InVivoMAb anti-mouse CD4
Product Details
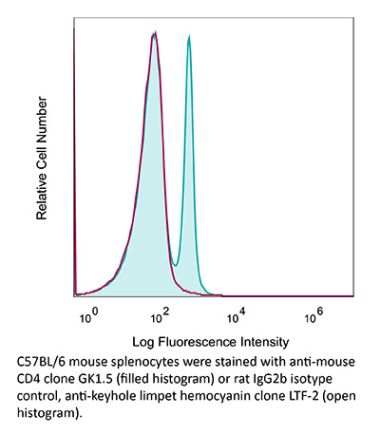
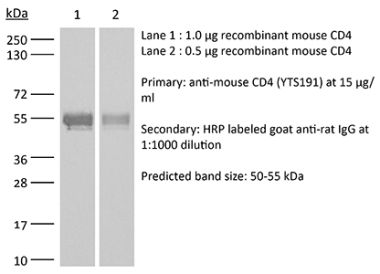
The YTS 177 monoclonal antibody reacts with the mouse CD4. The CD4 antigen is a 55 kDa cell surface type I membrane glycoprotein belonging to the immunoglobulin superfamily. CD4 acts as a co-receptor which in cooperation with the T cell receptor (TCR) interacts with class II MHC molecules displayed by antigen presenting cells (APC). CD4 is expressed by the majority of thymocytes, most helper T cells, a subset of NK-T cells and weakly by dendritic cells and macrophages. CD4 plays an important role in the development of T cells and is required for mature T cells to function optimally. The YTS 177 antibody has been shown to compete with clones GK1.5 and YTS 191 for CD4 binding. Additionally, the YTS 177 antibody has been reported to be non-depleting but binding does induce rapid internalization of CD4 on both CD4+ Foxp3- T cells and Foxp3+ regulatory T cells. Further, the YTS 177 clone has been shown to suppress or even prevent allograft rejection, allergic reactions and autoimmune responses.Specifications
| Isotype | Rat IgG2a |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse Spleen Cells |
| Reported Applications |
in vivo blockade of CD4+ T-cell responses Western blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
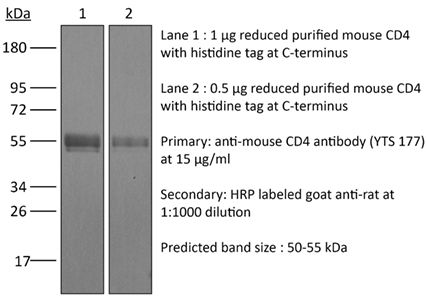
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107642 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Additional Formats
Recommended Products
in vivo blockade of CD4+ T-cell responses
Li, Z., et al. (2015). "Pre-treatment of allogeneic bone marrow recipients with the CXCR4 antagonist AMD3100 transiently enhances hematopoietic chimerism without promoting donor-specific skin allograft tolerance" Transpl Immunol 33(2): 125-129. PubMed
Hematopoietic chimerism established by allogeneic bone marrow transplantation is known to promote donor-specific organ allograft tolerance; however, clinical application is limited by the need for toxic host conditioning and “megadoses” of donor bone marrow cells. A potential solution to this problem has been suggested by the observation that recipient bone marrow mobilization by the CXCR4 antagonist AMD3100 promotes chimerism in congenic bone marrow transplantation experiments in mice. Here we report that a single subcutaneous dose of 10mg/kg AMD3100 in recipient C57BL/6 mice was able to enhance hematopoietic chimerism when complete MHC-mismatched BALB/c donor bone marrow cells were transplanted 1h after drug dosing. However, levels of chimerism measured 30days post-transplantation were not sustained when mice were reexamined on day 90 post-transplantation. Moreover, transient chimerism induced by this protocol did not support robust donor-specific skin allograft tolerance. Using the same transient immunosuppression protocol, we confirmed that “megadoses” of donor bone marrow cells could induce durable chimerism associated with donor-specific skin allograft tolerance without AMD3100 pre-treatment. We conclude that in this protocol AMD3100 pretreatment may empty bone marrow niches that become reoccupied by allogeneic donor hematopoietic progenitor cells but not by true long-lived donor hematopoietic stem cells, resulting in short-lived chimerism and failure to support durable donor-specific allograft tolerance.
in vivo blockade of CD4+ T-cell responses
Mayer, C. T., et al. (2014). "Anti-CD4 treatment inhibits autoimmunity in scurfy mice through the attenuation of co-stimulatory signals" J Autoimmun 50: 23-32. PubMed
A major concept in autoimmunity is that disruption of Foxp3(+) regulatory T cells (Tregs) predisposes to breach of tolerance. This is exemplified by the Foxp3-linked disorder termed IPEX (immunodysregulation, polyendocrinopathy, enteropathy, X-linked) which affects newborn children. There has been considerable clinical interest in the role of non-depleting anti-CD4 antibodies as a means of upregulating the function of Foxp3(+) Tregs in order to control detrimental inflammatory responses such as transplant rejection. However, according to the paradigm of a Treg-dependent mechanism of action, the effectiveness of anti-CD4 antibodies as a therapy for human autoimmune diseases is unclear considering that Treg function might be intrinsically impaired. Specifically, anti-CD4 therapy is expected to fail in patients suffering from the IPEX syndrome due to the lack of functional Foxp3(+) Tregs. Taking advantage of natural Foxp3 mutant scurfy (sf) mice closely resembling the IPEX syndrome, and genetically engineered mice depleted of Foxp3(+) Tregs, we report here that anti-CD4 treatment induces tolerance independent of Foxp3(+) Tregs. This so far undefined mechanism is dependent on the recessive non-infectious tolerization of autoreactive T cells. Treg-independent tolerance alone is powerful enough to suppress both the onset and severity of autoimmunity and reduces clinically relevant autoantibody levels and liver fibrosis. Mechanistically, tolerance induction requires the concomitant activation of autoreactive T cells and is associated with the down-regulation of the co-stimulatory TNF-receptor superfamily members OX40 and CD30 sustaining CD4(+) T cell survival. In the light of ongoing clinical trials, our results highlight an unexpected potency of anti-CD4 antibodies for the treatment of autoimmune diseases. Particularly, CD4 blockade might represent a novel therapeutic option for the human IPEX syndrome.
in vivo blockade of CD4+ T-cell responses
Rocca, C. J., et al. (2014). "rAAV9 combined with renal vein injection is optimal for kidney-targeted gene delivery: conclusion of a comparative study" Gene Ther 21(6): 618-628. PubMed
Effective gene therapy strategies for the treatment of kidney disorders remain elusive. We report an optimized kidney-targeted gene delivery strategy using recombinant adeno-associated virus (rAAV) administered via retrograde renal vein injection in mice. Renal vein injection of rAAV consistently resulted in superior kidney transduction compared with tail vein injection using as little as half the tail vein dose. We compared rAAV5, 6, 8 and 9, containing either green fluorescent protein (GFP) or luciferase reporter genes driven by the Cytomegalovirus promoter. We demonstrated that although rAAV6 and 8 injected via renal vein transduced the kidney, transgene expression was mainly restricted to the medulla. Transgene expression was systematically low after rAAV5 injection, attributed to T-cell immune response, which could be overcome by transient immunosuppression. However, rAAV9 was the only serotype that permitted high-transduction efficiency of both the cortex and medulla. Moreover, both the glomeruli and tubules were targeted, with a higher efficiency within the glomeruli. To improve the specificity of kidney-targeted gene delivery with rAAV9, we used the parathyroid hormone receptor ‘kidney-specific’ promoter. We obtained a more efficient transgene expression within the kidney, and a significant reduction in other tissues. Our work represents the first comprehensive and clinically relevant study for kidney gene delivery.
- Mus musculus (House mouse),
High-titer AAV disrupts cerebrovascular integrity and induces lymphocyte infiltration in adult mouse brain.
In Molecular Therapy. Methods Clinical Development on 14 December 2023 by Guo, Y., Chen, J., et al.
PubMed
The brain is often described as an "immune-privileged" organ due to the presence of the blood-brain-barrier (BBB), which limits the entry of immune cells. In general, intracranial injection of adeno-associated virus (AAV) is considered a relatively safe procedure. In this study, we discovered that AAV, a popular engineered viral vector for gene therapy, can disrupt the BBB and induce immune cell infiltration in a titer-dependent manner. First, our bulk RNA sequencing data revealed that injection of high-titer AAV significantly upregulated many genes involved in disrupting BBB integrity and antiviral adaptive immune responses. By using histologic analysis, we further demonstrated that the biological structure of the BBB was severely disrupted in the adult mouse brain. Meanwhile, we noticed abnormal leakage of blood components, including immune cells, within the brain parenchyma of high-titer AAV injected areas. Moreover, we identified that the majority of infiltrated immune cells were cytotoxic T lymphocytes (CTLs), which resulted in a massive loss of neurons at the site of AAV injection. In addition, antagonizing CTL function by administering antibodies significantly reduced neuronal toxicity induced by high-titer AAV. Collectively, our findings underscore potential severe side effects of intracranial injection of high-titer AAV, which might compromise proper data interpretation if unaware of. © 2023 The Authors.
- Mus musculus (House mouse)
Skeletal phenotype amelioration in mucopolysaccharidosis VI requires intervention at the earliest stages of postnatal development.
In JCI Insight on 8 November 2023 by Hwang-Wong, E., Amar, G., et al.
PubMed
Mucopolysaccharidosis VI (MPS VI) is a rare lysosomal disease arising from impaired function of the enzyme arylsulfatase B (ARSB). This impairment causes aberrant accumulation of dermatan sulfate, a glycosaminoglycan (GAG) abundant in cartilage. While clinical severity varies along with age at first symptom manifestation, MPS VI usually presents early and strongly affects the skeleton. Current enzyme replacement therapy (ERT) does not provide effective treatment for the skeletal manifestations of MPS VI. This lack of efficacy may be due to an inability of ERT to reach affected cells or to the irreversibility of the disease. To address the question of reversibility of skeletal phenotypes, we generated a conditional by inversion (COIN) mouse model of MPS VI, ArsbCOIN/COIN, wherein Arsb is initially null and can be restored to WT using Cre. We restored Arsb at different times during postnatal development, using a tamoxifen-dependent global Cre driver. By restoring Arsb at P7, P21, and P56-P70, we determined that skeletal phenotypes can be fully rescued if Arsb restoration occurs at P7, while only achieving partial rescue at P21 and no significant rescue at P56-P70. This work has highlighted the importance of early intervention in patients with MPS VI to maximize therapeutic impact.
- In Vivo,
- Mus musculus (House mouse),
- Immunology and Microbiology
Targeting of Cdc42 GTPase in regulatory T cells unleashes antitumor T-cell immunity.
In Journal for Immunotherapy of Cancer on 1 November 2022 by Kalim, K. W., Yang, J. Q., et al.
PubMed
Cancer immunotherapy has taken center stage in cancer treatment. However, the current immunotherapies only benefit a small proportion of patients with cancer, necessitating better understanding of the mechanisms of tumor immune evasion and improved cancer immunotherapy strategies. Regulatory T (Treg) cells play an important role in maintaining immune tolerance through inhibiting effector T-cell function. In the tumor microenvironment, Treg cells are used by tumor cells to counteract effector T cell-mediated tumor suppression. Targeting Treg cells may thus unleash the antitumor activity of effector T cells. While systemic depletion of Treg cells can cause excessive effector T-cell responses and subsequent autoimmune diseases, controlled targeting of Treg cells may benefit patients with cancer. Treg cells from Treg cell-specific heterozygous Cdc42 knockout mice, C57BL/6 mice treated with a Cdc42 inhibitor CASIN, and control mice were examined for their homeostasis and stability by flow cytometry. The autoimmune responses in Treg cell-specific heterozygous Cdc42 knockout mice, CASIN-treated C57BL/6 mice, and control mice were assessed by H&E staining and ELISA. Antitumor T-cell immunity in Treg cell-specific heterozygous Cdc42 knockout mice, CASIN-treated C57BL/6 mice, humanized NSGS mice, and control mice was assessed by challenging the mice with MC38 mouse colon cancer cells, KPC mouse pancreatic cancer cells, or HCT116 human colon cancer cells. Treg cell-specific heterozygous deletion or pharmacological targeting of Cdc42 with CASIN does not affect Treg cell numbers but induces Treg cell instability, leading to antitumor T-cell immunity without detectable autoimmune reactions. Cdc42 targeting causes an additive effect on immune checkpoint inhibitor anti-programmed cell death protein-1 antibody-induced T-cell response against mouse and human tumors. Mechanistically, Cdc42 targeting induces Treg cell instability and unleashes antitumor T-cell immunity through carbonic anhydrase I-mediated pH changes. Rational targeting of Cdc42 in Treg cells holds therapeutic promises in cancer immunotherapy. © Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
Targeting of Cdc42 GTPase in regulatory T cells unleashes anti-tumor T cell immunity
Preprint on BioRxiv : the Preprint Server for Biology on 24 September 2021 by Kalim, K. W., Yang, J., et al.
PubMed
Regulatory T (Treg) cells play an important role in maintaining immune tolerance through inhibiting effector T cell function. In the tumor microenvironment, Treg cells are utilized by tumor cells to counteract effector T cell-mediated tumor killing. Targeting Treg cells may thus unleash the anti-tumor activity of effector T cells. While systemic depletion of Treg cells can cause excessive effector T cell responses and subsequent autoimmune diseases, controlled targeting of Treg cells may benefit cancer patients. Here we show that Treg cell-specific heterozygous deletion or pharmacological targeting of Cdc42 GTPase does not affect Treg cell numbers but induces Treg cell plasticity, leading to anti-tumor T cell immunity without detectable autoimmune reactions. Cdc42 targeting potentiates an immune checkpoint blocker anti-PD-1 antibody-mediated T cell response against mouse and human tumors. Mechanistically, Cdc42 targeting induces Treg cell plasticity and unleashes antitumor T cell immunity through carbonic anhydrase I-mediated pH changes. Thus, rational targeting of Cdc42 in Treg cells holds therapeutic promises in cancer immunotherapy. h4>Significance/h4> Effector T lymphocytes promote autoimmune diseases but have potential to kill tumor cells. However, cancer cells can evade T cell-mediated killing in part by utilizing regulatory T (Treg) cells to inhibit effector T cell function. Here we show that Treg cell-specific heterozygous deletion of Cdc42 gene that encodes Cdc42 GTPase dampens Treg cell fitness through carbonic anhydrase I-mediated pH changes, leading to anti-tumor T cell immunity. Pharmacological targeting of Cdc42 mimics genetic deletion of Cdc42 in impairing Treg cell fitness and evoking anti-tumor T cell immunity. Importantly, Cdc42 targeting does not appear to cause systemic autoimmunity. Given that current cancer immunotherapies only demonstrate limited clinical efficacies, our findings may open a new avenue for cancer immunotherapy.
- In Vivo,
- Immu-depl,
- Mus musculus (House mouse),
- Immunology and Microbiology
Cytomegalovirus restricts ICOSL expression on antigen-presenting cells disabling T cell co-stimulation and contributing to immune evasion.
In eLife on 18 January 2021 by Angulo, G., Zeleznjak, J., et al.
PubMed
Viral infections are controlled, and very often cleared, by activated T lymphocytes. The inducible co-stimulator (ICOS) mediates its functions by binding to its ligand ICOSL, enhancing T-cell activation and optimal germinal center (GC) formation. Here, we show that ICOSL is heavily downmodulated during infection of antigen-presenting cells by different herpesviruses. We found that, in murine cytomegalovirus (MCMV), the immunoevasin m138/fcr-1 physically interacts with ICOSL, impeding its maturation and promoting its lysosomal degradation. This viral protein counteracts T-cell responses, in an ICOS-dependent manner, and limits virus control during the acute MCMV infection. Additionally, we report that blockade of ICOSL in MCMV-infected mice critically regulates the production of MCMV-specific antibodies due to a reduction of T follicular helper and GC B cells. Altogether, these findings reveal a novel mechanism evolved by MCMV to counteract adaptive immune surveillance, and demonstrates a role of the ICOS:ICOSL axis in the host defense against herpesviruses. © 2021, Angulo et al.