InVivoMAb anti-mouse VISTA
Product Description
Specifications
| Isotype | Armenian hamster IgG |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb polyclonal Armenian hamster IgG |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | EL4 cells overexpressing mouse VISTA-RFP and then boosted with VISTA-Ig fusion protein |
| Reported Applications |
in vivo blocking of VISTA signaling in vitro blocking of VISTA signaling Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_2736990 |
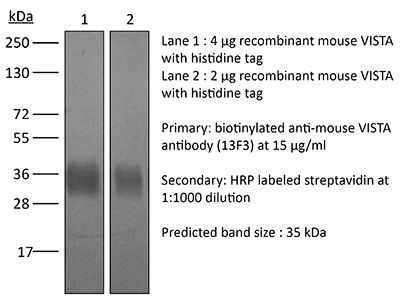
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo VISTA neutralization
Rosenbaum, S. R., et al (2020). "FOXD3 Regulates VISTA Expression in Melanoma" Cell Rep 30(2): 510-524.e516.
PubMed
Immune checkpoint inhibitors have improved patient survival in melanoma, but the innate resistance of many patients necessitates the investigation of alternative immune targets. Many immune checkpoint proteins lack proper characterization, including V-domain Ig suppressor of T cell activation (VISTA). VISTA expression on immune cells can suppress T cell activity; however, few studies have investigated its expression and regulation in cancer cells. In this study, we observe that VISTA is expressed in melanoma patient samples and cell lines. Tumor cell-specific expression of VISTA promotes tumor onset in vivo, associated with increased intratumoral T regulatory cells, and enhanced PDL-1 expression on tumor-infiltrating macrophages. VISTA transcript levels are regulated by the stemness factor Forkhead box D3 (FOXD3). BRAF inhibition upregulates FOXD3 and reduces VISTA expression. Overall, this study demonstrates melanoma cell expression of VISTA and its regulation by FOXD3, contributing to the rationale for therapeutic strategies that combine targeted inhibitors with immune checkpoint blockade.
in vivo VISTA neutralization
Sergent, P. A., et al (2018). "Blocking the VISTA pathway enhances disease progression in (NZB x NZW) F1 female mice" Lupus 27(2): 210-216.
PubMed
V-domain Ig suppressor of T-cell activation (VISTA) is a critical negative checkpoint molecule involved in regulating the immune response. Targeting the pathway with an antagonist anti-VISTA antibody designated 13F3 has been shown to enhance disease severity in experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis. To determine if VISTA plays a role in murine lupus, New Zealand Black x New Zealand White (BWF1) mice were treated with 13F3 or control hamster Ig and disease monitored. Onset of proteinuria was earlier and renal damage more profound in mice treated with 13F3. Cell subset analysis showed an increase of activated splenic T cells and inflammatory splenic myeloid cells, but no effect on B cells, in mice receiving 13F3. Examination of the kidney showed an increase in inflammatory myeloid cell infiltration with 13F3 treatment. This study along with previous EAE data, suggests that interventions that enhance VISTA regulatory activity may be effective for the treatment of autoimmune disease.
Flow Cytometry
Srivastava, R., et al (2018). "CXCL17 Chemokine-Dependent Mobilization of CXCR8(+)CD8(+) Effector Memory and Tissue-Resident Memory T Cells in the Vaginal Mucosa Is Associated with Protection against Genital Herpes" J Immunol 200(8): 2915-2926.
PubMed
Circulating conventional memory CD8(+) T cells (i.e., the CD8(+) effector memory T [TEM] cell and CD8(+) central memory T [TCM] cell subsets) and the noncirculating CD8(+) tissue-resident memory T (TRM) cell subset play a critical role in mucosal immunity. Mucosal chemokines, including the recently discovered CXCL17, are also important in mucosal immunity because they are homeostatically expressed in mucosal tissues. However, whether the CXCL17 chemokine contributes to the mobilization of memory CD8(+) T cell subsets to access infected mucosal tissues remains to be elucidated. In this study, we report that after intravaginal HSV type 1 infection of B6 mice, we detected high expression levels of CXCL17 and increased numbers of CD44(high)CD62L(low)CD8(+) TEM and CD103(high)CD8(+) TRM cells expressing CXCR8, the cognate receptor of CXCL17, in the vaginal mucosa (VM) of mice with reduced genital herpes infection and disease. In contrast to wild-type B6 mice, the CXCL17(-/-) mice developed 1) fewer CXCR8(+)CD8(+) TEM and TRM cells associated with more virus replication in the VM and more latency established in dorsal root ganglia, and 2) reduced numbers and frequencies of functional CD8(+) T cells in the VM. These findings suggest that the CXCL17/CXCR8 chemokine pathway plays a crucial role in mucosal vaginal immunity by promoting the mobilization of functional protective CD8(+) TEM and CD8(+) TRM cells, within this site of acute and recurrent herpes infection.
Flow Cytometry
Ceeraz, S., et al (2017). "VISTA Deficiency Accelerates the Development of Fatal Murine Lupus Nephritis" Arthritis Rheumatol 69(4): 814-825.
PubMed
OBJECTIVE: The targeting of negative checkpoint regulators as a means of augmenting antitumor immune responses is now an increasingly used and remarkably effective approach to the treatment of several human malignancies. The negative checkpoint regulator VISTA (V-domain Ig-containing suppressor of T cell activation; also known as programmed death 1 homolog or as death domain 1alpha) suppresses T cell responses and regulates myeloid activities. We proposed that exploitation of the VISTA pathway is a novel strategy for the treatment of human autoimmune disease, and therefore we undertook this study to determine the impact of VISTA genetic deficiency on lupus development in a lupus-prone mouse strain. METHODS: To evaluate whether genetic deficiency of VISTA affects the development of lupus, we interbred VISTA-deficient mice with Sle1.Sle3 mice, a well-characterized model of systemic lupus erythematosus (SLE). RESULTS: We demonstrated that the development of proteinuria and glomerulonephritis in these mice, designated Sle1.Sle3 VISTA(-/-) mice, was greatly accelerated and more severe compared to that in Sle1.Sle3 and C57BL/6 VISTA(-/-) mice. Analysis of cells from Sle1.Sle3 VISTA(-/-) mice showed enhanced activation of splenic CD4+ T cells and myeloid cell populations. No increase in titers of autoantibodies was seen in Sle1.Sle3 VISTA(-/-) mice. Most striking was a significant increase in proinflammatory cytokines, chemokines, and interferon (IFN)-regulated genes associated with SLE, such as IFNalpha, IFNgamma, tumor necrosis factor, interleukin-10, and CXCL10, in Sle1.Sle3 VISTA(-/-) mice. CONCLUSION: This study demonstrates for the first time that loss of VISTA in murine SLE exacerbates disease due to enhanced myeloid and T cell activation and cytokine production, including a robust IFNalpha signature, and supports a strategy of enhancement of the immunosuppressive activity of VISTA for the treatment of human lupus.
in vivo VISTA neutralization
Le Mercier, I., et al (2014). "VISTA Regulates the Development of Protective Antitumor Immunity" Cancer Res 74(7): 1933-1944.
PubMed
V-domain Ig suppressor of T-cell activation (VISTA) is a novel negative checkpoint ligand that is homologous to PD-L1 and suppresses T-cell activation. This study demonstrates the multiple mechanisms whereby VISTA relieves negative regulation by hematopoietic cells and enhances protective antitumor immunity. VISTA is highly expressed on myeloid cells and Foxp3(+)CD4(+) regulatory cells, but not on tumor cells within the tumor microenvironment (TME). VISTA monoclonal antibody (mAb) treatment increased the number of tumor-specific T cells in the periphery and enhanced the infiltration, proliferation, and effector function of tumor-reactive T cells within the TME. VISTA blockade altered the suppressive feature of the TME by decreasing the presence of monocytic myeloid-derived suppressor cells and increasing the presence of activated dendritic cells within the tumor microenvironment. In addition, VISTA blockade impaired the suppressive function and reduced the emergence of tumor-specific Foxp3(+)CD4(+) regulatory T cells. Consequently, VISTA mAb administration as a monotherapy significantly suppressed the growth of both transplantable and inducible melanoma. Initial studies explored a combinatorial regimen using VISTA blockade and a peptide-based cancer vaccine with TLR agonists as adjuvants. VISTA blockade synergized with the vaccine to effectively impair the growth of established tumors. Our study therefore establishes a foundation for designing VISTA-targeted approaches either as a monotherapy or in combination with additional immune-targeted strategies for cancer immunotherapy.
in vivo VISTA neutralization
in vitro VISTA neutralization
Wang, L., et al (2011). "VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses" J Exp Med 208(3): 577-592.
PubMed
The immunoglobulin (Ig) superfamily consists of many critical immune regulators, including the B7 family ligands and receptors. In this study, we identify a novel and structurally distinct Ig superfamily inhibitory ligand, whose extracellular domain bears homology to the B7 family ligand PD-L1. This molecule is designated V-domain Ig suppressor of T cell activation (VISTA). VISTA is primarily expressed on hematopoietic cells, and VISTA expression is highly regulated on myeloid antigen-presenting cells (APCs) and T cells. A soluble VISTA-Ig fusion protein or VISTA expression on APCs inhibits T cell proliferation and cytokine production in vitro. A VISTA-specific monoclonal antibody interferes with VISTA-induced suppression of T cell responses by VISTA-expressing APCs in vitro. Furthermore, anti-VISTA treatment exacerbates the development of the T cell-mediated autoimmune disease experimental autoimmune encephalomyelitis in mice. Finally, VISTA overexpression on tumor cells interferes with protective antitumor immunity in vivo in mice. These findings show that VISTA, a novel immunoregulatory molecule, has functional activities that are nonredundant with other Ig superfamily members and may play a role in the development of autoimmunity and immune surveillance in cancer.
Product Citations
-
-
Immunology and Microbiology
-
Pathology
Cytotoxic effector functions of T cells are not required for protective immunity against fatal Rickettsia typhi infection in a murine model of infection: Role of TH1 and TH17 cytokines in protection and pathology.
In PLoS Neglected Tropical Diseases on 1 February 2017 by Moderzynski, K., Heine, L., et al.
PubMed
Endemic typhus caused by Rickettsia (R.) typhi is an emerging febrile disease that can be fatal due to multiple organ pathology. Here we analyzed the requirements for protection against R. typhi by T cells in the CB17 SCID model of infection. BALB/c wild-type mice generate CD4+ TH1 and cytotoxic CD8+ T cells both of which are sporadically reactivated in persistent infection. Either adoptively transferred CD8+ or CD4+ T cells protected R. typhi-infected CB17 SCID mice from death and provided long-term control. CD8+ T cells lacking either IFNγ or Perforin were still protective, demonstrating that the cytotoxic function of CD8+ T cells is not essential for protection. Immune wild-type CD4+ T cells produced high amounts of IFNγ, induced the release of nitric oxide in R. typhi-infected macrophages and inhibited bacterial growth in vitro via IFNγ and TNFα. However, adoptive transfer of CD4+IFNγ-/- T cells still protected 30-90% of R. typhi-infected CB17 SCID mice. These cells acquired a TH17 phenotype, producing high amounts of IL-17A and IL-22 in addition to TNFα, and inhibited bacterial growth in vitro. Surprisingly, the neutralization of either TNFα or IL-17A in CD4+IFNγ-/- T cell recipient mice did not alter bacterial elimination by these cells in vivo, led to faster recovery and enhanced survival compared to isotype-treated animals. Thus, collectively these data show that although CD4+ TH1 cells are clearly efficient in protection against R. typhi, CD4+ TH17 cells are similarly protective if the harmful effects of combined production of TNFα and IL-17A can be inhibited.
-
-
-
Immunology and Microbiology
Decreased expression of the immune checkpoint regulator VISTA on neutrophils correlates with disease activity in autoimmune uveitis.
In FEBS J on 1 November 2025 by Qian, Y., Zhang, S., et al.
PubMed
Immune checkpoint V-domain Ig suppressor of T-cell activation (VISTA) exhibits distinct expression patterns and non-redundant immunoregulatory mechanisms in different autoimmune diseases. This study aims to investigate the expression of VISTA in patients with autoimmune uveitis and experimental autoimmune uveitis (EAU) mice, and explore its clinical significance and preliminary mechanisms in disease development. We found that VISTA expression on 12 subsets of peripheral blood immune cells was lower in autoimmune uveitis patients than in healthy volunteers, especially on neutrophils. The expression of neutrophil VISTA in active uveitis patients markedly increased when intraocular inflammation was ameliorated, indicating a significant correlation with disease activity. In vitro treatment of neutrophils from autoimmune uveitis patients with a VISTA antagonist markedly aggravated cell activation and neutrophil extracellular traps formation, whereas a VISTA agonist produced the opposite effect. Moreover, VISTA was constitutively expressed in the outer segments of retina in healthy mice, and decreased in EAU mice, reaching the lowest level of expression when the disease was at a peak stage. Taken together, this study investigates the relationship between neutrophil VISTA and the development of autoimmune uveitis, and provides new insights into the mechanisms and therapeutic roles of VISTA in autoimmune diseases.
-
-
-
Cancer Research
A Four Amino Acid Intracellular Motif of VISTA Blocks Growth Receptor Signaling in Cancer Cells to Induce Tumor Suppression.
In Cancer Res on 15 September 2025 by Zhao, Y., Andoh, T., et al.
PubMed
V-domain immunoglobulin suppressor of T-cell activation (VISTA, VSIR) is a key immune checkpoint receptor under investigation as a target for cancer immunotherapy. However, a better understanding of the signaling mechanisms of VISTA is needed to optimize the therapeutic potential. In this study, we identified a conserved four amino acid (NPGF) intracellular motif in VISTA that suppresses cell proliferation by constraining cell-intrinsic growth receptor signaling. A class of triple-negative breast cancers (TNBC) with high VISTA expression and low proliferative index was identified and characterized. The NPGF motif bound to the adapter protein NUMB and recruited Rab11 endosomal recycling machinery. The NPGF motif sequestered NUMB at endosomes, which interfered with EGFR trafficking and signaling to suppress tumor growth. These tumor-suppressive effects did not require canonical VISTA ligands or a functioning immune system. Mutation of the VISTA NPGF domain reverted VISTA-induced growth suppression in multiple breast cancer mouse models. The NPGF motif was also required for response of VISTA+ TNBCs to VISTA-blocking antibodies. These results define a mechanism by which VISTA recruits adapter proteins to control malignant epithelial cell growth and signaling. They also define distinct intracellular residues that are critical for response to therapeutic antibodies that could be exploited to improve immunotherapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
Targeting mTORC2 in lung squamous cell carcinoma improves anti-tumor immunity through the PSGL-1-VISTA axis.
In Cancer Gene Ther on 1 August 2025 by Ngwa, V. M., Hwang, Y., et al.
PubMed
Targeted therapies have improved survival for lung adenocarcinoma patients. However, similar advances are lacking for lung squamous carcinoma (LUSC). Advances in immunotherapy have shown some promise, but the overall response rate remains low in LUSC. Here, we demonstrate that the mTORC2 signaling pathway represents an actionable target in LUSC to improve anti-tumor immune responses. We show that genetic alterations affecting the mTORC2 pathway are common among patients with LUSC tumors, and targeting mTORC2 reduces LUSC tumor growth in mouse models. Transcriptomics reveal that mTORC2-deficient LUSC cells exhibit reduced expression of glycolytic and hypoxia-related genes. In agreement, loss of mTORC2 signaling decreases lactate levels in tumor-interstitial fluid, creating reduced acidity within the tumor microenvironment. Interestingly, mTORC2-deficient LUSC cells also exhibited reduced expression of the pH-sensitive VISTA ligand PSGL-1 in a HIF-2α dependent mechanism. LUSC patients, but not those with LUAD, display a positive correlation in expression between HIF-2α and PSGL-1, suggesting a distinct association among mTORC2, HIF-2α, and immune responses in LUSC. Indeed, mTORC2 loss-of-function enhanced CD8+ T cell activation in tumors, while use of anti-VISTA immunotherapy reduced LUSC tumor burden only in the presence of intact mTORC2 signaling. Collectively, these data describe an important role of mTORC2 signaling in LUSC tumors and demonstrate the therapeutic potential of targeting the mTORC2/PSGL-1/VISTA axis in patients that are non-responsive to current therapies.
-
-
-
Cancer Research
-
Immunology and Microbiology
Targeting mTORC2 in lung squamous cell carcinoma improves anti-tumor immunity through the PSGL-1-VISTA axis
In bioRxiv on 26 April 2025 by Ngwa, V. M., Hwang, Y., et al.
-
-
-
Cancer Research
Inhibitors of oncogenic Kras specifically prime CTLA4 blockade to transcriptionally reprogram Tregs and overcome resistance to suppress pancreas cancer
In bioRxiv on 4 March 2025 by Mahadevan, K. K., Maldonado, A. S., et al.
-
-
-
Immunology and Microbiology
-
Pharmacology
V-domain immunoglobulin suppressor of T-cell activation and programmed death receptor 1 dual checkpoint blockade enhances antitumour immunity and survival in glioblastoma.
In Br J Pharmacol on 1 March 2025 by Jin, S., Li, T., et al.
PubMed
The current therapy cannot meet the needs of glioblastoma (GBM). V-domain immunoglobulin suppressor of T-cell activation (VISTA) is significantly up-regulated in GBM patients; however, its therapeutic potential in GBM is still unclear.
-
-
-
In vivo experiments
-
Cancer Research
-
Immunology and Microbiology
METTL3-VISTA axis-based combination immunotherapy for APC truncation colorectal cancer.
In J Immunother Cancer on 9 December 2024 by Wu, L., Bai, R., et al.
PubMed
Although immune checkpoint blockade (ICB) therapy represents a bright spot in antitumor immunotherapy, its clinical benefits in colorectal cancer (CRC) are limited. Therefore, a new target for mediating CRC immunosuppression is urgently needed. Adenomatous polyposis coli (APC) mutations have been reported as early-stage characteristic events in CRC, but the role of truncated APC in the CRC immune microenvironment remains unclear and its clinical significance has yet to be explored.
-
-
-
Cancer Research
Identification of VISTA regulators in macrophages mediating cancer cell survival.
In Sci Adv on 29 November 2024 by Abdrabou, A. M., Ahmed, S., et al.
PubMed
Numerous human cancers have exhibited the ability to elude immune checkpoint blockade (ICB) therapies. This type of resistance can be mediated by immune-suppressive macrophages that limit antitumor immunity in the tumor microenvironment (TME). Here, we elucidate a strategy to shift macrophages into a proinflammatory state that down-regulates V domain immunoglobulin suppressor of T cell activation (VISTA) via inhibiting AhR and IRAK1. We used a high-throughput microfluidic platform combined with a genome-wide CRISPR knockout screen to identify regulators of VISTA levels. Functional characterization showed that the knockdown of these hits diminished VISTA surface levels on macrophages and sustained an antitumor phenotype. Furthermore, targeting of both AhR and IRAK1 in mouse models overcame resistance to ICB treatment. Tumor immunophenotyping indicated that infiltration of cytotoxic CD8+ cells, natural killer cells, and antitumor macrophages was substantially increased in treated mice. Collectively, AhR and IRAK1 are implicated as regulators of VISTA that coordinate a multifaceted barrier to antitumor immune responses.
-
-
-
Cardiovascular biology
VISTA Deficiency Exacerbates the Development of Pulmonary Fibrosis by Promoting Th17 Differentiation.
In J Inflamm Res on 24 June 2024 by Xie, H., Zhong, X., et al.
PubMed
Interstitial lung disease (ILD), characterized by pulmonary fibrosis (PF), represents the end-stage of various ILDs. The immune system plays an important role in the pathogenesis of PF. V-domain immunoglobulin suppressor of T-cell activation (VISTA) is an immune checkpoint with immune suppressive functions. However, its specific role in the development of PF and the underlying mechanisms remain to be elucidated.
-
-
-
Immunology and Microbiology
Combining toll-like receptor agonists with immune checkpoint blockade affects antitumor vaccine efficacy.
In J Immunother Cancer on 3 May 2024 by Jeon, D., Hill, E., et al.
PubMed
T cell checkpoint receptors are expressed when T cells are activated, and modulation of the expression or signaling of these receptors can alter the function of T cells and their antitumor efficacy. We previously found that T cells activated with cognate antigen had increases in the expression of PD-1, and this was attenuated in the presence of multiple toll-like receptor (TLR) agonists, notably TLR3 plus TLR9. In the current report, we sought to investigate whether combining TLR agonists with immune checkpoint blockade can further augment vaccine-mediated T cell antitumor immunity in murine tumor models.
-
-
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Aldehyde dehydrogenase 2-mediated aldehyde metabolism promotes tumor immune evasion by regulating the NOD/VISTA axis.
In J Immunother Cancer on 7 December 2023 by Chen, Y., Sun, J., et al.
PubMed
Aldehyde dehydrogenase 2 (ALDH2) is a crucial enzyme involved in endogenous aldehyde detoxification and has been implicated in tumor progression. However, its role in tumor immune evasion remains unclear.
-
-
-
Immunology and Microbiology
CD39 inhibition and VISTA blockade may overcome radiotherapy resistance by targeting exhausted CD8+ T cells and immunosuppressive myeloid cells.
In Cell Rep Med on 15 August 2023 by Zhang, Y., Hu, J., et al.
PubMed
Although radiotherapy (RT) has achieved great success in the treatment of non-small cell lung cancer (NSCLC), local relapses still occur and abscopal effects are rarely seen even when it is combined with immune checkpoint blockers (ICBs). Here, we characterize the dynamic changes of tumor-infiltrating immune cells after RT in a therapy-resistant murine tumor model using single-cell transcriptomes and T cell receptor sequencing. At the early stage, the innate and adaptive immune systems are activated. At the late stage, however, the tumor immune microenvironment (TIME) shifts into immunosuppressive properties. Our study reveals that inhibition of CD39 combined with RT preferentially decreases the percentage of exhausted CD8+ T cells. Moreover, we find that the combination of V-domain immunoglobulin suppressor of T cell activation (VISTA) blockade and RT synergistically reduces immunosuppressive myeloid cells. Clinically, high VISTA expression is associated with poor prognosis in patients with NSCLC. Altogether, our data provide deep insight into acquired resistance to RT from an immune perspective and present rational combination strategies.
-
-
-
Cancer Research
-
Immunology and Microbiology
Extracellular Vesicle Secretion by Leukemia Cells In Vivo Promotes CLL Progression by Hampering Antitumor T-cell Responses.
In Blood Cancer Discov on 6 January 2023 by Gargiulo, E., Viry, E., et al.
PubMed
Small extracellular vesicle (sEV, or exosome) communication among cells in the tumor microenvironment has been modeled mainly in cell culture, whereas their relevance in cancer pathogenesis and progression in vivo is less characterized. Here we investigated cancer-microenvironment interactions in vivo using mouse models of chronic lymphocytic leukemia (CLL). sEVs isolated directly from CLL tissue were enriched in specific miRNA and immune-checkpoint ligands. Distinct molecular components of tumor-derived sEVs altered CD8+ T-cell transcriptome, proteome, and metabolome, leading to decreased functions and cell exhaustion ex vivo and in vivo. Using antagomiRs and blocking antibodies, we defined specific cargo-mediated alterations on CD8+ T cells. Abrogating sEV biogenesis by Rab27a/b knockout dramatically delayed CLL pathogenesis. This phenotype was rescued by exogenous leukemic sEV or CD8+ T-cell depletion. Finally, high expression of sEV-related genes correlated with poor outcomes in CLL patients, suggesting sEV profiling as a prognostic tool. In conclusion, sEVs shape the immune microenvironment during CLL progression.
-
-
-
Immunology and Microbiology
Negative Immune Checkpoint Protein, VISTA, Regulates the CD4+ Treg Population During Sepsis Progression to Promote Acute Sepsis Recovery and Survival.
In Front Immunol on 12 April 2022 by Gray, C. C., Biron-Girard, B., et al.
PubMed
Sepsis is a systemic immune response to infection that is responsible for ~35% of in-hospital deaths and over 24 billion dollars in annual treatment costs. Strategic targeting of non-redundant negative immune checkpoint protein pathways can cater therapeutics to the individual septic patient and improve prognosis. B7-CD28 superfamily member V-domain Immunoglobulin Suppressor of T cell Activation (VISTA) is an ideal candidate for strategic targeting in sepsis. We hypothesized that immune checkpoint regulator, VISTA, controls T-regulatory cells (Treg), in response to septic challenge, thus playing a protective role/reducing septic morbidity/mortality. Further, we investigated if changes in morbidity/mortality are due to a Treg-mediated effect during the acute response to septic challenge. To test this, we used the cecal ligation and puncture model as a proxy for polymicrobial sepsis and assessed the phenotype of CD4+ Tregs in VISTA-gene deficient (VISTA-/-) and wild-type mice. We also measured changes in survival, soluble indices of tissue injury, and circulating cytokines in the VISTA-/- and wild-type mice. We found that in wild-type mice, CD4+ Tregs exhibit a significant upregulation of VISTA which correlates with higher Treg abundance in the spleen and small intestine following septic insult. However, VISTA-/- mice have reduced Treg abundance in these compartments met with a higher expression of Foxp3, CTLA4, and CD25 compared to wild-type mice. VISTA-/- mice also have a significant survival deficit, higher levels of soluble indicators of liver injury (i.e., ALT, AST, bilirubin), and increased circulating proinflammatory cytokines (i.e., IL-6, IL-10, TNFα, IL-17F, IL-23, and MCP-1) following septic challenge. To elucidate the role of Tregs in VISTA-/- sepsis mortality, we adoptively transferred VISTA-expressing Tregs into VISTA-/- mice. This adoptive transfer rescued VISTA-/- survival to wild-type levels. Taken together, we propose a protective Treg-mediated role for VISTA by which inflammation-induced tissue injury is suppressed and improves survival in early-stage murine sepsis. Thus, enhancing VISTA expression or adoptively transferring VISTA+ Tregs in early-stage sepsis may provide a novel therapeutic approach to ameliorate inflammation-induced death.
-
-
-
In vivo experiments
-
Cancer Research
-
Immunology and Microbiology
The allergy mediator histamine confers resistance to immunotherapy in cancer patients via activation of the macrophage histamine receptor H1.
In Cancer Cell on 10 January 2022 by Li, H., Xiao, Y., et al.
PubMed
Reinvigoration of antitumor immunity remains an unmet challenge. Our retrospective analyses revealed that cancer patients who took antihistamines during immunotherapy treatment had significantly improved survival. We uncovered that histamine and histamine receptor H1 (HRH1) are frequently increased in the tumor microenvironment and induce T cell dysfunction. Mechanistically, HRH1-activated macrophages polarize toward an M2-like immunosuppressive phenotype with increased expression of the immune checkpoint VISTA, rendering T cells dysfunctional. HRH1 knockout or antihistamine treatment reverted macrophage immunosuppression, revitalized T cell cytotoxic function, and restored immunotherapy response. Allergy, via the histamine-HRH1 axis, facilitated tumor growth and induced immunotherapy resistance in mice and humans. Importantly, cancer patients with low plasma histamine levels had a more than tripled objective response rate to anti-PD-1 treatment compared with patients with high plasma histamine. Altogether, pre-existing allergy or high histamine levels in cancer patients can dampen immunotherapy responses and warrant prospectively exploring antihistamines as adjuvant agents for combinatorial immunotherapy.
-
-
-
Immunology and Microbiology
VISTA Blockade Aggravates Bone Loss in Experimental Murine Apical Periodontitis.
In Front Immunol on 26 October 2021 by Yang, F., Zhang, Y., et al.
PubMed
V-domain Ig suppressor of T cell activation (VISTA) is a novel coinhibitory immune checkpoint molecule that maintains immune homeostasis. The present study explored the role of VISTA in human and murine inflammatory tissues of apical periodontitis (AP). VISTA was upregulated in inflammatory tissues of human AP. In mice, the expression of VISTA gradually increased with the development of mouse experimental apical periodontitis (MAP), the CD3+ T cells, CD11b+ myeloid cells, and FOXP3+ regulatory T cells also gradually accumulated. Moreover, a blockade of VISTA using a mouse in vivo anti-VISTA antibody aggravated periapical bone loss and enhanced the infiltration of immune cells in an experimental mouse periapical periodontitis model. The collective results suggest that VISTA serves as a negative regulator of the development and bone loss of apical periodontitis.
-
-
-
Flow cytometry/Cell sorting
-
Immunology and Microbiology
CNS-Native Myeloid Cells Drive Immune Suppression in the Brain Metastatic Niche through Cxcl10.
In Cell on 25 November 2020 by Guldner, I. H., Wang, Q., et al.
PubMed
Brain metastasis (br-met) develops in an immunologically unique br-met niche. Central nervous system-native myeloid cells (CNS-myeloids) and bone-marrow-derived myeloid cells (BMDMs) cooperatively regulate brain immunity. The phenotypic heterogeneity and specific roles of these myeloid subsets in shaping the br-met niche to regulate br-met outgrowth have not been fully revealed. Applying multimodal single-cell analyses, we elucidated a heterogeneous but spatially defined CNS-myeloid response during br-met outgrowth. We found Ccr2+ BMDMs minimally influenced br-met while CNS-myeloid promoted br-met outgrowth. Additionally, br-met-associated CNS-myeloid exhibited downregulation of Cx3cr1. Cx3cr1 knockout in CNS-myeloid increased br-met incidence, leading to an enriched interferon response signature and Cxcl10 upregulation. Significantly, neutralization of Cxcl10 reduced br-met, while rCxcl10 increased br-met and recruited VISTAHi PD-L1+ CNS-myeloid to br-met lesions. Inhibiting VISTA- and PD-L1-signaling relieved immune suppression and reduced br-met burden. Our results demonstrate that loss of Cx3cr1 in CNS-myeloid triggers a Cxcl10-mediated vicious cycle, cultivating a br-met-promoting, immune-suppressive niche.
-
-
-
Blocking experiments
-
Blocking experiments
-
Cancer Research
FOXD3 Regulates VISTA Expression in Melanoma.
In Cell Rep on 14 January 2020 by Rosenbaum, S. R., Knecht, M., et al.
PubMed
Immune checkpoint inhibitors have improved patient survival in melanoma, but the innate resistance of many patients necessitates the investigation of alternative immune targets. Many immune checkpoint proteins lack proper characterization, including V-domain Ig suppressor of T cell activation (VISTA). VISTA expression on immune cells can suppress T cell activity; however, few studies have investigated its expression and regulation in cancer cells. In this study, we observe that VISTA is expressed in melanoma patient samples and cell lines. Tumor cell-specific expression of VISTA promotes tumor onset in vivo, associated with increased intratumoral T regulatory cells, and enhanced PDL-1 expression on tumor-infiltrating macrophages. VISTA transcript levels are regulated by the stemness factor Forkhead box D3 (FOXD3). BRAF inhibition upregulates FOXD3 and reduces VISTA expression. Overall, this study demonstrates melanoma cell expression of VISTA and its regulation by FOXD3, contributing to the rationale for therapeutic strategies that combine targeted inhibitors with immune checkpoint blockade.
-