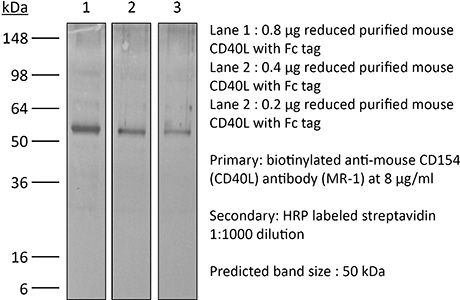
InVivoPlus anti-mouse CD40L (CD154)
Product Details
The MR-1 monoclonal antibody reacts with mouse CD154 also known as CD40 ligand. CD154 exists as a 39 kDa accessory molecule and belongs to the TNF superfamily of cytokines. CD154 is primarily expressed on the surface of activated CD4+ T lymphocytes but can also be expressed by platelets, mast cells, macrophages, basophils, NK cells, B lymphocytes, CD8+ T lymphocytes as well as non-hematopoietic cells including smooth muscle cells, endothelial cells, and epithelial cells. CD154 signals through CD40 and is thought to play a key role in T and B lymphocyte costimulation. The MR-1 monoclonal antibody has been reported to inhibit in vitro activation of B lymphocytes by blocking the binding of CD154 with CD40 on T helper cells as well as inhibit the formation of germinal centers and disrupt antigen-specific T cell responses. Additionally, the MR-1 antibody blocks interactions of T cells and antigen-presenting cells in vitro and blocks the development of experimental autoimmune disease in vivo.Specifications
| Isotype | Armenian hamster IgG |
|---|---|
| Recommended Isotype Control(s) | InVivoPlus polyclonal Armenian hamster IgG |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Activated mouse Th1 clone D1.6 |
| Reported Applications |
in vivo blocking of CD40/CD40L signaling in vitro blocking of CD40/CD40L signaling Western blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin* |
<1EU/mg (<0.001EU/μg) Determined by LAL gel clotting assay |
| Aggregation* |
<5% Determined by DLS |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 μM filtered |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_1107601 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests* |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Additional Formats
Recommended Products
in vivo blocking of CD40/CD40L signaling
Pasqual, G., et al. (2018). "Monitoring T cell-dendritic cell interactions in vivo by intercellular enzymatic labelling" Nature 553(7689): 496-500. PubMed
Interactions between different cell types are essential for multiple biological processes, including immunity, embryonic development and neuronal signalling. Although the dynamics of cell-cell interactions can be monitored in vivo by intravital microscopy, this approach does not provide any information on the receptors and ligands involved or enable the isolation of interacting cells for downstream analysis. Here we describe a complementary approach that uses bacterial sortase A-mediated cell labelling across synapses of immune cells to identify receptor-ligand interactions between cells in living mice, by generating a signal that can subsequently be detected ex vivo by flow cytometry. We call this approach for the labelling of ‘kiss-and-run’ interactions between immune cells ‘Labelling Immune Partnerships by SorTagging Intercellular Contacts’ (LIPSTIC). Using LIPSTIC, we show that interactions between dendritic cells and CD4(+) T cells during T-cell priming in vivo occur in two distinct modalities: an early, cognate stage, during which CD40-CD40L interactions occur specifically between T cells and antigen-loaded dendritic cells; and a later, non-cognate stage during which these interactions no longer require prior engagement of the T-cell receptor. Therefore, LIPSTIC enables the direct measurement of dynamic cell-cell interactions both in vitro and in vivo. Given its flexibility for use with different receptor-ligand pairs and a range of detectable labels, we expect that this approach will be of use to any field of biology requiring quantification of intercellular communication.
in vivo blocking of CD40/CD40L signaling
Brasseit, J., et al. (2015). "CD4 T cells are required for both development and maintenance of disease in a new mouse model of reversible colitis" Mucosal Immunol. doi : 10.1038/mi.2015.93. PubMed
Current therapies to treat inflammatory bowel diseases have limited efficacy, significant side effects, and often wane over time. Little is known about the cellular and molecular mechanisms operative in the process of mucosal healing from colitis. To study such events, we developed a new model of reversible colitis in which adoptive transfer of CD4+CD45RBhi T cells into Helicobacter typhlonius-colonized lymphopenic mice resulted in a rapid onset of colonic inflammation that was reversible through depletion of colitogenic T cells. Remission was associated with an improved clinical and histopathological score, reduced immune cell infiltration to the intestinal mucosa, altered intestinal gene expression profiles, regeneration of the colonic mucus layer, and the restoration of epithelial barrier integrity. Notably, colitogenic T cells were not only critical for induction of colitis but also for maintenance of disease. Depletion of colitogenic T cells resulted in a rapid drop in tumor necrosis factor alpha (TNFalpha) levels associated with reduced infiltration of inflammatory immune cells to sites of inflammation. Although neutralization of TNFalpha prevented the onset of colitis, anti-TNFalpha treatment of mice with established disease failed to resolve colonic inflammation. Collectively, this new model of reversible colitis provides an important research tool to study the dynamics of mucosal healing in chronic intestinal remitting-relapsing disorders.
in vivo blocking of CD40/CD40L signaling
Conde, P., et al. (2015). "DC-SIGN(+) Macrophages Control the Induction of Transplantation Tolerance" Immunity 42(6): 1143-1158. PubMed
Tissue effector cells of the monocyte lineage can differentiate into different cell types with specific cell function depending on their environment. The phenotype, developmental requirements, and functional mechanisms of immune protective macrophages that mediate the induction of transplantation tolerance remain elusive. Here, we demonstrate that costimulatory blockade favored accumulation of DC-SIGN-expressing macrophages that inhibited CD8(+) T cell immunity and promoted CD4(+)Foxp3(+) Treg cell expansion in numbers. Mechanistically, that simultaneous DC-SIGN engagement by fucosylated ligands and TLR4 signaling was required for production of immunoregulatory IL-10 associated with prolonged allograft survival. Deletion of DC-SIGN-expressing macrophages in vivo, interfering with their CSF1-dependent development, or preventing the DC-SIGN signaling pathway abrogated tolerance. Together, the results provide new insights into the tolerogenic effects of costimulatory blockade and identify DC-SIGN(+) suppressive macrophages as crucial mediators of immunological tolerance with the concomitant therapeutic implications in the clinic.
in vivo blocking of CD40/CD40L signaling
Awe, O., et al. (2015). "PU.1 Expression in T Follicular Helper Cells Limits CD40L-Dependent Germinal Center B Cell Development" J Immunol . PubMed
PU.1 is an ETS family transcription factor that is important for the development of multiple hematopoietic cell lineages. Previous work demonstrated a critical role for PU.1 in promoting Th9 development and in limiting Th2 cytokine production. Whether PU.1 has functions in other Th lineages is not clear. In this study, we examined the effects of ectopic expression of PU.1 in CD4+ T cells and observed decreased expression of genes involved with the function of T follicular helper (Tfh) cells, including Il21 and Tnfsf5 (encoding CD40L). T cells from conditional mutant mice that lack expression of PU.1 in T cells (Sfpi1lck-/-) demonstrated increased production of CD40L and IL-21 in vitro. Following adjuvant-dependent or adjuvant-independent immunization, we observed that Sfpi1lck-/- mice had increased numbers of Tfh cells, increased germinal center B cells (GCB cells), and increased Ab production in vivo. This correlated with increased expression of IL-21 and CD40L in Tfh cells from Sfpi1lck-/- mice compared with control mice. Finally, although blockade of IL-21 did not affect GCB cells in Sfpi1lck-/- mice, anti-CD40L treatment of immunized Sfpi1lck-/- mice decreased GCB cell numbers and Ag-specific Ig concentrations. Together, these data indicate an inhibitory role for PU.1 in the function of Tfh cells, germinal centers, and Tfh-dependent humoral immunity.
in vivo blocking of CD40/CD40L signaling
Miller, M. L., et al. (2015). "Spontaneous restoration of transplantation tolerance after acute rejection" Nat Commun 6: 7566. PubMed
Transplantation is a cure for end-stage organ failure but, in the absence of pharmacological immunosuppression, allogeneic organs are acutely rejected. Such rejection invariably results in allosensitization and accelerated rejection of secondary donor-matched grafts. Transplantation tolerance can be induced in animals and a subset of humans, and enables long-term acceptance of allografts without maintenance immunosuppression. However, graft rejection can occur long after a state of transplantation tolerance has been acquired. When such an allograft is rejected, it has been assumed that the same rules of allosensitization apply as to non-tolerant hosts and that immunological tolerance is permanently lost. Using a mouse model of cardiac transplantation, we show that when Listeria monocytogenes infection precipitates acute rejection, thus abrogating transplantation tolerance, the donor-specific tolerant state re-emerges, allowing spontaneous acceptance of a donor-matched second transplant. These data demonstrate a setting in which the memory of allograft tolerance dominates over the memory of transplant rejection.
in vivo blocking of CD40/CD40L signaling, in vitro blocking of CD40/CD40L signaling
Krummey, S. M., et al. (2014). "Candida-elicited murine Th17 cells express high Ctla-4 compared with Th1 cells and are resistant to costimulation blockade" J Immunol 192(5): 2495-2504. PubMed
Effector and memory T cells may cross-react with allogeneic Ags to mediate graft rejection. Whereas the costimulation properties of Th1 cells are well studied, relatively little is known about the costimulation requirements of microbe-elicited Th17 cells. The costimulation blocker CTLA-4 Ig has been ineffective in the treatment of several Th17-driven autoimmune diseases and is associated with severe acute rejection following renal transplantation, leading us to investigate whether Th17 cells play a role in CD28/CTLA-4 blockade-resistant alloreactivity. We established an Ag-specific model in which Th1 and Th17 cells were elicited via Mycobacterium tuberculosis and Candida albicans immunization, respectively. C. albicans immunization elicited a higher frequency of Th17 cells and conferred resistance to costimulation blockade following transplantation. Compared with the M. tuberculosis group, C. albicans-elicited Th17 cells contained a higher frequency of IL-17(+)IFN-gamma(+) producers and a lower frequency of IL-10(+) and IL-10(+)IL-17(+) cells. Importantly, Th17 cells differentially regulated the CD28/CTLA-4 pathway, expressing similarly high CD28 but significantly greater amounts of CTLA-4 compared with Th1 cells. Ex vivo blockade experiments demonstrated that Th17 cells are more sensitive to CTLA-4 coinhibition and therefore less susceptible to CTLA-4 Ig. These novel insights into the differential regulation of CTLA-4 coinhibition on CD4(+) T cells have implications for the immunomodulation of pathologic T cell responses during transplantation and autoimmunity.
in vivo blocking of CD40/CD40L signaling
Marshall, D., et al. (2014). "Differential requirement for IL-2 and IL-15 during bifurcated development of thymic regulatory T cells" J Immunol 193(11): 5525-5533. PubMed
The developmental pathways of regulatory T cells (T(reg)) generation in the thymus are not fully understood. In this study, we reconstituted thymic development of Zap70-deficient thymocytes with a tetracycline-inducible Zap70 transgene to allow temporal dissection of T(reg) development. We find that T(reg) develop with distinctive kinetics, first appearing by day 4 among CD4 single-positive (SP) thymocytes. Accepted models of CD25(+)Foxp3(+) T(reg) selection suggest development via CD25(+)Foxp3(-) CD4 SP precursors. In contrast, our kinetic analysis revealed the presence of abundant CD25(-)Foxp3(+) cells that are highly efficient at maturing to CD25(+)Foxp3(+) cells in response to IL-2. CD25(-)Foxp3(+) cells more closely resembled mature T(reg) both with respect to kinetics of development and avidity for self-peptide MHC. These population also exhibited distinct requirements for cytokines during their development. CD25(-)Foxp3(+) cells were IL-15 dependent, whereas generation of CD25(+)Foxp3(+) specifically required IL-2. Finally, we found that IL-2 and IL-15 arose from distinct sources in vivo. IL-15 was of stromal origin, whereas IL-2 was of exclusively from hemopoetic cells that depended on intact CD4 lineage development but not either Ag-experienced or NKT cells.
in vivo blocking of CD40/CD40L signaling
Ballesteros-Tato, A., et al. (2014). "Epitope-specific regulation of memory programming by differential duration of antigen presentation to influenza-specific CD8(+) T cells" Immunity 41(1): 127-140. PubMed
Memory CD8(+) T cells are programmed during the primary response for robust secondary responsiveness. Here we show that CD8(+) T cells responding to different epitopes of influenza virus received qualitatively different signals during the primary response that altered their secondary responsiveness. Nucleoprotein (NP)-specific CD8(+) T cells encountered antigen on CD40-licensed, CD70-expressing, CD103(-)CD11b(hi) dendritic cells (DCs) at later times in the primary response. As a consequence, they maintained CD25 expression and responded to interleukin-2 (IL-2) and CD27, which together programmed their robust secondary proliferative capacity and interferon-gamma (IFN-gamma)-producing ability. In contrast, polymerase (PA)-specific CD8(+) T cells did not encounter antigen-bearing, CD40-activated DCs at later times in the primary response, did not receive CD27 and CD25 signals, and were not programmed to become memory CD8(+) T cells with strong proliferative and cytokine-producing ability. As a result, CD8(+) T cells responding to abundant antigens, like NP, dominated the secondary response.
in vivo blocking of CD40/CD40L signaling
Baumjohann, D., et al. (2013). "Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype" Immunity 38(3): 596-605. PubMed
T follicular helper (Tfh) cells provide help to B cells and are crucial for establishment of germinal center (GC) reactions, including production of high-affinity antibodies and generation of memory B cells and long-lived plasma cells. Here we report that the magnitude of the Tfh cell response was dictated by the amount of antigen and directly correlated with the magnitude of the GC B cell response. In addition, maintenance of the Tfh cell phenotype required sustained antigenic stimulation by GC B cells. In lymphopenic conditions, a strong and prolonged Tfh cell response led to bystander B cell activation, hypergammaglobulinemia, and production of poly- and self-reactive antibodies. These data demonstrate that antigen dose determines the size and duration of the Tfh cell response and GC reaction, highlight the transient nature of the Tfh cell phenotype, and suggest a link between overstimulation of Tfh cells and the development of dysregulated humoral immune responses.
in vivo blocking of CD40/CD40L signaling
Taylor, J. J., et al. (2012). "A germinal center-independent pathway generates unswitched memory B cells early in the primary response" J Exp Med 209(3): 597-606. PubMed
Memory B cells can be produced from the classical germinal center (GC) pathway or a less understood GC-independent route. We used antigen-based cell enrichment to assess the relative contributions of these pathways to the polyclonal memory B cell pool. We identified a CD38(+) GL7(+) B cell precursor population that differentiated directly into IgM(+) or isotype-switched (sw) Ig(+) memory B cells in a GC-independent fashion in response to strong CD40 stimulation. Alternatively, CD38(+) GL7(+) B cell precursors had the potential to become Bcl-6(+) GC cells that then generated primarily swIg(+) memory B cells. These results demonstrate that early IgM(+) and swIg(+) memory B cells are products of a GC-independent pathway, whereas later switched Ig(+) memory B cells are products of GC cells.
in vivo blocking of CD40/CD40L signaling
Hofstetter, A. R., et al. (2012). "MHC class Ib-restricted CD8 T cells differ in dependence on CD4 T cell help and CD28 costimulation over the course of mouse polyomavirus infection" J Immunol 188(7): 3071-3079. PubMed
We recently identified a protective MHC class Ib-restricted CD8 T cell response to infection with mouse polyomavirus. These CD8 T cells recognize a peptide from aa 139-147 of the VP2 viral capsid protein bound to the nonpolymorphic H-2Q9 molecule, a member of the Qa-2 family of beta(2)m-associated MHC class Ib molecules. Q9:VP2.139-specific CD8 T cells exhibit an unusual inflationary response characterized by a gradual expansion over 3 mo followed by a stable maintenance phase. We previously demonstrated that Q9:VP2.139-specific CD8 T cells are dependent on Ag for expansion, but not for long-term maintenance. In this study, we tested the hypothesis that the expansion and maintenance components of the Q9:VP2.139-specific T cell response are differentially dependent on CD4 T cell help and CD28 costimulation. Depletion of CD4(+) cells and CD28/CD40L blockade impaired expansion of Q9:VP2.139-specific CD8 T cells, and intrinsic CD28 signaling was sufficient for expansion. In contrast, CD4 T cell insufficiency, but not CD28/CD40L blockade, resulted in a decline in frequency of Q9:VP2.139-specific CD8 T cells during the maintenance phase. These results indicate that the Q9:VP2.139-specific CD8 T cell response to mouse polyomavirus infection depends on CD4 T cell help and CD28 costimulation for inflationary expansion, but only on CD4 T cell help for maintenance.
in vivo blocking of CD40/CD40L signaling
West, E. E., et al. (2011). "Tight regulation of memory CD8(+) T cells limits their effectiveness during sustained high viral load" Immunity 35(2): 285-298. PubMed
To design successful vaccines for chronic diseases, an understanding of memory CD8(+) T cell responses to persistent antigen restimulation is critical. However, most studies comparing memory and naive cell responses have been performed only in rapidly cleared acute infections. Herein, by comparing the responses of memory and naive CD8(+) T cells to acute and chronic lymphocytic choriomeningitis virus infection, we show that memory cells dominated over naive cells and were protective when present in sufficient numbers to quickly reduce infection. In contrast, when infection was not rapidly reduced, because of high antigen load or persistence, memory cells were quickly lost, unlike naive cells. This loss of memory cells was due to a block in sustaining cell proliferation, selective regulation by the inhibitory receptor 2B4, and increased reliance on CD4(+) T cell help. Thus, emphasizing the importance of designing vaccines that elicit effective CD4(+) T cell help and rapidly control infection.
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
Tumor-specific CD4 T cells instruct monocyte fate in pancreatic ductal adenocarcinoma.
In Cell Reports on 25 July 2023 by Patterson, M. T., Burrack, A. L., et al.
PubMed
Pancreatic ductal adenocarcinoma (PDA) orchestrates a suppressive tumor microenvironment that fosters immunotherapy resistance. Tumor-associated macrophages (TAMs) are the principal immune cell infiltrating PDA and are heterogeneous. Here, by employing macrophage fate-mapping approaches and single-cell RNA sequencing, we show that monocytes give rise to most macrophage subsets in PDA. Tumor-specific CD4, but not CD8, T cells promote monocyte differentiation into MHCIIhi anti-tumor macrophages. By conditional major histocompatibility complex (MHC) class II deletion on monocyte-derived macrophages, we show that tumor antigen presentation is required for instructing monocyte differentiation into anti-tumor macrophages, promoting Th1 cells, abrogating Treg cells, and mitigating CD8 T cell exhaustion. Non-redundant IFNγ and CD40 promote MHCIIhi anti-tumor macrophages. Intratumoral monocytes adopt a pro-tumor fate indistinguishable from that of tissue-resident macrophages following loss of macrophage MHC class II or tumor-specific CD4 T cells. Thus, tumor antigen presentation by macrophages to CD4 T cells dictates TAM fate and is a major determinant of macrophage heterogeneity in cancer. Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
- Mus musculus (House mouse),
- Immunology and Microbiology
MAIT cells activate dendritic cells to promote TFH cell differentiation and induce humoral immunity.
In Cell Reports on 25 April 2023 by Pankhurst, T. E., Buick, K. H., et al.
PubMed
Protective immune responses against respiratory pathogens, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza virus, are initiated by the mucosal immune system. However, most licensed vaccines are administered parenterally and are largely ineffective at inducing mucosal immunity. The development of safe and effective mucosal vaccines has been hampered by the lack of a suitable mucosal adjuvant. In this study we explore a class of adjuvant that harnesses mucosal-associated invariant T (MAIT) cells. We show evidence that intranasal immunization of MAIT cell agonists co-administered with protein, including the spike receptor binding domain from SARS-CoV-2 virus and hemagglutinin from influenza virus, induce protective humoral immunity and immunoglobulin A production. MAIT cell adjuvant activity is mediated by CD40L-dependent activation of dendritic cells and subsequent priming of T follicular helper cells. In summary, we show that MAIT cells are promising vaccine targets that can be utilized as cellular adjuvants in mucosal vaccines. Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
Cancer-targeted photoimmunotherapy induces antitumor immunity and can be augmented by anti-PD-1 therapy for durable anticancer responses in an immunologically active murine tumor model.
In Cancer Immunology, Immunotherapy : CII on 1 January 2023 by Hsu, M. A., Okamura, S. M., et al.
PubMed
The complex immunosuppressive nature of solid tumor microenvironments poses a significant challenge to generating efficacious and durable anticancer responses. Photoimmunotherapy is a cancer treatment strategy by which an antibody is conjugated with a non-toxic light-activatable dye. Following administration of the conjugate and binding to the target tumor, subsequent local laser illumination activates the dye, resulting in highly specific target cell membrane disruption. Here we demonstrate that photoimmunotherapy treatment elicited tumor necrosis, thus inducing immunogenic cell death characterized by the release of damage-associated molecular patterns (DAMPs). Photoimmunotherapy-killed tumor cells activated dendritic cells (DC), leading to the production of proinflammatory cytokines, T cell stimulation, priming antigen-specific T cells, and durable memory T cell responses, which led complete responder mice to effectively reject new tumors upon rechallenge. PD-1 blockade in combination with photoimmunotherapy enhanced overall anticancer efficacy, including against anti-PD-1-resistant tumors. The combination treatment also elicited abscopal anticancer activity, as observed by reduction of distal, non-illuminated tumors, further demonstrating the ability of photoimmunotherapy to harness local and peripheral T cell responses. With this work we therefore delineate the immune mechanisms of action for photoimmunotherapy and demonstrate the potential for cancer-targeted photoimmunotherapy to be combined with other immunotherapy approaches for augmented, durable anticancer efficacy. Moreover, we demonstrate responses utilizing various immunocompetent mouse models, as well as in vitro data from human cells, suggesting broad translational potential. © 2022. The Author(s).
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
Tumor-specific CD4 T cells instruct monocyte differentiation in pancreatic ductal adenocarcinoma
Preprint on BioRxiv : the Preprint Server for Biology on 10 November 2022 by Patterson, M. T., Burrack, A. L., et al.
PubMed
Summary Pancreatic ductal adenocarcinoma (PDA) is a lethal malignancy resistant to immunotherapy. The pancreatic tumor microenvironment is shaped and maintained by myeloid cells that outnumber tumor cells. Here, using monocyte fate-mapping PDA mouse models and human tumor tissues, we identify monocytes give rise to most heterogeneous macrophage subpopulations in PDA. We show that monocyte differentiation is governed by the local presence of CD4, but not CD8, T cells. We demonstrate that tumor specific CD4 T cells induce monocyte differentiation into antitumor MHCII hi proinflammatory macrophages dependent on non-redundant IFNγ and CD40 signaling pathways that suppress tumor growth. Pancreatic tissue-resident macrophages exhibit an immunosuppressive pro-tumor state that is refractory to the modulatory effects of antitumor CD4 T cells. Intratumoral monocytes adopt a pro-tumor fate indistinguishable from tissue-resident macrophages following CD4 T cell depletion. Thus, tumor-specific CD4 T cell governance of monocyte fate promotes immune-mediated control of solid tumors. Highlights ▪ Circulating monocytes are progenitors to most heterogeneous macrophage subsets in PDA ▪ Monocyte-derived macrophage acquisition of an MHCII hi phenotype is dependent on tumor-specific CD4 T cells ▪ In the absence of CD4 T cells, monocyte-derived macrophages acquire tissue resident macrophage traits and tumors rapidly progress ▪ IFNγ and CD40 signaling are nonredundant and critical determinants of intratumoral monocyte fate
- Mus musculus (House mouse),
- Immunology and Microbiology
BCL6 controls contact-dependent help delivery during follicular T-B cell interactions.
In Immunity on 12 October 2021 by Liu, D., Yan, J., et al.
PubMed
BCL6 is required for development of follicular T helper (Tfh) cells to support germinal center (GC) formation. However, it is not clear what unique functions programmed by BCL6 can explain its absolute essentiality in T cells for GC formation. We found that ablation of one Bcl6 allele did not appreciably alter early T cell activation and follicular localization but inhibited GC formation and Tfh cell maintenance. BCL6 impinged on Tfh calcium signaling and also controlled Tfh entanglement with and CD40L delivery to B cells. Amounts of BCL6 protein and nominal frequencies of Tfh cells markedly changed within hours after strengths of T-B cell interactions were altered in vivo, while CD40L overexpression rectified both defective GC formation and Tfh cell maintenance because of the BCL6 haploinsufficiency. Our results reveal BCL6 functions in Tfh cells that are essential for GC formation and suggest that BCL6 helps maintain Tfh cell phenotypes in a T cell non-autonomous manner. Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.