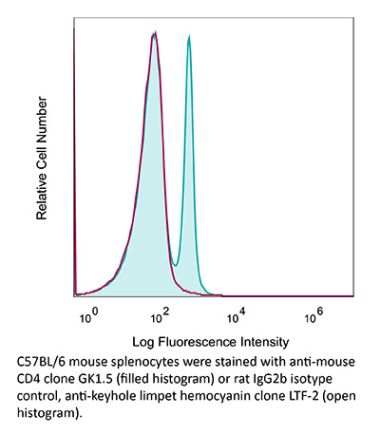
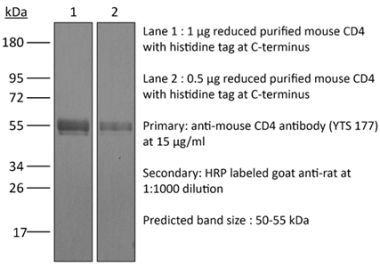
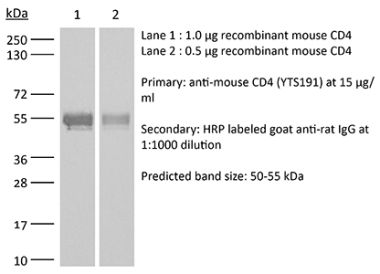
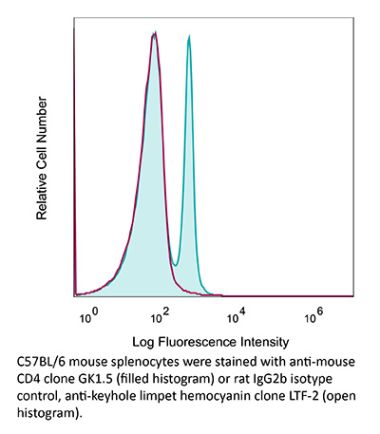
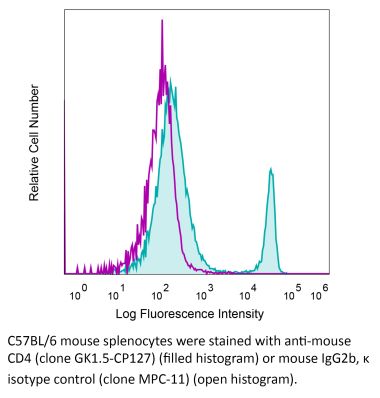
InVivoMAb anti-human CD4
Product Details
The OKT-4 monoclonal antibody reacts with the human CD4. The CD4 antigen is a 55 kDa cell surface type I membrane glycoprotein belonging to the immunoglobulin superfamily. CD4 acts as a co-receptor which in cooperation with the T cell receptor (TCR) interacts with class II MHC molecules displayed by antigen presenting cells (APC). CD4 is expressed by the majority of thymocytes, most helper T cells, a subset of NK-T cells and weakly by dendritic cells and macrophages. CD4 plays an important role in the development of T cells and is required for mature T cells to function optimally.Specifications
| Isotype | Mouse IgG2b, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG2b isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Human peripheral T cells |
| Reported Applications |
in vitro T cell stimulation/activation in vivo CD4+ T cell depletion in humanized mice Flow cytometry Immunoprecipitation |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
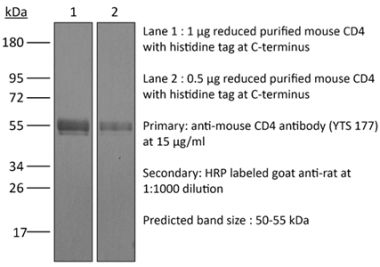
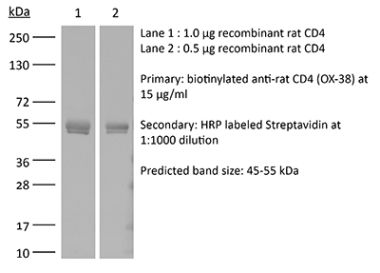
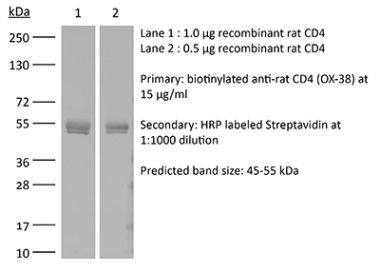
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107638 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
in vivo CD4+ T cell depletion in humanized mice
Danzer, H., et al. (2020). "Human Fcγ-receptor IIb modulates pathogen-specific versus self-reactive antibody responses in lyme arthritis" Elife 9 . PubMed
Pathogen-specific antibody responses need to be tightly regulated to generate protective but limit self-reactive immune responses. While loss of humoral tolerance has been associated with microbial infections, the pathways involved in balancing protective versus autoreactive antibody responses in humans are incompletely understood. Studies in classical mouse model systems have provided evidence that balancing of immune responses through inhibitory receptors is an important quality control checkpoint. Genetic differences between inbred mouse models and the outbred human population and allelic receptor variants not present in mice; however, argue for caution when directly translating these findings to the human system. By studying Borrelia burgdorferi infection in humanized mice reconstituted with human hematopoietic stem cells from donors homozygous for a functional or a non-functional FcγRIIb allele, we show that the human inhibitory FcγRIIb is a critical checkpoint balancing protective and autoreactive immune responses, linking infection with induction of autoimmunity in the human immune system.
in vivo CD4+ T cell depletion in humanized mice
Homet Moreno, B., et al. (2016). "Response to Programmed Cell Death-1 Blockade in a Murine Melanoma Syngeneic Model Requires Costimulation, CD4, and CD8 T Cells" Cancer Immunol Res 4(10): 845-857. PubMed
The programmed cell death protein 1 (PD-1) limits effector T-cell functions in peripheral tissues, and its inhibition leads to clinical benefit in different cancers. To better understand how PD-1 blockade therapy modulates the tumor-host interactions, we evaluated three syngeneic murine tumor models, the BRAF(V600E)-driven YUMM1.1 and YUMM2.1 melanomas, and the carcinogen-induced murine colon adenocarcinoma MC38. The YUMM cell lines were established from mice with melanocyte-specific BRAF(V600E) mutation and PTEN loss (BRAF(V600E)/PTEN(-/-)). Anti-PD-1 or anti-PD-L1 therapy engendered strong antitumor activity against MC38 and YUMM2.1, but not YUMM1.1. PD-L1 expression did not differ between the three models at baseline or upon interferon stimulation. Whereas mutational load was high in MC38, it was lower in both YUMM models. In YUMM2.1, the antitumor activity of PD-1 blockade had a critical requirement for both CD4 and CD8 T cells, as well as CD28 and CD80/86 costimulation, with an increase in CD11c(+)CD11b(+)MHC-II(high) dendritic cells and tumor-associated macrophages in the tumors after PD-1 blockade. Compared with YUMM1.1, YUMM2.1 exhibited a more inflammatory profile by RNA sequencing analysis, with an increase in expression of chemokine-trafficking genes that are related to immune cell recruitment and T-cell priming. In conclusion, response to PD-1 blockade therapy in tumor models requires CD4 and CD8 T cells and costimulation that is mediated by dendritic cells and macrophages.
in vitro T cell stimulation/activation
Klammt, C., et al. (2015). "T cell receptor dwell times control the kinase activity of Zap70" Nat Immunol 16(9): 961-969. PubMed
Kinase recruitment to membrane receptors is essential for signal transduction. However, the underlying regulatory mechanisms are poorly understood. We investigated how conformational changes control T cell receptor (TCR) association and activity of the kinase Zap70. Structural analysis showed that TCR binding or phosphorylation of Zap70 triggers a transition from a closed, autoinhibited conformation to an open conformation. Using Zap70 mutants with defined conformations, we found that TCR dwell times controlled Zap70 activity. The closed conformation minimized TCR dwell times and thereby prevented activation by membrane-associated kinases. Parallel recruitment of coreceptor-associated Lck kinase to the TCR ensured Zap70 phosphorylation and stabilized Zap70 TCR binding. Our study suggests that the dynamics of cytosolic enzyme recruitment to the plasma membrane regulate the activity and function of receptors lacking intrinsic catalytic activity.
in vivo CD4+ T cell depletion in humanized mice
Billerbeck, E., et al. (2013). "Characterization of human antiviral adaptive immune responses during hepatotropic virus infection in HLA-transgenic human immune system mice" J Immunol 191(4): 1753-1764. PubMed
Humanized mice have emerged as a promising model to study human immunity in vivo. Although they are susceptible to many pathogens exhibiting an almost exclusive human tropism, human immune responses to infection remain functionally impaired. It has recently been demonstrated that the expression of HLA molecules improves human immunity to lymphotropic virus infections in humanized mice. However, little is known about the extent of functional human immune responses in nonlymphoid tissues, such as in the liver, and the role of HLA expression in this context. Therefore, we analyzed human antiviral immunity in humanized mice during a hepatotropic adenovirus infection. We compared immune responses of conventional humanized NOD SCID IL-2Rgamma-deficient (NSG) mice to those of a novel NOD SCID IL-2Rgamma-deficient strain transgenic for both HLA-A*0201 and a chimeric HLA-DR*0101 molecule. Using a firefly luciferase-expressing adenovirus and in vivo bioluminescence imaging, we demonstrate a human T cell-dependent partial clearance of adenovirus-infected cells from the liver of HLA-transgenic humanized mice. This correlated with liver infiltration and activation of T cells, as well as the detection of Ag-specific humoral and cellular immune responses. When infected with a hepatitis C virus NS3-expressing adenovirus, HLA-transgenic humanized mice mounted an HLA-A*0201-restricted hepatitis C virus NS3-specific CD8(+) T cell response. In conclusion, our study provides evidence for the generation of partial functional antiviral immune responses against a hepatotropic pathogen in humanized HLA-transgenic mice. The adenovirus reporter system used in our study may serve as simple in vivo method to evaluate future strategies for improving human intrahepatic immune responses in humanized mice.
Flow Cytometry, Immunoprecipitation
Hepburn, T. W., et al. (2003). "Antibody-mediated stripping of CD4 from lymphocyte cell surface in patients with rheumatoid arthritis" Rheumatology (Oxford) 42(1): 54-61. PubMed
Kinase recruitment to membrane receptors is essential for signal transduction. However, the underlying regulatory mechanisms are poorly understood. We investigated how conformational changes control T cell receptor (TCR) association and activity of the kinase Zap70. Structural analysis showed that TCR binding or phosphorylation of Zap70 triggers a transition from a closed, autoinhibited conformation to an open conformation. Using Zap70 mutants with defined conformations, we found that TCR dwell times controlled Zap70 activity. The closed conformation minimized TCR dwell times and thereby prevented activation by membrane-associated kinases. Parallel recruitment of coreceptor-associated Lck kinase to the TCR ensured Zap70 phosphorylation and stabilized Zap70 TCR binding. Our study suggests that the dynamics of cytosolic enzyme recruitment to the plasma membrane regulate the activity and function of receptors lacking intrinsic catalytic activity.
- Cancer Research,
- Immunology and Microbiology
Human CD4 cytotoxic T lymphocytes mediate potent tumor control in humanized immune system mice.
In Communications Biology on 25 April 2023 by Lin, W., Singh, V., et al.
PubMed
Efficacy of immune checkpoint inhibitors in cancers can be limited by CD8 T cell dysfunction or HLA-I down-regulation. Tumor control mechanisms independent of CD8/HLA-I axis would overcome these limitations. Here, we report potent CD4 T cell-mediated tumor regression and memory responses in humanized immune system (HIS) mice implanted with HT-29 colorectal tumors. The regressing tumors showed increased CD4 cytotoxic T lymphocyte (CTL) infiltration and enhanced tumor HLA-II expression compared to progressing tumors. The intratumoral CD4 T cell subset associated with tumor regression expressed multiple cytotoxic markers and exhibited clonal expansion. Notably, tumor control was abrogated by depletion of CD4 but not CD8 T cells. CD4 T cells derived from tumor-regressing mice exhibited HLA-II-dependent and tumor-specific killing ex vivo. Taken together, our study demonstrates a critical role of human CD4 CTLs in mediating tumor clearance independent of CD8 T cells and provides a platform to study human anti-tumor immunity in vivo. © 2023. The Author(s).
- In Vivo,
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
PAD-2-mediated citrullination of nucleophosmin provides an effective target for tumor immunotherapy.
In Journal for Immunotherapy of Cancer on 1 February 2022 by Choudhury, R. H., Symonds, P., et al.
PubMed
The enzymatic conversion of arginine to citrulline is involved in gene and protein regulation and in alerting the immune system to stressed cells, including tumor cells. Nucleophosmin (NPM) is a nuclear protein that plays key roles in cellular metabolism including ribosome biogenesis, mRNA processing and chromatin remodeling and is regulated by citrullination. In this study, we explored if the same citrullinated arginines within NPM are involved in gene regulation and immune activation. HLA-DP4 and HLA-DR4 transgenic mice were immunized with 22 citrullinated NPM overlapping peptides and immune responses to the peptides were assessed by ex vivo ELISpot assays. Antitumor immunity of NPM targeted vaccination was assessed by challenging transgenic mice with B16F1 HHDII/iDP4, B16F1 HHDII/PAD2KOcDP4, B16F1 HHDII and Lewis lung carcinoma cells/cDP4 cells subcutaneously. Peripheral blood mononuclear cells isolated from healthy donors were stimulated with NPM266-285cit peptides with/without CD45RO+memory cell depletion to assess if the responses in human were naïve or memory. In contrast to NPM regulation, which is mediated by peptidylarginine deiminase (PAD4) citrullination of arginine at position 197, only citrullinated NPM266-285 peptide induced a citrulline-specific CD4 T cell response in transgenic mice models expressing human HLA-DP4 or HLA-DR4. Vaccinations with the NPM266-285cit peptide stimulated antitumor responses that resulted in dramatic tumor therapy, greatly improved survival, and protected against rechallenge without further vaccination. The antitumor response was lost if MHCII expression on the tumor cells was knocked out demonstrating direct presentation of the NPM266-285cit epitope in tumors. This antitumor response was lost in B16 tumors lacking PAD2 enzyme indicating NPM266cit is citrullinated by PAD2 in this model. Assessment of the T cell repertoire in healthy individuals and patients with lung cancer also showed CD4 T cells that respond to NPM266-285cit. The proliferative CD4 responses displayed a Th1 profile as they were accompanied with increased IFNγ and granzyme B expression. Depletion of CD45RO+ memory cells prior to stimulation suggested that responses originated from a naïve population in healthy donors. This study indicates PAD2 can citrullinate the nuclear antigen NPM at position 277 which can be targeted by CD4 T cells for antitumor therapy. This is distinct from PAD4 citrullination of arginine 197 within NPM which results in its transport from the nucleoli to the nucleoplasm. © Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
- In Vivo,
- Immu-depl,
- Mus musculus (House mouse)
Human Fcγ-receptor IIb modulates pathogen-specific versus self-reactive antibody responses in lyme arthritis.
In eLife on 2 July 2020 by Danzer, H., Glaesner, J., et al.
PubMed
Pathogen-specific antibody responses need to be tightly regulated to generate protective but limit self-reactive immune responses. While loss of humoral tolerance has been associated with microbial infections, the pathways involved in balancing protective versus autoreactive antibody responses in humans are incompletely understood. Studies in classical mouse model systems have provided evidence that balancing of immune responses through inhibitory receptors is an important quality control checkpoint. Genetic differences between inbred mouse models and the outbred human population and allelic receptor variants not present in mice; however, argue for caution when directly translating these findings to the human system. By studying Borrelia burgdorferi infection in humanized mice reconstituted with human hematopoietic stem cells from donors homozygous for a functional or a non-functional FcγRIIb allele, we show that the human inhibitory FcγRIIb is a critical checkpoint balancing protective and autoreactive immune responses, linking infection with induction of autoimmunity in the human immune system. © 2020, Danzer et al.
- Immunology and Microbiology
EBV persistence without its EBNA3A and 3C oncogenes in vivo.
In PLoS Pathogens on 1 April 2018 by Murer, A., McHugh, D., et al.
PubMed
The oncogenic Epstein Barr virus (EBV) infects the majority of the human population and usually persists within its host for life without symptoms. The EBV oncoproteins nuclear antigen 3A (EBNA3A) and 3C (EBNA3C) are required for B cell transformation in vitro and are expressed in EBV associated immunoblastic lymphomas in vivo. In order to address the necessity of EBNA3A and EBNA3C for persistent EBV infection in vivo, we infected NOD-scid γcnull mice with reconstituted human immune system components (huNSG mice) with recombinant EBV mutants devoid of EBNA3A or EBNA3C expression. These EBV mutants established latent infection in secondary lymphoid organs of infected huNSG mice for at least 3 months, but did not cause tumor formation. Low level viral persistence in the absence of EBNA3A or EBNA3C seemed to be supported primarily by proliferation with the expression of early latent EBV gene products transitioning into absent viral protein expression without elevated lytic replication. In vitro, EBNA3A and EBNA3C deficient EBV infected B cells could be rescued from apoptosis through CD40 stimulation, mimicking T cell help in secondary lymphoid tissues. Thus, even in the absence of the oncogenes EBNA3A and 3C, EBV can access a latent gene expression pattern that is reminiscent of EBV persistence in healthy virus carriers without prior expression of its whole growth transforming program.
- In Vitro,
- Conjugation,
- Chemical,
- Genetics,
- FC/FACS,
- Homo sapiens (Human)
Hit-and-run programming of therapeutic cytoreagents using mRNA nanocarriers.
In Nature Communications on 30 August 2017 by Moffett, H. F., Coon, M. E., et al.
PubMed
Therapies based on immune cells have been applied for diseases ranging from cancer to diabetes. However, the viral and electroporation methods used to create cytoreagents are complex and expensive. Consequently, we develop targeted mRNA nanocarriers that are simply mixed with cells to reprogram them via transient expression. Here, we describe three examples to establish that the approach is simple and generalizable. First, we demonstrate that nanocarriers delivering mRNA encoding a genome-editing agent can efficiently knock-out selected genes in anti-cancer T-cells. Second, we imprint a long-lived phenotype exhibiting improved antitumor activities into T-cells by transfecting them with mRNAs that encode a key transcription factor of memory formation. Third, we show how mRNA nanocarriers can program hematopoietic stem cells with improved self-renewal properties. The simplicity of the approach contrasts with the complex protocols currently used to program therapeutic cells, so our methods will likely facilitate manufacturing of cytoreagents.Current widely used viral and electroporation methods for creating therapeutic cell-based products are complex and expensive. Here, the authors develop targeted mRNA nanocarriers that can transiently program gene expression by simply mixing them with cells, to improve their therapeutic potential.
- In Vivo,
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
Response to Programmed Cell Death-1 Blockade in a Murine Melanoma Syngeneic Model Requires Costimulation, CD4, and CD8 T Cells.
In Cancer Immunology Research on 1 October 2016 by Homet Moreno, B., Zaretsky, J. M., et al.
PubMed
The programmed cell death protein 1 (PD-1) limits effector T-cell functions in peripheral tissues, and its inhibition leads to clinical benefit in different cancers. To better understand how PD-1 blockade therapy modulates the tumor-host interactions, we evaluated three syngeneic murine tumor models, the BRAFV600E-driven YUMM1.1 and YUMM2.1 melanomas, and the carcinogen-induced murine colon adenocarcinoma MC38. The YUMM cell lines were established from mice with melanocyte-specific BRAFV600E mutation and PTEN loss (BRAFV600E/PTEN-/-). Anti-PD-1 or anti-PD-L1 therapy engendered strong antitumor activity against MC38 and YUMM2.1, but not YUMM1.1. PD-L1 expression did not differ between the three models at baseline or upon interferon stimulation. Whereas mutational load was high in MC38, it was lower in both YUMM models. In YUMM2.1, the antitumor activity of PD-1 blockade had a critical requirement for both CD4 and CD8 T cells, as well as CD28 and CD80/86 costimulation, with an increase in CD11c+CD11b+MHC-IIhigh dendritic cells and tumor-associated macrophages in the tumors after PD-1 blockade. Compared with YUMM1.1, YUMM2.1 exhibited a more inflammatory profile by RNA sequencing analysis, with an increase in expression of chemokine-trafficking genes that are related to immune cell recruitment and T-cell priming. In conclusion, response to PD-1 blockade therapy in tumor models requires CD4 and CD8 T cells and costimulation that is mediated by dendritic cells and macrophages. Cancer Immunol Res; 4(10); 845-57. ©2016 AACR.©2016 American Association for Cancer Research.
- Immunology and Microbiology
Characterization of human antiviral adaptive immune responses during hepatotropic virus infection in HLA-transgenic human immune system mice.
In The Journal of Immunology on 15 August 2013 by Billerbeck, E., Horwitz, J. A., et al.
PubMed
Humanized mice have emerged as a promising model to study human immunity in vivo. Although they are susceptible to many pathogens exhibiting an almost exclusive human tropism, human immune responses to infection remain functionally impaired. It has recently been demonstrated that the expression of HLA molecules improves human immunity to lymphotropic virus infections in humanized mice. However, little is known about the extent of functional human immune responses in nonlymphoid tissues, such as in the liver, and the role of HLA expression in this context. Therefore, we analyzed human antiviral immunity in humanized mice during a hepatotropic adenovirus infection. We compared immune responses of conventional humanized NOD SCID IL-2Rγ-deficient (NSG) mice to those of a novel NOD SCID IL-2Rγ-deficient strain transgenic for both HLA-A*0201 and a chimeric HLA-DR*0101 molecule. Using a firefly luciferase-expressing adenovirus and in vivo bioluminescence imaging, we demonstrate a human T cell-dependent partial clearance of adenovirus-infected cells from the liver of HLA-transgenic humanized mice. This correlated with liver infiltration and activation of T cells, as well as the detection of Ag-specific humoral and cellular immune responses. When infected with a hepatitis C virus NS3-expressing adenovirus, HLA-transgenic humanized mice mounted an HLA-A*0201-restricted hepatitis C virus NS3-specific CD8(+) T cell response. In conclusion, our study provides evidence for the generation of partial functional antiviral immune responses against a hepatotropic pathogen in humanized HLA-transgenic mice. The adenovirus reporter system used in our study may serve as simple in vivo method to evaluate future strategies for improving human intrahepatic immune responses in humanized mice.