, the secondary antibody must not be anti-rat IgG2b.Specifications
| Isotype | Rat IgG2b, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoPlus rat IgG2b isotype control, anti-keyhole limpet hemocyanin |
| Recommended Dilution Buffer | InVivoPure pH 8.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | BALB/c mouse macrophage cell line J774 |
| Reported Applications |
in vivo Fc receptor blocking Fc receptor blocking, flow cytometry Fc receptor blocking, immunofluorescence |
| Formulation |
PBS, pH 8.0 Contains no stabilizers or preservatives |
| Aggregation* |
<5% Determined by SEC |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2736987 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests* |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Additional Formats
Recommended Products
Fc receptor blocking, Flow Cytometry
Pasqual, G., et al. (2018). "Monitoring T cell-dendritic cell interactions in vivo by intercellular enzymatic labelling" Nature 553(7689): 496-500. PubMed
Interactions between different cell types are essential for multiple biological processes, including immunity, embryonic development and neuronal signalling. Although the dynamics of cell-cell interactions can be monitored in vivo by intravital microscopy, this approach does not provide any information on the receptors and ligands involved or enable the isolation of interacting cells for downstream analysis. Here we describe a complementary approach that uses bacterial sortase A-mediated cell labelling across synapses of immune cells to identify receptor-ligand interactions between cells in living mice, by generating a signal that can subsequently be detected ex vivo by flow cytometry. We call this approach for the labelling of ‘kiss-and-run’ interactions between immune cells ‘Labelling Immune Partnerships by SorTagging Intercellular Contacts’ (LIPSTIC). Using LIPSTIC, we show that interactions between dendritic cells and CD4(+) T cells during T-cell priming in vivo occur in two distinct modalities: an early, cognate stage, during which CD40-CD40L interactions occur specifically between T cells and antigen-loaded dendritic cells; and a later, non-cognate stage during which these interactions no longer require prior engagement of the T-cell receptor. Therefore, LIPSTIC enables the direct measurement of dynamic cell-cell interactions both in vitro and in vivo. Given its flexibility for use with different receptor-ligand pairs and a range of detectable labels, we expect that this approach will be of use to any field of biology requiring quantification of intercellular communication.
in vivo Fc receptor blocking
Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, Miller MA, Carlson JC, Freeman GJ, Anthony RM, Weissleder R, Pittet MJ. (2017). "In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy" Sci Transl Med 9(389):eaal3604. PubMed
Monoclonal antibodies (mAbs) targeting the immune checkpoint anti-programmed cell death protein 1 (aPD-1) have demonstrated impressive benefits for the treatment of some cancers; however, these drugs are not always effective, and we still have a limited understanding of the mechanisms that contribute to their efficacy or lack thereof. We used in vivo imaging to uncover the fate and activity of aPD-1 mAbs in real time and at subcellular resolution in mice. We show that aPD-1 mAbs effectively bind PD-1+ tumor-infiltrating CD8+ T cells at early time points after administration. However, this engagement is transient, and aPD-1 mAbs are captured within minutes from the T cell surface by PD-1- tumor-associated macrophages. We further show that macrophage accrual of aPD-1 mAbs depends both on the drug's Fc domain glycan and on Fcγ receptors (FcγRs) expressed by host myeloid cells and extend these findings to the human setting. Finally, we demonstrate that in vivo blockade of FcγRs before aPD-1 mAb administration substantially prolongs aPD-1 mAb binding to tumor-infiltrating CD8+ T cells and enhances immunotherapy-induced tumor regression in mice. These investigations yield insight into aPD-1 target engagement in vivo and identify specific Fc/FcγR interactions that can be modulated to improve checkpoint blockade therapy.
in vivo Fc receptor blocking
Yu, X., et al. (2015). "A monoclonal antibody with anti-D-like activity in murine immune thrombocytopenia requires Fc domain function for immune thrombocytopenia ameliorative effects" Transfusion 55(6 Pt 2): 1501-1511. PubMed
BACKGROUND: The mechanism of action of anti-D in ameliorating immune thrombocytopenia (ITP) remains unclear. The monoclonal antibody (MoAb) Ter119, which targets murine red blood cells (RBCs), has been shown to mimic the effect of anti-D in improving antibody-mediated murine ITP. The mechanism of Ter119-mediated ITP amelioration, especially the role of the antigen-binding and Fc domains, remains untested. A functional Fc domain is crucial for many therapeutic MoAb activity; therefore, the requirement of Ter119 Fc domain in ITP amelioration is investigated using outbred CD-1 mice. STUDY DESIGN AND METHODS: Ter119 variants, including Ter119 F(ab’)2 fragments, deglycosylated Ter119, and afucosylated Ter119, were generated to test their effect in ameliorating antibody-induced murine ITP. In vivo inhibition of FcgammaRIII and FcgammaRIIB was achieved using the Fab fragment of the FcgammaRIII/FcgammaRIIB-specific MoAb 2.4G2. RESULTS: Ter119 F(ab’)2 fragments and deglycosylated Ter119 were unable to ameliorate murine ITP or mediate phagocytosis of RBCs by RAW264.7 macrophages in vitro. Inhibition of FcgammaRIII and FcgammaRIIB, as well as Ter119 defucosylation, do not affect Ter119-mediated ITP amelioration. CONCLUSION: The Fc domain of Ter119, as well as its Fc glycosylation, is required for Ter119-mediated ITP amelioration. Moreover, both Fc and Fc glycosylation are required for Ter119-mediated phagocytosis in vitro. These findings demonstrate the importance of the Fc domain in a therapeutic MoAb with anti-D-like activity.
Fc receptor blocking, Flow Cytometry
Liu, X., et al. (2015). "CD47 blockade triggers T cell-mediated destruction of immunogenic tumors" Nat Med 21(10): 1209-1215. PubMed
Macrophage phagocytosis of tumor cells mediated by CD47-specific blocking antibodies has been proposed to be the major effector mechanism in xenograft models. Here, using syngeneic immunocompetent mouse tumor models, we reveal that the therapeutic effects of CD47 blockade depend on dendritic cell but not macrophage cross-priming of T cell responses. The therapeutic effects of anti-CD47 antibody therapy were abrogated in T cell-deficient mice. In addition, the antitumor effects of CD47 blockade required expression of the cytosolic DNA sensor STING, but neither MyD88 nor TRIF, in CD11c(+) cells, suggesting that cytosolic sensing of DNA from tumor cells is enhanced by anti-CD47 treatment, further bridging the innate and adaptive responses. Notably, the timing of administration of standard chemotherapy markedly impacted the induction of antitumor T cell responses by CD47 blockade. Together, our findings indicate that CD47 blockade drives T cell-mediated elimination of immunogenic tumors.
Fc receptor blocking, Flow Cytometry
Peske, J. D., et al. (2015). "Effector lymphocyte-induced lymph node-like vasculature enables naive T-cell entry into tumours and enhanced anti-tumour immunity" Nat Commun 6: 7114. PubMed
The presence of lymph node (LN)-like vasculature in tumours, characterized by expression of peripheral node addressin and chemokine CCL21, is correlated with T-cell infiltration and positive prognosis in breast cancer and melanoma patients. However, mechanisms controlling the development of LN-like vasculature and how it might contribute to a beneficial outcome for cancer patients are unknown. Here we demonstrate that LN-like vasculature is present in murine models of melanoma and lung carcinoma. It enables infiltration by naive T cells that significantly delay tumour outgrowth after intratumoral activation. Development of this vasculature is controlled by a mechanism involving effector CD8 T cells and NK cells that secrete LTalpha3 and IFNgamma. LN-like vasculature is also associated with organized aggregates of B lymphocytes and gp38(+) fibroblasts, which resemble tertiary lymphoid organs that develop in models of chronic inflammation. These results establish LN-like vasculature as both a consequence of and key contributor to anti-tumour immunity.
Fc receptor blocking, Flow Cytometry
Arbelaez, C. A., et al. (2015). "IL-7/IL-7 Receptor Signaling Differentially Affects Effector CD4+ T Cell Subsets Involved in Experimental Autoimmune Encephalomyelitis" J Immunol 195(5): 1974-1983. PubMed
IL-17-producing CD4(+) T (Th17) cells, along with IFN-gamma-expressing Th1 cells, represent two major pathogenic T cell subsets in experimental autoimmune encephalomyelitis (EAE), the animal model of multiple sclerosis (MS). The cytokines and transcription factors involved in the development and effector functions of Th1 and Th17 cells have been largely characterized. Among them, IL-23 is essential for the generation of stable and encephalitogenic Th17 cells and for the development of EAE. The IL-7/IL-7R signaling axis participates in cell survival, and perturbation of this pathway has been associated with enhanced susceptibility to MS. A link between IL-23-driven pathogenic T cells and IL-7/IL-7R signaling has previously been proposed, but has not been formally addressed. In the current study, we showed that Th17 cells from mice with EAE express high levels of IL-7Ralpha compared with Th1 cells. Using mice that constitutively express IL-7Ralpha on T cells, we determined that sustained IL-7R expression in IL-23R-deficient mice could not drive pathogenic T cells and the development of EAE. IL-7 inhibited the differentiation of Th17 cells, but promoted IFN-gamma and GM-CSF secretion in vitro. In vivo IL-7/anti-IL-7 mAb complexes selectively expanded and enhanced the proliferation of CXCR3-expressing Th1 cells, but did not impact Th17 cells and EAE development in wild-type and IL-23R-deficient mice. Importantly, high IL-7 expression was detected in the CNS during EAE and could drive the plasticity of Th17 cells to IFN-gamma-producing T cells. Together, these data address the contribution of IL-23/IL-23R and IL-7/IL-7R signaling in Th17 and Th1 cell dynamics during CNS autoimmunity.
Fc receptor blocking, Flow Cytometry
Leon, B., et al. (2014). "FoxP3+ regulatory T cells promote influenza-specific Tfh responses by controlling IL-2 availability" Nat Commun 5: 3495. PubMed
Here, we test the role of FoxP3(+) regulatory T cells (Tregs) in controlling T follicular helper (Tfh) and germinal centre (GC) B-cell responses to influenza. In contrast to the idea that Tregs suppress T-cell responses, we find that Treg depletion severely reduces the Tfh cell response to influenza virus. Furthermore, Treg depletion prevents the accumulation of influenza-specific GCs. These effects are not due to alterations in TGFbeta availability or a precursor-progeny relationship between Tregs and Tfh cells, but are instead mediated by increased availability of IL-2, which suppresses the differentiation of Tfh cells and as a consequence, compromises the GC B response. Thus, Tregs promote influenza-specific GC responses by preventing excessive IL-2 signalling, which suppresses Tfh cell differentiation.
Fc receptor blocking, Flow Cytometry
Deng, L., et al. (2014). "Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice" J Clin Invest 124(2): 687-695. PubMed
High-dose ionizing irradiation (IR) results in direct tumor cell death and augments tumor-specific immunity, which enhances tumor control both locally and distantly. Unfortunately, local relapses often occur following IR treatment, indicating that IR-induced responses are inadequate to maintain antitumor immunity. Therapeutic blockade of the T cell negative regulator programmed death-ligand 1 (PD-L1, also called B7-H1) can enhance T cell effector function when PD-L1 is expressed in chronically inflamed tissues and tumors. Here, we demonstrate that PD-L1 was upregulated in the tumor microenvironment after IR. Administration of anti-PD-L1 enhanced the efficacy of IR through a cytotoxic T cell-dependent mechanism. Concomitant with IR-mediated tumor regression, we observed that IR and anti-PD-L1 synergistically reduced the local accumulation of tumor-infiltrating myeloid-derived suppressor cells (MDSCs), which suppress T cells and alter the tumor immune microenvironment. Furthermore, activation of cytotoxic T cells with combination therapy mediated the reduction of MDSCs in tumors through the cytotoxic actions of TNF. Our data provide evidence for a close interaction between IR, T cells, and the PD-L1/PD-1 axis and establish a basis for the rational design of combination therapy with immune modulators and radiotherapy.
Fc receptor blocking, Flow Cytometry
Muppidi, J. R., et al. (2014). "Loss of signalling via Galpha13 in germinal centre B-cell-derived lymphoma" Nature 516(7530): 254-258. PubMed
Germinal centre B-cell-like diffuse large B-cell lymphoma (GCB-DLBCL) is a common malignancy, yet the signalling pathways that are deregulated and the factors leading to its systemic dissemination are poorly defined. Work in mice showed that sphingosine-1-phosphate receptor-2 (S1PR2), a Galpha12 and Galpha13 coupled receptor, promotes growth regulation and local confinement of germinal centre B cells. Recent deep sequencing studies of GCB-DLBCL have revealed mutations in many genes in this cancer, including in GNA13 (encoding Galpha13) and S1PR2 (refs 5,6, 7). Here we show, using in vitro and in vivo assays, that GCB-DLBCL-associated mutations occurring in S1PR2 frequently disrupt the receptor’s Akt and migration inhibitory functions. Galpha13-deficient mouse germinal centre B cells and human GCB-DLBCL cells were unable to suppress pAkt and migration in response to S1P, and Galpha13-deficient mice developed germinal centre B-cell-derived lymphoma. Germinal centre B cells, unlike most lymphocytes, are tightly confined in lymphoid organs and do not recirculate. Remarkably, deficiency in Galpha13, but not S1PR2, led to germinal centre B-cell dissemination into lymph and blood. GCB-DLBCL cell lines frequently carried mutations in the Galpha13 effector ARHGEF1, and Arhgef1 deficiency also led to germinal centre B-cell dissemination. The incomplete phenocopy of Galpha13- and S1PR2 deficiency led us to discover that P2RY8, an orphan receptor that is mutated in GCB-DLBCL and another germinal centre B-cell-derived malignancy, Burkitt’s lymphoma, also represses germinal centre B-cell growth and promotes confinement via Galpha13. These findings identify a Galpha13-dependent pathway that exerts dual actions in suppressing growth and blocking dissemination of germinal centre B cells that is frequently disrupted in germinal centre B-cell-derived lymphoma.
Fc receptor blocking, Flow Cytometry
Heesch, K., et al. (2014). "The function of the chemokine receptor CXCR6 in the T cell response of mice against Listeria monocytogenes" PLoS One 9(5): e97701. PubMed
The chemokine receptor CXCR6 is expressed on different T cell subsets and up-regulated following T cell activation. CXCR6 has been implicated in the localization of cells to the liver due to the constitutive expression of its ligand CXCL16 on liver sinusoidal endothelial cells. Here, we analyzed the role of CXCR6 in CD8+ T cell responses to infection of mice with Listeria monocytogenes. CD8+ T cells responding to listerial antigens acquired high expression levels of CXCR6. However, deficiency of mice in CXCR6 did not impair control of the L. monocytogenes infection. CXCR6-deficient mice were able to generate listeria-specific CD4+ and CD8+ T cell responses and showed accumulation of T cells in the infected liver. In transfer assays, we detected reduced accumulation of listeria-specific CXCR6-deficient CD8+ T cells in the liver at early time points post infection. Though, CXCR6 was dispensable at later time points of the CD8+ T cell response. When transferred CD8+ T cells were followed for extended time periods, we observed a decline in CXCR6-deficient CD8+ T cells. The manifestation of this cell loss depended on the tissue analyzed. In conclusion, our results demonstrate that CXCR6 is not required for the formation of a T cell response to L. monocytogenes and for the accumulation of T cells in the infected liver but CXCR6 appears to influence long-term survival and tissue distribution of activated cells.
Fc receptor blocking, Immunofluorescence
Brinkman CC, Rouhani SJ, Srinivasan N, Engelhard VH. (2013). "Peripheral tissue homing receptors enable T cell entry into lymph nodes and affect the anatomical distribution of memory cells" J Immunol 191(5):2412-25. PubMed
Peripheral tissue homing receptors enable T cells to access inflamed nonlymphoid tissues. In this study, we show that two such molecules, E-selectin ligand and α4β1 integrin, enable activated and memory T cells to enter lymph nodes (LN) as well. This affects the quantitative and qualitative distribution of these cells among regional LN beds. CD8 memory T cells in LN that express these molecules were mostly CD62L(lo) and would normally be classified as effector memory cells. However, similar to central memory cells, they expanded upon Ag re-encounter. This led to differences in the magnitude of the recall response that depended on the route of immunization. These novel cells share properties of both central and effector memory cells and reside in LN based on previously undescribed mechanisms of entry.
- FC/FACS,
- Mus musculus (House mouse)
Epitranscriptional regulation of TGF-β pseudoreceptor BAMBI by m6A/YTHDF2 drives extrinsic radioresistance.
In The Journal of Clinical Investigation on 15 December 2023 by Wang, L., Si, W., et al.
PubMed
Activation of TGF-β signaling serves as an extrinsic resistance mechanism that limits the potential for radiotherapy. Bone morphogenetic protein and activin membrane-bound inhibitor (BAMBI) antagonizes TGF-β signaling and is implicated in cancer progression. However, the molecular mechanisms of BAMBI regulation in immune cells and its impact on antitumor immunity after radiation have not been established. Here, we show that ionizing radiation (IR) specifically reduces BAMBI expression in immunosuppressive myeloid-derived suppressor cells (MDSCs) in both murine models and humans. Mechanistically, YTH N6-methyladenosine RNA-binding protein F2 (YTHDF2) directly binds and degrades Bambi transcripts in an N6-methyladenosine-dependent (m6A-dependent) manner, and this relies on NF-κB signaling. BAMBI suppresses the tumor-infiltrating capacity and suppression function of MDSCs via inhibiting TGF-β signaling. Adeno-associated viral delivery of Bambi (AAV-Bambi) to the tumor microenvironment boosts the antitumor effects of radiotherapy and radioimmunotherapy combinations. Intriguingly, combination of AAV-Bambi and IR not only improves local tumor control, but also suppresses distant metastasis, further supporting its clinical translation potential. Our findings uncover a surprising role of BAMBI in myeloid cells, unveiling a potential therapeutic strategy for overcoming extrinsic radioresistance.
- Immunology and Microbiology,
- Cell Biology,
- Biochemistry and Molecular biology
Metabolic adaptation supports enhanced macrophage efferocytosis in limited-oxygen environments.
In Cell Metabolism on 7 February 2023 by Wang, Y. T., Trzeciak, A. J., et al.
PubMed
Apoptotic cell (AC) clearance (efferocytosis) is performed by phagocytes, such as macrophages, that inhabit harsh physiological environments. Here, we find that macrophages display enhanced efferocytosis under prolonged (chronic) physiological hypoxia, characterized by increased internalization and accelerated degradation of ACs. Transcriptional and translational analyses revealed that chronic physiological hypoxia induces two distinct but complimentary states. The first, "primed" state, consists of concomitant transcription and translation of metabolic programs in AC-naive macrophages that persist during efferocytosis. The second, "poised" state, consists of transcription, but not translation, of phagocyte function programs in AC-naive macrophages that are translated during efferocytosis. Mechanistically, macrophages efficiently flux glucose into a noncanonical pentose phosphate pathway (PPP) loop to enhance NADPH production. PPP-derived NADPH directly supports enhanced efferocytosis under physiological hypoxia by ensuring phagolysosomal maturation and redox homeostasis. Thus, macrophages residing under physiological hypoxia adopt states that support cell fitness and ensure performance of essential homeostatic functions rapidly and safely. Copyright © 2022 Elsevier Inc. All rights reserved.
- Immunology and Microbiology
Engineered human cytokine/antibody fusion proteins expand regulatory T cells and confer autoimmune disease protection.
In Cell Reports on 18 October 2022 by VanDyke, D., Iglesias, M., et al.
PubMed
Low-dose human interleukin-2 (hIL-2) treatment is used clinically to treat autoimmune disorders due to the cytokine's preferential expansion of immunosuppressive regulatory T cells (Tregs). However, off-target immune cell activation and short serum half-life limit the clinical potential of IL-2 treatment. Recent work showed that complexes comprising hIL-2 and the anti-hIL-2 antibody F5111 overcome these limitations by preferentially stimulating Tregs over immune effector cells. Although promising, therapeutic translation of this approach is complicated by the need to optimize dosing ratios and by the instability of the cytokine/antibody complex. We leverage structural insights to engineer a single-chain hIL-2/F5111 antibody fusion protein, termed F5111 immunocytokine (IC), which potently and selectively activates and expands Tregs. F5111 IC confers protection in mouse models of colitis and checkpoint inhibitor-induced diabetes mellitus. These results provide a roadmap for IC design and establish a Treg-biased immunotherapy that could be clinically translated for autoimmune disease treatment. Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
- Mus musculus (House mouse),
- Immunology and Microbiology
Engineered human cytokine/antibody fusion proteins expand regulatory T cells and confer autoimmune disease protection
Preprint on BioRxiv : the Preprint Server for Biology on 29 May 2022 by VanDyke, D., Iglesias, M., et al.
PubMed
h4>Summary/h4> Low dose human interleukin-2 (hIL-2) treatment is used clinically to treat autoimmune disorders due to the cytokine’s preferential expansion of immunosuppressive regulatory T cells (T Reg s). However, high toxicity, short serum half-life, and off-target immune cell activation limit the clinical potential of IL-2 treatment. Recent work showed that complexes comprising hIL-2 and the anti-hIL-2 antibody F5111 overcome these limitations by preferentially stimulating T Reg s over immune effector cells. Although promising, therapeutic translation of this approach is complicated by the need to optimize dosing ratios and by the instability of the cytokine/antibody complex. We leveraged structural insights to engineer a single-chain hIL-2/F5111 antibody fusion protein, termed F5111 immunocytokine (IC), that potently and selectively activates and expands T Reg s. F5111 IC conferred protection in mouse models of colitis and checkpoint inhibitor-induced diabetes mellitus. These results provide a roadmap for IC design and establish a T Reg -biased immunotherapy that could be clinically translated for autoimmune disease treatment.
- Cancer Research
Circulating Tregs Accumulate in Omental Tumors and Acquire Adipose-Resident Features.
In Cancer Immunology Research on 3 May 2022 by Liu, M., Starenki, D., et al.
PubMed
Tumors that metastasize in the peritoneal cavity typically end up in the omental adipose tissue, a particularly immune-suppressive environment that includes specialized adipose-resident regulatory T cells (Treg). Tregs rapidly accumulate in the omentum after tumor implantation and potently suppress antitumor immunity. However, it is unclear whether these Tregs are recruited from the circulation or derived from preexisting adipose-resident Tregs by clonal expansion. Here we show that Tregs in tumor-bearing omenta predominantly have thymus-derived characteristics. Moreover, naïve tumor antigen-specific CD4+ T cells fail to differentiate into Tregs in tumor-bearing omenta. In fact, Tregs derived from the pretumor repertoire are sufficient to suppress antitumor immunity and promote tumor growth. However, tumor implantation in the omentum does not promote Treg clonal expansion, but instead leads to increased clonal diversity. Parabiosis experiments show that despite tissue-resident (noncirculating) characteristics of omental Tregs in naïve mice, tumor implantation promotes a rapid influx of circulating Tregs, many of which come from the spleen. Finally, we show that newly recruited Tregs rapidly acquire characteristics of adipose-resident Tregs in tumor-bearing omenta. These data demonstrate that most Tregs in omental tumors are recruited from the circulation and adapt to their environment by altering their homing, transcriptional, and metabolic properties. ©2022 American Association for Cancer Research.
- Immunology and Microbiology,
- Neuroscience,
- Cell Culture,
- Mus musculus (House mouse)
Microglia Require CD4 T Cells to Complete the Fetal-to-Adult Transition.
In Cell on 6 August 2020 by Pasciuto, E., Burton, O. T., et al.
PubMed
The brain is a site of relative immune privilege. Although CD4 T cells have been reported in the central nervous system, their presence in the healthy brain remains controversial, and their function remains largely unknown. We used a combination of imaging, single cell, and surgical approaches to identify a CD69+ CD4 T cell population in both the mouse and human brain, distinct from circulating CD4 T cells. The brain-resident population was derived through in situ differentiation from activated circulatory cells and was shaped by self-antigen and the peripheral microbiome. Single-cell sequencing revealed that in the absence of murine CD4 T cells, resident microglia remained suspended between the fetal and adult states. This maturation defect resulted in excess immature neuronal synapses and behavioral abnormalities. These results illuminate a role for CD4 T cells in brain development and a potential interconnected dynamic between the evolution of the immunological and neurological systems. VIDEO ABSTRACT.Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
- Cancer Research
Novel, genetically induced mouse model that recapitulates the histological morphology and immunosuppressive tumor microenvironment of metastatic peritoneal carcinomatosis.
In Journal for Immunotherapy of Cancer on 1 February 2020 by Tseng, S. H., Park, S. T., et al.
PubMed
Peritoneal carcinomatosis is a hallmark of advanced peritoneal tumor progression, particularly for tubal/ovarian high-grade serous carcinomas (HGSCs). Patients with peritoneal carcinomatosis have poor survival rates and are difficult to treat clinically due to widespread tumor dissemination in the peritoneal cavity. We developed a clinically relevant, genetically induced, peritoneal carcinomatosis model that recapitulates the histological morphology and immunosuppressive state of the tumor microenvironment of metastatic peritoneal HGSCs by intraperitoneally injecting shp53, AKT, c-Myc, luciferase and sleeping beauty transposase, followed by electroporation (EP) in the peritoneal cavity of immunocompetent mice (intraperitoneal (IP)/EP mice). Similar to the spread of human ovarian cancers, IP/EP mice displayed multiple tumor nodules attached to the surface of the abdomen. Histopathological analysis indicated that these tumors were epithelial in origin. These IP/EP mice also displayed a loss of CD3+ T cell infiltration in tumors, highly expressed inhibitory checkpoint molecules in tumor-infiltrating and global CD4+ and CD8+ T cells, and increased levels of transforming growth factor-β in the ascites, all of which contribute to the promotion of tumor growth. Overall, our tumor model recapitulates clinical peritoneal HGSC metastasis, which makes it ideal for preclinical drug screening, testing of immunotherapy-based therapeutics and studying of the tumor biology of peritoneal carcinomatosis. © Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
- Cancer Research,
- Immunology and Microbiology
A universal anti-cancer vaccine: Chimeric invariant chain potentiates the inhibition of melanoma progression and the improvement of survival.
In International Journal of Cancer on 15 February 2019 by Sharbi-Yunger, A., Grees, M., et al.
PubMed
For many years, clinicians and scientists attempt to develop methods to stimulate the immune system to target malignant cells. Recent data suggest that effective cancer vaccination requires combination immunotherapies to overcome tumor immune evasion. Through presentation of both MHC-I and II molecules, DCs-based vaccine platforms are effective in generating detectable CD4 and CD8 T cell responses against tumor-associated antigens. Several platforms include DC transfection with mRNA of the desired tumor antigen. These DCs are then delivered to the host and elicit an immune response against the antigen of interest. We have recently established an mRNA genetic platform which induced specific CD8+ cytotoxic T cell response by DC vaccination against melanoma. In our study, an MHC-II mRNA DCs vaccine platform was developed to activate CD4+ T cells and to enhance the anti-tumor response. The invariant chain (Ii) was modified and the semi-peptide CLIP was replaced with an MHC-II binding peptide sequences of melanoma antigens. These chimeric MHC-II constructs are presented by DCs and induce proliferation of tumor specific CD4+ T cells. When administered in combination with the MHC-I platform into tumor bearing mice, these constructs were able to inhibit tumor growth, and improve mouse survival. Deciphering the immunological mechanism of action, we observed an efficient CTLs killing in addition to higher levels of Th1 and Th2 subsets in the groups immunized with a combination of the MHC-I and MHC-II constructs. These universal constructs can be applied in multiple combinations and offer an attractive opportunity to improve cancer treatment. © 2018 UICC.
- Immunology and Microbiology
A Minimum Epitope Overlap between Infections Strongly Narrows the Emerging T Cell Repertoire.
In Cell Reports on 11 October 2016 by Oberle, S. G., Hanna-El-Daher, L., et al.
PubMed
Many infections are caused by pathogens that are similar, but not identical, to previously encountered viruses, bacteria, or vaccines. In such re-infections, pathogens introduce known antigens, which are recognized by memory T cells and new antigens that activate naive T cells. How preexisting memory T cells impact the repertoire of T cells responding to new antigens is still largely unknown. We demonstrate that even a minimum epitope overlap between infections strongly increases the activation threshold and narrows the diversity of T cells recruited in response to new antigens. Thus, minimal cross-reactivity between infections can significantly impact the outcome of a subsequent immune response. Interestingly, we found that non-transferrable memory T cells are most effective in raising the activation threshold. Our findings have implications for designing vaccines and suggest that vaccines meant to target low-affinity T cells are less effective when they contain a strong CD8 T cell epitope that has previously been encountered. Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
- Cancer Research,
- Immunology and Microbiology
NIAM-deficient mice are predisposed to the development of proliferative lesions including B-cell lymphomas.
In PLoS ONE on 14 November 2014 by Reed, S. M., Hagen, J., et al.
PubMed
Nuclear Interactor of ARF and Mdm2 (NIAM, gene designation Tbrg1) is a largely unstudied inhibitor of cell proliferation that helps maintain chromosomal stability. It is a novel activator of the ARF-Mdm2-Tip60-p53 tumor suppressor pathway as well as other undefined pathways important for genome maintenance. To examine its predicted role as a tumor suppressor, we generated NIAM mutant (NIAM(m/m)) mice homozygous for a β-galactosidase expressing gene-trap cassette in the endogenous gene. The mutant mice expressed significantly lower levels of NIAM protein in tissues compared to wild-type animals. Fifty percent of aged NIAM deficient mice (14 to 21 months) developed proliferative lesions, including a uterine hemangioma, pulmonary papillary adenoma, and a Harderian gland adenoma. No age-matched wild-type or NIAM(+/m) heterozygous animals developed lesions. In the spleen, NIAM(m/m) mice had prominent white pulp expansion which correlated with enhanced increased reactive lymphoid hyperplasia and evidence of systemic inflammation. Notably, 17% of NIAM mutant mice had splenic white pulp features indicating early B-cell lymphoma. This correlated with selective expansion of marginal zone B cells in the spleens of younger, tumor-free NIAM-deficient mice. Unexpectedly, basal p53 expression and activity was largely unaffected by NIAM loss in isolated splenic B cells. In sum, NIAM down-regulation in vivo results in a significant predisposition to developing benign tumors or early stage cancers. These mice represent an outstanding platform for dissecting NIAM's role in tumorigenesis and various anti-cancer pathways, including p53 signaling.
T follicular helper cell dynamics in germinal centers.
In Science on 9 August 2013 by Shulman, Z., Gitlin, A. D., et al.
PubMed
T follicular helper (T(FH)) cells are a specialized subset of effector T cells that provide help to and thereby select high-affinity B cells in germinal centers (GCs). To examine the dynamic behavior of T(FH) cells in GCs in mice, we used two-photon microscopy in combination with a photoactivatable fluorescent reporter. Unlike GC B cells, which are clonally restricted, T(FH) cells distributed among all GCs in lymph nodes and continually emigrated into the follicle and neighboring GCs. Moreover, newly activated T(FH) cells invaded preexisting GCs, where they contributed to B cell selection and plasmablast differentiation. Our data suggest that the dynamic exchange of T(FH) cells between GCs ensures maximal diversification of T cell help and that their ability to enter ongoing GCs accommodates antigenic variation during the immune response.