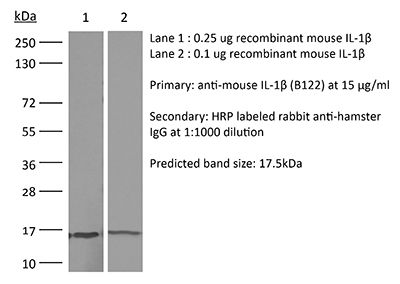
InVivoMAb anti-mouse/rat IL-1β
Product Description
Specifications
| Isotype | Armenian hamster IgG |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb polyclonal Armenian hamster IgG |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Recombinant mouse IL-1β |
| Reported Applications |
in vivo IL-1β neutralization in vitro IL-1β neutralization ELISA |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687727 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo IL-1β neutralization
Coffelt, S. B., et al (2015). "IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis" Nature 522(7556): 345-348.
PubMed
Metastatic disease remains the primary cause of death for patients with breast cancer. The different steps of the metastatic cascade rely on reciprocal interactions between cancer cells and their microenvironment. Within this local microenvironment and in distant organs, immune cells and their mediators are known to facilitate metastasis formation. However, the precise contribution of tumour-induced systemic inflammation to metastasis and the mechanisms regulating systemic inflammation are poorly understood. Here we show that tumours maximize their chance of metastasizing by evoking a systemic inflammatory cascade in mouse models of spontaneous breast cancer metastasis. We mechanistically demonstrate that interleukin (IL)-1beta elicits IL-17 expression from gamma delta (gammadelta) T cells, resulting in systemic, granulocyte colony-stimulating factor (G-CSF)-dependent expansion and polarization of neutrophils in mice bearing mammary tumours. Tumour-induced neutrophils acquire the ability to suppress cytotoxic T lymphocytes carrying the CD8 antigen, which limit the establishment of metastases. Neutralization of IL-17 or G-CSF and absence of gammadelta T cells prevents neutrophil accumulation and downregulates the T-cell-suppressive phenotype of neutrophils. Moreover, the absence of gammadelta T cells or neutrophils profoundly reduces pulmonary and lymph node metastases without influencing primary tumour progression. Our data indicate that targeting this novel cancer-cell-initiated domino effect within the immune system–the gammadelta T cell/IL-17/neutrophil axis–represents a new strategy to inhibit metastatic disease.
in vivo IL-1β neutralization
Copenhaver, A. M., et al (2015). "IL-1R signaling enables bystander cells to overcome bacterial blockade of host protein synthesis" Proc Natl Acad Sci U S A 112(24): 7557-7562.
PubMed
The innate immune system is critical for host defense against microbial pathogens, yet many pathogens express virulence factors that impair immune function. Here, we used the bacterial pathogen Legionella pneumophila to understand how the immune system successfully overcomes pathogen subversion mechanisms. L. pneumophila replicates within macrophages by using a type IV secretion system to translocate bacterial effectors into the host cell cytosol. As a consequence of effector delivery, host protein synthesis is blocked at several steps, including translation initiation and elongation. Despite this translation block, infected cells robustly produce proinflammatory cytokines, but the basis for this is poorly understood. By using a reporter system that specifically discriminates between infected and uninfected cells within a population, we demonstrate here that infected macrophages produced IL-1alpha and IL-1beta, but were poor producers of IL-6, TNF, and IL-12, which are critical mediators of host protection. Uninfected bystander cells robustly produced IL-6, TNF, and IL-12, and this bystander response required IL-1 receptor (IL-1R) signaling during early pulmonary infection. Our data demonstrate functional heterogeneity in production of critical protective cytokines and suggest that collaboration between infected and uninfected cells enables the immune system to bypass pathogen-mediated translation inhibition to generate an effective immune response.
in vivo IL-1β neutralization
Hernandez, P. P., et al (2015). "Interferon-lambda and interleukin 22 act synergistically for the induction of interferon-stimulated genes and control of rotavirus infection" Nat Immunol 16(7): 698-707.
PubMed
The epithelium is the main entry point for many viruses, but the processes that protect barrier surfaces against viral infections are incompletely understood. Here we identified interleukin 22 (IL-22) produced by innate lymphoid cell group 3 (ILC3) as an amplifier of signaling via interferon-lambda (IFN-lambda), a synergism needed to curtail the replication of rotavirus, the leading cause of childhood gastroenteritis. Cooperation between the receptor for IL-22 and the receptor for IFN-lambda, both of which were ‘preferentially’ expressed by intestinal epithelial cells (IECs), was required for optimal activation of the transcription factor STAT1 and expression of interferon-stimulated genes (ISGs). These data suggested that epithelial cells are protected against viral replication by co-option of two evolutionarily related cytokine networks. These data may inform the design of novel immunotherapy for viral infections that are sensitive to interferons.
in vivo IL-1β neutralization
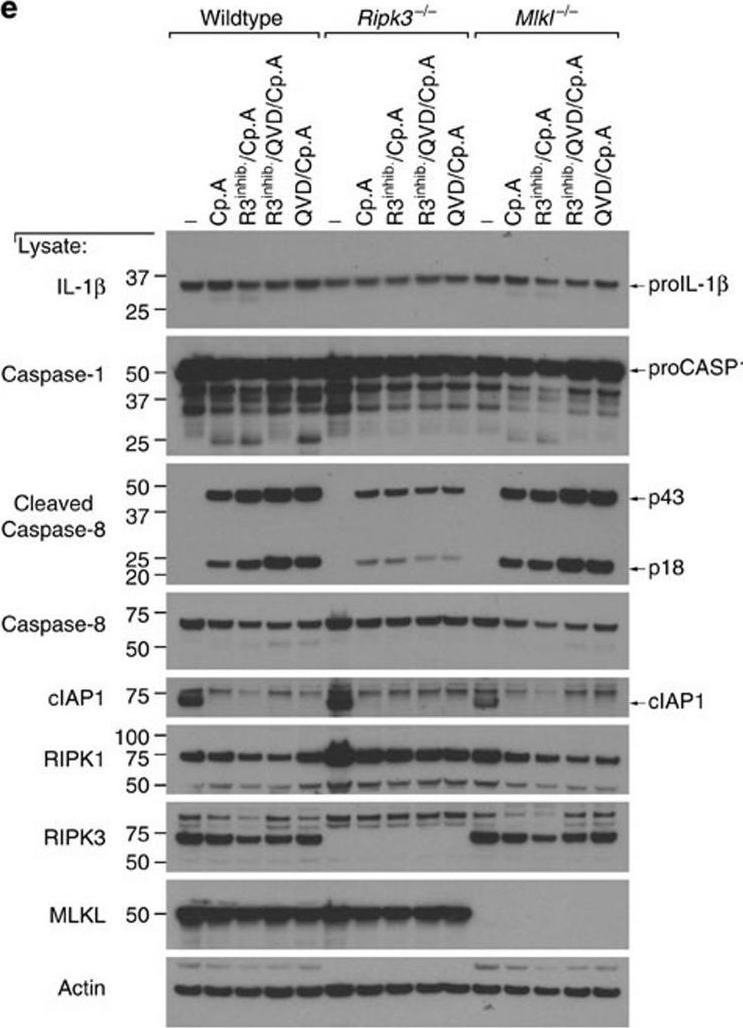
Lawlor, K. E., et al (2015). "RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL" Nat Commun 6: 6282.
PubMed
RIPK3 and its substrate MLKL are essential for necroptosis, a lytic cell death proposed to cause inflammation via the release of intracellular molecules. Whether and how RIPK3 might drive inflammation in a manner independent of MLKL and cell lysis remains unclear. Here we show that following LPS treatment, or LPS-induced necroptosis, the TLR adaptor protein TRIF and inhibitor of apoptosis proteins (IAPs: X-linked IAP, cellular IAP1 and IAP2) regulate RIPK3 and MLKL ubiquitylation. Hence, when IAPs are absent, LPS triggers RIPK3 to activate caspase-8, promoting apoptosis and NLRP3-caspase-1 activation, independent of RIPK3 kinase activity and MLKL. In contrast, in the absence of both IAPs and caspase-8, RIPK3 kinase activity and MLKL are essential for TLR-induced NLRP3 activation. Consistent with in vitro experiments, interleukin-1 (IL-1)-dependent autoantibody-mediated arthritis is exacerbated in mice lacking IAPs, and is reduced by deletion of RIPK3, but not MLKL. Therefore RIPK3 can promote NLRP3 inflammasome and IL-1beta inflammatory responses independent of MLKL and necroptotic cell death.
in vivo IL-1β neutralization
Russell, R. F., et al (2015). "Partial Attenuation of Respiratory Syncytial Virus with a Deletion of a Small Hydrophobic Gene Is Associated with Elevated Interleukin-1beta Responses" J Virol 89(17): 8974-8981.
PubMed
The small hydrophobic (SH) gene of respiratory syncytial virus (RSV), a major cause of infant hospitalization, encodes a viroporin of unknown function. SH gene knockout virus (RSV DeltaSH) is partially attenuated in vivo, but not in vitro, suggesting that the SH protein may have an immunomodulatory role. RSV DeltaSH has been tested as a live attenuated vaccine in humans and cattle, and here we demonstrate that it protected against viral rechallenge in mice. We compared the immune response to infection with RSV wild type and RSV DeltaSH in vivo using BALB/c mice and in vitro using epithelial cells, neutrophils, and macrophages. Strikingly, the interleukin-1beta (IL-1beta) response to RSV DeltaSH infection was greater than to wild-type RSV, in spite of a decreased viral load, and when IL-1beta was blocked in vivo, the viral load returned to wild-type levels. A significantly greater IL-1beta response to RSV DeltaSH was also detected in vitro, with higher-magnitude responses in neutrophils and macrophages than in epithelial cells. Depleting macrophages (with clodronate liposome) and neutrophils (with anti-Ly6G/1A8) demonstrated the contribution of these cells to the IL-1beta response in vivo, the first demonstration of neutrophilic IL-1beta production in response to viral lung infection. In this study, we describe an increased IL-1beta response to RSV DeltaSH, which may explain the attenuation in vivo and supports targeting the SH gene in live attenuated vaccines. IMPORTANCE: There is a pressing need for a vaccine for respiratory syncytial virus (RSV). A number of live attenuated RSV vaccine strains have been developed in which the small hydrophobic (SH) gene has been deleted, even though the function of the SH protein is unknown. The structure of the SH protein has recently been solved, showing it is a pore-forming protein (viroporin). Here, we demonstrate that the IL-1beta response to RSV DeltaSH is greater in spite of a lower viral load, which contributes to the attenuation in vivo. This potentially suggests a novel method by which viruses can evade the host response. As all Pneumovirinae and some Paramyxovirinae carry similar SH genes, this new understanding may also enable the development of live attenuated vaccines for both RSV and other members of the Paramyxoviridae.
in vivo IL-1β neutralization
Khmaladze, I., et al (2014). "Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice" Proc Natl Acad Sci U S A 111(35): E3669-3678.
PubMed
Psoriasis (Ps) and psoriasis arthritis (PsA) are poorly understood common diseases, induced by unknown environmental factors, affecting skin and articular joints. A single i.p. exposure to mannan from Saccharomyces cerevisiae induced an acute inflammation in inbred mouse strains resembling human Ps and PsA-like disease, whereas multiple injections induced a relapsing disease. Exacerbation of disease severity was observed in mice deficient for generation of reactive oxygen species (ROS). Interestingly, restoration of ROS production, specifically in macrophages, ameliorated both skin and joint disease. Neutralization of IL-17A, mainly produced by gammadelta T cells, completely blocked disease symptoms. Furthermore, mice depleted of granulocytes were resistant to disease development. In contrast, certain acute inflammatory mediators (C5, Fcgamma receptor III, mast cells, and histamine) and adaptive immune players (alphabeta T and B cells) were redundant in disease induction. Hence, we propose that mannan-induced activation of macrophages leads to TNF-alpha secretion and stimulation of local gammadelta T cells secreting IL-17A. The combined action of activated macrophages and IL-17A produced in situ drives neutrophil infiltration in the epidermis and dermis of the skin, leading to disease manifestations. Thus, our finding suggests a new mechanism triggered by exposure to exogenous microbial components, such as mannan, that can induce and exacerbate Ps and PsA.
in vivo IL-1β neutralization
Chen, K. W., et al (2014). "The neutrophil NLRC4 inflammasome selectively promotes IL-1beta maturation without pyroptosis during acute Salmonella challenge" Cell Rep 8(2): 570-582.
PubMed
The macrophage NLRC4 inflammasome drives potent innate immune responses against Salmonella by eliciting caspase-1-dependent proinflammatory cytokine production (e.g., interleukin-1beta [IL-1beta]) and pyroptotic cell death. However, the potential contribution of other cell types to inflammasome-mediated host defense against Salmonella was unclear. Here, we demonstrate that neutrophils, typically viewed as cellular targets of IL-1beta, themselves activate the NLRC4 inflammasome during acute Salmonella infection and are a major cell compartment for IL-1beta production during acute peritoneal challenge in vivo. Importantly, unlike macrophages, neutrophils do not undergo pyroptosis upon NLRC4 inflammasome activation. The resistance of neutrophils to pyroptotic death is unique among inflammasome-signaling cells so far described and allows neutrophils to sustain IL-1beta production at a site of infection without compromising the crucial inflammasome-independent antimicrobial effector functions that would be lost if neutrophils rapidly lysed upon caspase-1 activation. Inflammasome pathway modification in neutrophils thus maximizes host proinflammatory and antimicrobial responses during pathogen challenge.
in vivo IL-1β neutralization
Gopinath, S., et al (2014). "Role of disease-associated tolerance in infectious superspreaders" Proc Natl Acad Sci U S A 111(44): 15780-15785.
PubMed
Natural populations show striking heterogeneity in their ability to transmit disease. For example, a minority of infected individuals known as superspreaders carries out the majority of pathogen transmission events. In a mouse model of Salmonella infection, a subset of infected hosts becomes superspreaders, shedding high levels of bacteria (>10(8) cfu per g of feces) but remain asymptomatic with a dampened systemic immune state. Here we show that superspreader hosts remain asymptomatic when they are treated with oral antibiotics. In contrast, nonsuperspreader Salmonella-infected hosts that are treated with oral antibiotics rapidly shed superspreader levels of the pathogen but display signs of morbidity. This morbidity is linked to an increase in inflammatory myeloid cells in the spleen followed by increased production of acute-phase proteins and proinflammatory cytokines. The degree of colonic inflammation is similar in antibiotic-treated superspreader and nonsuperspreader hosts, indicating that the superspreader hosts are tolerant of antibiotic-mediated perturbations in the intestinal tract. Importantly, neutralization of acute-phase proinflammatory cytokines in antibiotic-induced superspreaders suppresses the expansion of inflammatory myeloid cells and reduces morbidity. We describe a unique disease-associated tolerance to oral antibiotics in superspreaders that facilitates continued transmission of the pathogen.
in vivo IL-1β neutralization
Botelho, F. M., et al (2011). "IL-1alpha/IL-1R1 expression in chronic obstructive pulmonary disease and mechanistic relevance to smoke-induced neutrophilia in mice" PLoS One 6(12): e28457.
PubMed
BACKGROUND: Cigarette smoking is the main risk factor for the development of chronic obstructive pulmonary disease (COPD), a major cause of morbidity and mortality worldwide. Despite this, the cellular and molecular mechanisms that contribute to COPD pathogenesis are still poorly understood. METHODOLOGY AND PRINCIPAL FINDINGS: The objective of this study was to assess IL-1 alpha and beta expression in COPD patients and to investigate their respective roles in perpetuating cigarette smoke-induced inflammation. Functional studies were pursued in smoke-exposed mice using gene-deficient animals, as well as blocking antibodies for IL-1alpha and beta. Here, we demonstrate an underappreciated role for IL-1alpha expression in COPD. While a strong correlation existed between IL-1alpha and beta levels in patients during stable disease and periods of exacerbation, neutrophilic inflammation was shown to be IL-1alpha-dependent, and IL-1beta- and caspase-1-independent in a murine model of cigarette smoke exposure. As IL-1alpha was predominantly expressed by hematopoietic cells in COPD patients and in mice exposed to cigarette smoke, studies pursued in bone marrow chimeric mice demonstrated that the crosstalk between IL-1alpha+ hematopoietic cells and the IL-1R1+ epithelial cells regulates smoke-induced inflammation. IL-1alpha/IL-1R1-dependent activation of the airway epithelium also led to exacerbated inflammatory responses in H1N1 influenza virus infected smoke-exposed mice, a previously reported model of COPD exacerbation. CONCLUSIONS AND SIGNIFICANCE: This study provides compelling evidence that IL-1alpha is central to the initiation of smoke-induced neutrophilic inflammation and suggests that IL-1alpha/IL-1R1 targeted therapies may be relevant for limiting inflammation and exacerbations in COPD.
in vitro IL-1β neutralization
ELISA
Gonzalez-Navajas, J. M., et al (2010). "Interleukin 1 receptor signaling regulates DUBA expression and facilitates Toll-like receptor 9-driven antiinflammatory cytokine production" J Exp Med 207(13): 2799-2807.
PubMed
The interleukin 1 receptor (IL-1R) and the Toll-like receptors (TLRs) are highly homologous innate immune receptors that provide the first line of defense against infection. We show that IL-1R type I (IL-1RI) is essential for TLR9-dependent activation of tumor necrosis factor receptor-associated factor 3 (TRAF3) and for production of the antiinflammatory cytokines IL-10 and type I interferon (IFN). Noncanonical K63-linked ubiquitination of TRAF3, which is essential for type I IFN and IL-10 production, was impaired in Il1r1(-/-) CD11c(+) dendritic cells. In contrast, degradative ubiquitination of TRAF3 was not affected in the absence of IL-1R1 signaling. Deubiquitinating enzyme A (DUBA), which selectively cleaves K63-linked ubiquitin chains from TRAF3, was up-regulated in the absence of IL-1R1 signaling. DUBA short interference RNA augmented the TLR9-dependent type I IFN response. Mice deficient in IL-1RI signaling showed reduced expression of IL-10 and type I IFN and increased susceptibility to dextran sulphate sodium-induced colitis and failed to mount a protective type I IFN response after TLR9 ligand (CpG) administration. Our data identifies a new molecular pathway by which IL-1 signaling attenuates TLR9-mediated proinflammatory responses.
ELISA
Gekara, N. O., et al (2009). "Signals triggered by a bacterial pore-forming toxin contribute to toll-like receptor redundancy in gram-positive bacterial recognition" J Infect Dis 199(1): 124-133.
PubMed
BACKGROUND: Toll-like receptor (TLR) 2 is the principal recognition receptor for gram-positive microbes. However, in some gram-positive bacterial infections, TLR2 is dispensable. One of the outstanding questions regarding host-bacteria interactions is why TLR2 is essential in some infections but dispensable in others. METHODS: We used a combination of bacterial plating, flow cytometry, enzyme-linked immunosorbent assay, and reverse-transcriptase polymerase chain reaction to analyze the inflammatory responses induced by Listeria monocytogenes and its toxin listeriolysin O (LLO) in vitro and in vivo. We analyzed wild-type, TLR2(-/-)-, TLR4(-/-)-, MyD88(-/-)-, interleukin (IL)-1beta(-/-)-, and IL-18(-/-)-deficient mice and the bone marrow-derived mast cells obtained from these respective groups. RESULTS: TLR2(-/-) mice had unaltered L. monocytogenes clearance and did not experience impairment of cytokine/chemokine induction and neutrophil mobilization by L. monocytogenes or purified LLO, but they were unresponsive to the LLO-deficient mutant L. monocytogenes (LmDeltahly). We show that L. monocytogenes and LLO mediate such responses in part via interleukin (IL)-1beta and IL-18-MyD88 pathways. CONCLUSIONS: The results illustrate that signals triggered by LLO contribute to TLR2 redundancy in recognition of L. monocytogenes. Under normal conditions, multiple and, sometimes, redundant pathways cooperate to induce a rapid antimicrobial defense. When one signaling pathway-in this case, TLR2-is removed from the system, the other pathways are still capable of mounting a sufficient response to ensure survival of the host.
Product Citations
-
-
Immunology and Microbiology
Harnessing the dual immunomodulatory function of myeloid-derived suppressor cells to reshape the inflammatory microenvironment for osteoarthritis therapy.
In Mater Today Bio on 1 December 2025 by Guo, Z., Chen, T., et al.
PubMed
Osteoarthritis (OA) pathogenesis is profoundly influenced by dysregulated immune dynamics, where persistent interleukin-17 (IL-17)/T helper 17 (Th17) cell mediated inflammation coordinates with failed regenerative processes to perpetuate joint destruction. Here, we unveil the role of myeloid-derived suppressor cells (MDSCs) as dual-phase regulators that paradoxically orchestrate both inflammatory escalation and tissue repair in OA progression. Intra-articular administration of MDSCs in OA mice amplified IL-17 dependent inflammatory cascades and chemokine-driven leukocyte recruitment, revealing a context-dependent pro-inflammatory phenotype. Unexpectedly, MDSC depletion failed to attenuate joint damage, implying their indispensable yet multifaceted role in OA pathogenesis. Mechanistically, MDSCs exhibited functional plasticity by upregulating arginase-1 to polarize M2 macrophages, fostering a regenerative niche alongside their inflammatory activity. To resolve this duality, we developed a bio-responsive hydrogel-microsphere system integrating transforming growth factor β1 (TGF-β1) and interleukin-1 β1 antibody (anti-IL-1β) loaded mesoporous silica nanoparticles (MSNs). This spatiotemporally controlled platform selectively suppressed MDSC-mediated Th17 cell expansion while harnessing their intrinsic capacity to drive M2 macrophage polarization and chondrogenesis. The resultant shift from a pro-inflammatory to pro-regenerative microenvironment significantly attenuated cartilage erosion and restored joint integrity in OA models. Our findings redefine MDSCs as bifunctional immune orchestrators in OA and establish precision biomaterial guided immune decoding as a paradigm-shifting therapeutic strategy. By engineering MDSCs plasticity through antagonistic cytokine delivery, this work provides a blueprint for microenvironment remodeling in degenerative joint diseases.
-
-
-
Cancer Research
Purinergic Receptor Nanoimmunoamplifiers Potentiate Chemoimmunotherapy Efficacy in Hepatocellular Carcinoma.
In Biomater Res on 10 November 2025 by Zhang, J., Zhang, J., et al.
PubMed
The effectiveness of chemoimmunotherapy for hepatocellular carcinoma (HCC) is hindered by the weak immunogenicity of chemotherapy-induced immunogenic cell death (ICD). This limitation primarily stems from the insufficient activation of the extracellular adenosine triphosphate (eATP)/P2X7 purinergic receptor (P2X7R)/NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome pathway in dendritic cells (DCs). To address this challenge, we designed ivermectin-MnO2 nanocomplexes (IMNs) as P2X7R-targeted nanoimmunoamplifiers to enhance the immunogenicity of chemotherapy-induced ICD. The ivermectin component of IMN enhanced liposomal doxorubicin (LD)-induced ICD and increased P2X7R sensitivity to eATP. Additionally, the MnO2 component of IMN alleviated tumor hypoxia and down-regulated CD39/CD73 expression, thereby preventing eATP degradation. These combined strategies robustly activated the eATP/P2X7R/NLRP3 inflammasome cascade in DCs, eliciting a potent antitumor immune response. In combination with anti-PD-L1 antibody and LD, IMN effectively inhibited tumor growth in orthotopic, subcutaneous, and metastatic HCC mouse models. Our study underscores the crucial role of IMN in amplifying the NLRP3 inflammasome cascade in DCs during ICD, presenting a promising strategy to enhance the efficacy of HCC chemoimmunotherapy.
-
-
-
Neuroscience
Paraventricular nucleus CRH neurons regulate acute lung injury via sympathetic nerve-neutrophil axis.
In Nat Commun on 6 October 2025 by Li, H., Liu, T., et al.
PubMed
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are severe conditions with high morbidity and mortality with limited effective therapies. Neuroimmune interactions play a critical role in lung homeostasis, but it remains unclear if specific brain regions regulate lung inflammation. Here, we perform anatomical tracing, chemogenetic modulation, and pharmacological interventions in male mice and identify a neural circuit from corticotropin-releasing hormone neurons in the paraventricular nucleus of the hypothalamus (CRHPVN neurons) to the lung. The activation of these neurons protects mice from ALI and promotes survival, reduces neutrophil infiltration and effector functions in the lung, whereas inhibiting CRHPVN neurons worsens ALI. The protective effect is mediated by increased sympathetic nervous activity, with locally released norepinephrine modulating neutrophil functions via β2-AR-β-arrestin2 signaling to inhibit the NF-κB pathway. These findings uncover a brain-lung neural circuit that modulates immune responses during ALI, offering a potential therapeutic target for ALI and ARDS.
-
-
-
Immunology and Microbiology
FABP5 in skin macrophages mediates saturated fat-induced IL-1β signaling in psoriatic inflammation.
In Cell Rep on 23 September 2025 by Yu, J., Hao, J., et al.
PubMed
High fat diet (HFD)-induced obesity increases the risk and severity of psoriasis. However, the immunoregulatory effects of different HFDs on psoriasis pathogenesis remains poorly understood. Here, mimicking human dietary fat profiles, four HFDs-saturated, monounsaturated, omega-6, and omega-3 fats-were designed and used to induce obesity in mice. Despite comparable obesity levels across groups, only the saturated HFD exacerbated imiquimod (IMQ)-induced psoriasis. This exacerbation correlated with elevated levels of IL-1β-producing macrophages, IL-17A-producing γδ T cells, and neutrophils within psoriatic lesions. Mechanistically, saturated fatty acids (FAs) promoted IL-1β/IL-17A signaling via fatty acid-binding protein 5 (FABP5)-mediated mitochondrial FA oxidation and extracellular ATP release in skin macrophages. Deletion of FABP5, either globally or specifically in macrophages, attenuated IL-1β/IL-17A signaling and alleviated IMQ-induced psoriasis. These findings identify FABP5 as a key mediator of saturated HFD-driven psoriasis via the IL-1β/IL-17 axis, offering insights into the interplay between dietary fats, obesity, and psoriasis.
-
-
-
Immunology and Microbiology
-
Cancer Research
TIM3+ breast cancer cells license immune evasion during micrometastasis outbreak.
In Cancer Cell on 11 August 2025 by Rozalén, C., Sangrador, I., et al.
PubMed
In metastasis, the dynamics of tumor-immune interactions during micrometastasis remain unclear. Identifying the vulnerabilities of micrometastases before outbreaking into macrometastases can reveal therapeutic opportunities for metastasis. Here, we report a function of T cell immunoglobulin and mucin domain 3 (TIM3) in tumor cells during micrometastasis using breast cancer (BC) metastasis mouse models. TIM3 is highly upregulated in micrometastases, promoting survival, stemness, and immune escape. TIM3+ tumor cells are specifically selected during early seeding of micrometastasis. Mechanistically, TIM3 increases β-catenin/interleukin-1β (IL-1β) signaling, leading to stemness and immune-evasion by inducing immunosuppressive γδ T cells and reducing CD8 T cells during micrometastasis. Clinical data confirm increased TIM3+ tumor cells in BC metastasis and TIM3+ tumor cells as a biomarker of poor outcome in BC patients. (Neo)adjuvant TIM3 blockade reduces the metastatic seeding and incidence in preclinical models. These findings unveil a specific mechanism of micrometastasis immune-evasion and the potential use of TIM3 blockade for subclinical metastasis.
-
-
-
Cancer Research
Understanding and reversing mammary tumor-driven reprogramming of myelopoiesis to reduce metastatic spread.
In Cancer Cell on 14 July 2025 by Garner, H., Martinovic, M., et al.
PubMed
Tumor-induced systemic accumulation and polarization of neutrophils to an immunosuppressive phenotype is a potent driver of metastasis formation. Yet, how mammary tumors reprogram granulopoiesis at the molecular level and when tumor imprinting occurs during neutrophil development remains underexplored. Here, we combined single-cell, chromatin and functional analyses to unravel the tumor-driven reprogramming of granulopoiesis in the bone marrow, along with intervention studies aimed at reversing this process. We observe that mammary tumors accelerate commitment to the neutrophil lineage at the expense of lymphopoiesis and erythropoiesis without stimulating the development of a novel myeloid lineage. Moreover, tumor-directed immunosuppressive imprinting of neutrophils starts early in hematopoiesis. Treatment with anti-IL-1β normalizes tumor-induced granulopoiesis, reducing neutrophil immunosuppressive phenotype and mitigating metastatic spread. Together, these data provide molecular insights into the aberrant, tumor-driven neutrophil differentiation pathway leading to metastasis-promoting chronic inflammation and how it can be reversed to reduce metastatic spread.
-
-
-
Cell Biology
-
COVID-19
IL-1β drives SARS-CoV-2-induced disease independently of the inflammasome and pyroptosis signalling.
In Cell Death Differ on 1 July 2025 by Bader, S. M., Scherer, L., et al.
PubMed
Excessive inflammation and cytokine release are hallmarks of severe COVID-19. Certain programmed cell death processes can drive inflammation, however, their role in the pathogenesis of severe COVID-19 is unclear. Pyroptosis is a pro-inflammatory form of regulated cell death initiated by inflammasomes and executed by the pore-forming protein gasdermin D (GSDMD). Using an established mouse adapted SARS-CoV-2 virus and a panel of gene-targeted mice we found that deletion of the inflammasome (NLRP1/3 and the adaptor ASC) and pore forming proteins involved in pyroptosis (GSDMA/C/D/E) only marginally reduced IL-1β levels and did not impact disease outcome or viral loads. Furthermore, we found that SARS-CoV-2 infection did not trigger GSDMD activation in mouse lungs. Finally, we did not observe any difference between WT animals and mice with compound deficiencies in the pro-inflammatory initiator caspases (C1/11/12-/-). This indicates that the classical canonical and non-canonical pro-inflammatory caspases known to process and activate pro-IL-1β, pro-IL-18 and GSDMD do not substantially contribute to SARS-CoV-2 pathogenesis. However, the loss of IL-1β, but not the absence of IL-18, ameliorated disease and enhanced survival in SARS-CoV-2 infected animals compared to wildtype mice. Collectively, these findings demonstrate that IL-1β is an important factor contributing to severe SARS-CoV-2 disease, but its release was largely independent of inflammasome and pyroptotic pathways.
-
-
-
Biochemistry and Molecular biology
-
Cancer Research
-
Immunology and Microbiology
Sulfasalazine combined with anti-IL-1β mAb induces ferroptosis and immune modulation in oral squamous cell carcinoma.
In Cell Mol Life Sci on 28 May 2025 by Zhou, R., Zhou, J., et al.
PubMed
Oral squamous cell carcinoma (OSCC), one of the most prevalent and aggressive forms of head and neck squamous cell carcinoma, has a five-year survival rate of about 50% ~ 60%, emphasizing the urgent need for more effective therapeutic strategies. Solute carrier family 7 member 11 (SLC7A11) is overexpressed in various cancers and represents a potential therapeutic target. Sulfasalazine (SAS), a Food and Drug Administration-approved drug, is a potent inhibiter of SLC7A11. However, SAS can also increase the levels of pro-inflammatory cytokines such as IL-1β, which may suppress the immune response. Here, we investigate the effect of SAS combined with anti-IL-1β monoclonal antibody (anti-IL-1β mAb) as a novel treatment strategy for OSCC. In this study, SLC7A11 was markedly increased in OSCC tissues, and high SLC7A11 expression predicted poor prognosis. SAS treatment was shown to suppress OSCC cell proliferation and trigger ferroptosis, as evidenced by elevated reactive oxygen species, reduced glutathione and enhanced lipid peroxidation. SAS also elevated IL-1β levels, leading to T cell exhaustion. Combining SAS with anti-IL-1β mAb reversed T cell exhaustion and amplified the anti-tumor effects in vitro. In the 4-nitroquinoline-1-oxide-induced oral cancergenisis model, the combination treatment significantly inhibited oral carcinogenesis compared to monotherapy. Our results suggest that combining SAS with anti-IL-1β mAb enhances the anti-tumor efficacy against OSCC through tumor growth inhibition and immune modulation, offering a promising therapeutic strategy.
-
-
-
Stem Cells and Developmental Biology
-
Immunology and Microbiology
-
Cell Biology
-
Biochemistry and Molecular biology
The P2X7R/NLRP3 inflammasome axis suppresses enthesis regeneration through inflammatory and metabolic macrophage-stem cell cross-talk.
In Sci Adv on 25 April 2025 by Gao, H., Wang, L., et al.
PubMed
The regeneration of the enthesis remains a formidable challenge in regenerative medicine. However, key regulators underlying unsatisfactory regeneration remain poorly understood. This study reveals that the purinergic receptor P2X7 (P2X7R)/Nod-like receptor family protein 3 (NLRP3) inflammasome axis suppresses enthesis regeneration by amplifying IL-1β-mediated inflammatory cross-talk and suppressing docosatrienoic acid (DTA) metabolic cross-talk. NLRP3 inflammasomes were activated in macrophages following enthesis injury, thereby impairing the histological and functional recovery of the injured enthesis. Single-cell RNA sequencing (scRNA-seq) indicated that Nlrp3 knockout attenuated pathological inflammation and ameliorated the detrimental effects of IL-1β signaling cross-talk. Furthermore, NLRP3 inflammasomes suppressed the secretion of anti-inflammatory cytokines (IL-10 and IL-13) and DTA. The NLRP3 inflammasome-mediated secretome reduced differentiation and migration of stem cells. Neutralizing IL-1β or replenishing docosatrienoic acid accelerated enthesis regeneration. Moreover, conditional knockout of P2rx7 in myeloid cells attenuated NLRP3 inflammasome activation and facilitated enthesis regeneration. This study demonstrates that the P2X7R/NLRP3 inflammasome axis represents a promising therapeutic target for enthesis repair.
-
-
-
Cancer Research
Ferroptotic Neutrophils Induce Immunosuppression and Chemoresistance in Breast Cancer.
In Cancer Res on 1 February 2025 by Zeng, W., Zhang, R., et al.
PubMed
Inducing ferroptosis in tumor cells is emerging as a strategy for treating malignancies that are refractory to traditional treatment modalities. However, the consequences of ferroptosis of immune cells in the tumor microenvironment need to be better understood in order to realize the potential of this approach. In this study, we discovered that neutrophils in chemoresistant breast cancer are highly sensitive to ferroptosis. Reduction of the acyltransferase MOAT1 in chemoresistance-associated neutrophils induced phospholipid reprogramming, switching the preference from monounsaturated fatty acids to polyunsaturated fatty acids, which increased their susceptibility to ferroptosis. Ferroptotic neutrophils secreted PGE2, IDO, and oxidized lipids that suppressed the proliferation and cytotoxicity of antitumor CD8+ T cells. Furthermore, neutrophil ferroptosis was closely related to a distinct subset of IL1β+CXCL3+CD4+ (Fer-CD4) T lymphocytes, which were enriched in chemoresistant tumors. Fer-CD4 T cells orchestrated neutrophil ferroptosis by modulating MOAT1 expression via IL1β/IL1R1/NF-κB signaling. Moreover, Fer-CD4 T cells secreted CXCL3, IL8, and S100A9 to replenish the neutrophil pool in the tumor microenvironment. Ferroptotic neutrophils in turn fostered Fer-CD4 T-cell differentiation. In spontaneous tumorigenesis mouse models, targeting IL1β+ CD4+ T cells or IL1R1+ neutrophils broke the cross-talk, restraining neutrophil ferroptosis, enhancing antitumor immunity, and overcoming chemoresistance. Overall, these findings uncover the role of neutrophil ferroptosis in shaping the immune landscape and propose appealing targets for restoring immunosurveillance and chemosensitivity in breast cancer. Significance: In chemoresistant breast cancer, IL1β+CXCL3+CD4+ T cells mediate neutrophil ferroptosis that suppresses antitumor immunity, indicating that interfering with this intercellular cross-talk could be an attractive strategy to reverse chemoresistance.
-
-
-
Cancer Research
-
Immunology and Microbiology
Tumor-colonized Streptococcus mutans metabolically reprograms tumor microenvironment and promotes oral squamous cell carcinoma.
In Microbiome on 5 October 2024 by Zhou, J., Hu, Z., et al.
PubMed
Oral squamous cell carcinoma (OSCC) remains a major death cause in head and neck cancers, but the exact pathogenesis mechanisms of OSCC are largely unclear.
-
-
-
Immunocytochemistry-immunofluorescence
-
Cell Biology
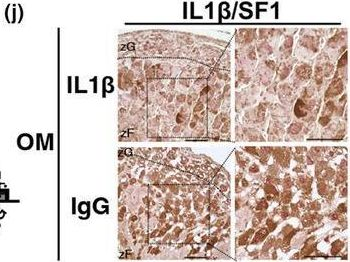
Accumulation of senescent cells in the adrenal gland induces hypersecretion of corticosterone via IL1β secretion.
In Aging Cell on 1 September 2024 by Okudaira, N., Akimoto, M. H., et al.
PubMed
Aging progresses through the interaction of metabolic processes, including changes in the immune and endocrine systems. Glucocorticoids (GCs), which are regulated by the hypothalamic-pituitary-adrenal (HPA) axis, play an important role in regulating metabolism and immune responses. However, the age-related changes in the secretion mechanisms of GCs remain elusive. Here, we found that corticosterone (CORT) secretion follows a circadian rhythm in young mice, whereas it oversecreted throughout the day in aged mice >18 months old, resulting in the disappearance of diurnal variation. Furthermore, senescent cells progressively accumulated in the zF of the adrenal gland as mice aged beyond 18 months. This accumulation was accompanied by an increase in the number of Ad4BP/SF1 (SF1), a key transcription factor, strongly expressing cells (SF1-high positive: HP). Removal of senescent cells with senolytics, dasatinib, and quercetin resulted in the reduction of the number of SF1-HP cells and recovery of CORT diurnal oscillation in 24-month-old mice. Similarly, administration of a neutralizing antibody against IL1β, which was found to be strongly expressed in the adrenocortical cells of the zF, resulted in a marked decrease in SF1-HP cells and restoration of the CORT circadian rhythm. Our findings suggest that the disappearance of CORT diurnal oscillation is a characteristic of aging individuals and is caused by the secretion of IL1β, one of the SASPs, from senescent cells that accumulate in the zF of the adrenal cortex. These findings provide a novel insight into aging. Age-related hypersecretory GCs could be a potential therapeutic target for aging-related diseases.
-
-
-
COVID-19
IL-1b drives SARS-CoV-2 disease in vivo, independently of the inflammasome and pyroptotic signalling
In Research Square on 28 August 2024 by Doerflinger, M., Bader, S. M., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
NR0B2 re-educates myeloid immune cells to reduce regulatory T cell expansion and progression of breast and other solid tumors.
In Cancer Lett on 10 August 2024 by Vidana Gamage, H. E., Shahoei, S. H., et al.
PubMed
Although survival from breast cancer has dramatically increased, many will develop recurrent, metastatic disease. Unfortunately, survival for this stage of disease remains very low. Activating the immune system has incredible promise since it has the potential to be curative. However, immune checkpoint blockade (ICB) which works through T cells has been largely disappointing for metastatic breast cancer. One reason for this is a suppressive myeloid immune compartment that is unaffected by ICB. Cholesterol metabolism and proteins involved in cholesterol homeostasis play important regulatory roles in myeloid cells. Here, we demonstrate that NR0B2, a nuclear receptor involved in negative feedback of cholesterol metabolism, works in several myeloid cell types to impair subsequent expansion of regulatory T cells (Tregs); Tregs being a subset known to be highly immune suppressive and associated with poor therapeutic response. Within myeloid cells, NR0B2 serves to decrease many aspects of the inflammasome, ultimately resulting in decreased IL1β; IL1β driving Treg expansion. Importantly, mice lacking NR0B2 exhibit accelerated tumor growth. Thus, NR0B2 represents an important node in myeloid cells dictating ensuing Treg expansion and tumor growth, thereby representing a novel therapeutic target to re-educate these cells, having impact across different solid tumor types. Indeed, a paper co-published in this issue demonstrates the therapeutic utility of targeting NR0B2.
-
-
-
Cancer Research
Development of NR0B2 as a therapeutic target for the re-education of tumor associated myeloid cells.
In Cancer Lett on 10 August 2024 by Vidana Gamage, H. E., Albright, S. T., et al.
PubMed
Immune checkpoint blockade (ICB) has had limited utility in several solid tumors such as breast cancer, a major cause of cancer-related mortality in women. Therefore, there is considerable interest in alternate strategies to promote an anti-cancer immune response. A paper co-published in this issue describes how NR0B2, a protein involved in cholesterol homeostasis, functions within myeloid immune cells to modulate the inflammasome and reduce the expansion of immune-suppressive regulatory T cells (Treg). Here, we develop NR0B2 as a potential therapeutic target. NR0B2 in tumors is associated with improved survival for several cancer types including breast. Importantly, NR0B2 expression is also prognostic of ICB success. Within breast tumors, NR0B2 expression is inversely associated with FOXP3, a marker of Tregs. While a described agonist (DSHN) had some efficacy, it required high doses and long treatment times. Therefore, we designed and screened several derivatives. A methyl ester derivative (DSHN-OMe) emerged as superior in terms of (1) cellular uptake, (2) ability to regulate expected expression of genes, (3) suppression of Treg expansion using in vitro co-culture systems, and (4) efficacy against the growth of primary and metastatic tumors. This work identifies NR0B2 as a target to re-educate myeloid immune cells and a novel ligand with significant anti-tumor efficacy in preclinical models.
-
-
-
Biochemistry and Molecular biology
-
Cell Biology
Oxylipins and metabolites from pyroptotic cells act as promoters of tissue repair.
In Nature on 1 July 2024 by Mehrotra, P., Maschalidi, S., et al.
PubMed
Pyroptosis is a lytic cell death mode that helps limit the spread of infections and is also linked to pathology in sterile inflammatory diseases and autoimmune diseases1-4. During pyroptosis, inflammasome activation and the engagement of caspase-1 lead to cell death, along with the maturation and secretion of the inflammatory cytokine interleukin-1β (IL-1β). The dominant effect of IL-1β in promoting tissue inflammation has clouded the potential influence of other factors released from pyroptotic cells. Here, using a system in which macrophages are induced to undergo pyroptosis without IL-1β or IL-1α release (denoted Pyro-1), we identify unexpected beneficial effects of the Pyro-1 secretome. First, we noted that the Pyro-1 supernatants upregulated gene signatures linked to migration, cellular proliferation and wound healing. Consistent with this gene signature, Pyro-1 supernatants boosted migration of primary fibroblasts and macrophages, and promoted faster wound closure in vitro and improved tissue repair in vivo. In mechanistic studies, lipidomics and metabolomics of the Pyro-1 supernatants identified the presence of both oxylipins and metabolites, linking them to pro-wound-healing effects. Focusing specifically on the oxylipin prostaglandin E2 (PGE2), we find that its synthesis is induced de novo during pyroptosis, downstream of caspase-1 activation and cyclooxygenase-2 activity; further, PGE2 synthesis occurs late in pyroptosis, with its release dependent on gasdermin D pores opened during pyroptosis. As for the pyroptotic metabolites, they link to immune cell infiltration into the wounds, and polarization to CD301+ macrophages. Collectively, these data advance the concept that the pyroptotic secretome possesses oxylipins and metabolites with tissue repair properties that may be harnessed therapeutically.
-
-
-
Immunology and Microbiology
Intense impact of IL-1β expressing inflammatory macrophages in acute aortic dissection.
In Sci Rep on 28 June 2024 by Inoue, T., Emoto, T., et al.
PubMed
There is no treatment for acute aortic dissection (AAD) targeting inflammatory cells. We aimed to identify the new therapeutic targets associated with inflammatory cells. We characterized the specific distribution of myeloid cells of both human type A AAD samples and a murine AAD model generated using angiotensin II (ANGII) and β-aminopropionitrile (BAPN) by single-cell RNA sequencing (scRNA-seq). We also examined the effect of an anti-interleukin-1β (IL-1β) antibody in the murine AAD model. IL1B+ inflammatory macrophages and classical monocytes were increased in human AAD samples. Trajectory analysis demonstrated that IL1B+ inflammatory macrophages differentiated from S100A8/9/12+ classical monocytes uniquely observed in the aorta of AAD. We found increased infiltration of neutrophils and monocytes with the expression of inflammatory cytokines in the aorta and accumulation of inflammatory macrophages before the onset of macroscopic AAD in the murine AAD model. In blocking experiments using an anti-IL-1β antibody, it improved survival of murine AAD model by preventing elastin degradation. We observed the accumulation of inflammatory macrophages expressing IL-1β in both human AAD samples and in a murine AAD model. Anti-IL-1β antibody could improve the mortality rate in mice, suggesting that it may be a treatment option for AAD.
-
-
-
Cell Biology
MicroRNA-7 deficiency ameliorates d-galactose-induced aging in mice by regulating senescence of Kupffer cells.
In Aging Cell on 1 June 2024 by Wang, Y., Qiu, H., et al.
PubMed
Aging is intricately linked to immune system dysfunction. Recent studies have highlighted the biological function of microRNA-7 (miR-7) as a novel regulator of immune cell function and related diseases. However, the potential role of miR-7 in aging remains unexplored. Here, we investigated the contribution of miR-7 to d-gal-induced aging in mice, focusing on its regulation of senescent Kupffer cells. Our findings revealed that miR-7 deficiency significantly ameliorated the aging process, characterized by enhanced CD4+ T-cell activation. However, the adoptive transfer of miR-7-deficient CD4+T cells failed to improve the age-related phenotype. Further analysis showed that miR-7 deficiency significantly reduced IL-1β production in liver tissue, and inhibiting IL-1β in vivo slowed down the aging process in mice. Notably, IL-1β is mainly produced by senescent Kupffer cells in the liver tissue of aging mice, and miR-7 expression was significantly up-regulated in these cells. Mechanistically, KLF4, a target of miR-7, was down-regulated in senescent Kupffer cells in aging mouse model. Furthermore, miR-7 deficiency also modulated the NF-κB activation and IL-1β production in senescent Kupffer cells through KLF4. In conclusion, our findings unveil the role of miR-7 in d-gal-induced aging in mice, highlighting its regulation of KLF4/NF-κB/IL-1β pathways in senescent Kupffer cells. This research may enhance our understanding of miRNA-based aging immune cells and offer new avenues for new intervention strategies in aging process.
-
-
-
Immunology and Microbiology
-
Flow cytometry/Cell sorting
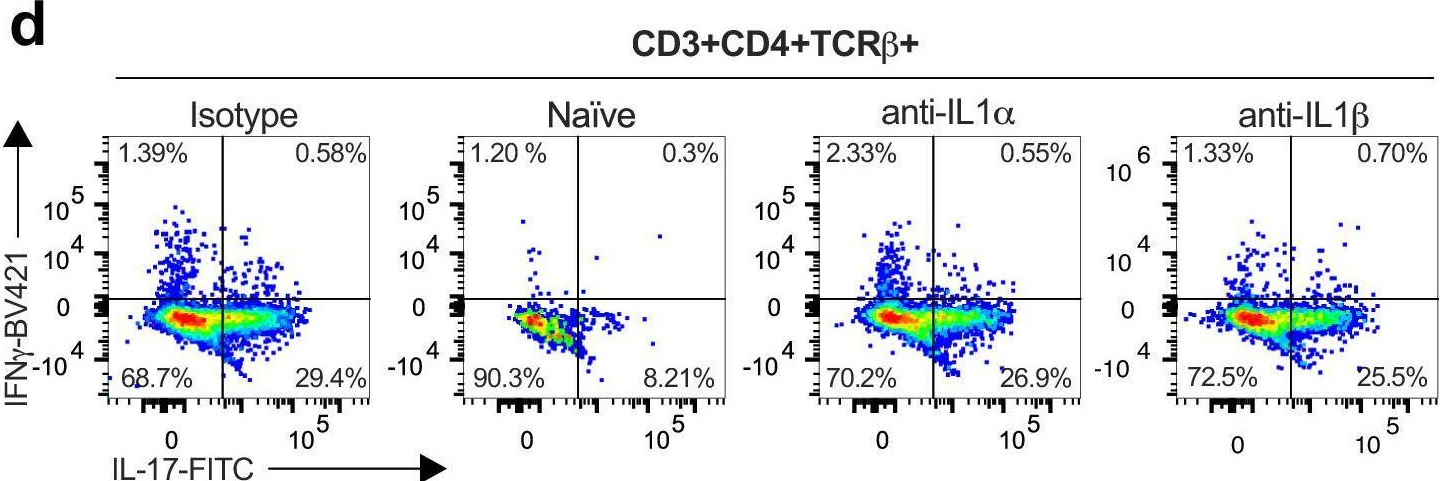
Vaccine-elicited IL-1R signaling results in Th17 TRM-mediated immunity.
In Commun Biol on 9 April 2024 by Hoffmann, J. P., Srivastava, A., et al.
PubMed
Lung tissue resident memory (TRM) cells are thought to play crucial roles in lung host defense. We have recently shown that immunization with the adjuvant LTA1 (derived from the A1 domain of E. coli heat labile toxin) admixed with OmpX from K. pneumoniae can elicit antigen specific lung Th17 TRM cells that provide serotype independent immunity to members of the Enterobacteriaceae family. However, the upstream requirements to generate these cells are unclear. Single-cell RNA-seq showed that vaccine-elicited Th17 TRM cells expressed high levels of IL-1R1, suggesting that IL-1 family members may be critical to generate these cells. Using a combination of genetic and antibody neutralization approaches, we show that Th17 TRM cells can be generated independent of caspase-1 but are compromised when IL-1α is neutralized. Moreover IL-1α could serve as a molecular adjuvant to generate lung Th17 TRM cells independent of LTA1. Taken together, these data suggest that IL-1α plays a major role in vaccine-mediated lung Th17 TRM generation.
-
-
-
Immunology and Microbiology
ZEB1 shapes AML immunological niches, suppressing CD8 T cell activity while fostering Th17 cell expansion.
In Cell Rep on 27 February 2024 by Bassani, B., Simonetti, G., et al.
PubMed
Acute myeloid leukemia (AML) progression is influenced by immune suppression induced by leukemia cells. ZEB1, a critical transcription factor in epithelial-to-mesenchymal transition, demonstrates immune regulatory functions in AML. Silencing ZEB1 in leukemic cells reduces engraftment and extramedullary disease in immune-competent mice, activating CD8 T lymphocytes and limiting Th17 cell expansion. ZEB1 in AML cells directly promotes Th17 cell development that, in turn, creates a self-sustaining loop and a pro-invasive phenotype, favoring transforming growth factor β (TGF-β), interleukin-23 (IL-23), and SOCS2 gene transcription. In bone marrow biopsies from AML patients, immunohistochemistry shows a direct correlation between ZEB1 and Th17. Also, the analysis of ZEB1 expression in larger datasets identifies two distinct AML groups, ZEB1high and ZEB1low, each with specific immunological and molecular traits. ZEB1high patients exhibit increased IL-17, SOCS2, and TGF-β pathways and a negative association with overall survival. This unveils ZEB1's dual role in AML, entwining pro-tumoral and immune regulatory capacities in AML blasts.
-