Catalog #CP060
RecombiMAb anti-mouse IL-10R (CD210)
Clone
1B1.3A-CP060
Reactivities
Mouse
Isotype
Mouse IgG2a, kappa
(switched from Rat IgG1, kappa)
(switched from Rat IgG1, kappa)
You may also be interested in:
Product Description
The 1B1.3A-CP060 monoclonal antibody is a recombinant, chimeric version of the original 1B1.3A clone. The variable domain sequences are identical to the original 1B1.3A but the constant region sequences have been switched from Rat IgG1, κ to mouse IgG2a, κ for use in murine models. Species-matched chimeric antibodies exhibit regulated effector functions—including Fc receptor binding and complement activation—and cause less immunogenicity and formation of anti-drug antibodies (ADAs) than xenogenic antibodies in animal models. The highly controlled sequence and lack of genetic drift in recombinant antibodies provide more reliable and reproducible results compared to hybridoma derived antibodies.
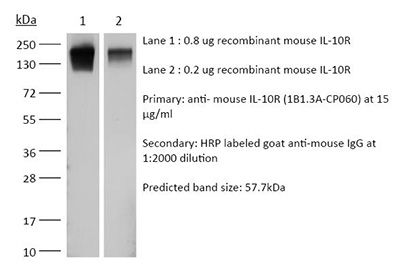
The 1B1.3A monoclonal antibody reacts with mouse IL-10R (IL-10 receptor) also known as CD210. The IL-10R is a class II cytokine receptor and is expressed by a variety of cell types including thymocytes, T lymphocytes, B lymphocytes, NK cells, monocytes, and macrophages. Upon binding IL-10, IL-10R stimulation results in many pleiotropic, effects in immunoregulation and inflammation. IL-10R downregulates the expression of pro-inflammatory cytokines, MHC class II antigens, and co-stimulatory molecules on macrophages. It also enhances B lymphocyte survival, proliferation, and antibody production. IL-10R signaling can block NF-κB activity and is involved in the regulation of the JAK-STAT signaling pathway. The 1B1.3A antibody is a neutralizing antibody and has been shown to block the binding of human IL-10, which cross-reacts with the mouse IL-10R. However, this clone does not recognize the human IL-10R.
The 1B1.3A monoclonal antibody reacts with mouse IL-10R (IL-10 receptor) also known as CD210. The IL-10R is a class II cytokine receptor and is expressed by a variety of cell types including thymocytes, T lymphocytes, B lymphocytes, NK cells, monocytes, and macrophages. Upon binding IL-10, IL-10R stimulation results in many pleiotropic, effects in immunoregulation and inflammation. IL-10R downregulates the expression of pro-inflammatory cytokines, MHC class II antigens, and co-stimulatory molecules on macrophages. It also enhances B lymphocyte survival, proliferation, and antibody production. IL-10R signaling can block NF-κB activity and is involved in the regulation of the JAK-STAT signaling pathway. The 1B1.3A antibody is a neutralizing antibody and has been shown to block the binding of human IL-10, which cross-reacts with the mouse IL-10R. However, this clone does not recognize the human IL-10R.
Specifications
| Isotype | Mouse IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | RecombiMAb mouse IgG2a isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Reported Applications |
Flow Cytometry Western blot in vitro blocking of IL-10R signaling in vivo blocking of IL-10/IL-10R signaling *Reported for the original rat IgG1 1B1.3A antibody |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from HEK293 cell supernatant in an animal-free facility |
| Purification | Protein G |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |