InVivoMAb anti-mouse IL-6R
Product Description
Specifications
| Isotype | Rat IgG2b, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2b isotype control, anti-keyhole limpet hemocyanin |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | OKT-4 hybridoma cells |
| Reported Applications |
in vivo blocking of IL-6/IL-6R signaling in vitro blocking of IL-6R signaling |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A High Salt |
| RRID | AB_1107588 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo blocking of IL-6/IL-6R signaling
Tsukamoto, H., et al (2015). "IL-6-mediated environmental conditioning of defective Th1 differentiation dampens antitumour immune responses in old age" Nat Commun 6: 6702.
PubMed
Decline in immune function and inflammation concomitantly develop with ageing. Here we focus on the impact of this inflammatory environment on T cells, and demonstrate that in contrast to successful tumour elimination in young mice, replenishment of tumour-specific CD4(+) T cells fails to induce tumour regression in aged hosts. The impaired antitumour effect of CD4(+) T cells with their defective Th1 differentiation in an aged environment is restored by interleukin (IL)-6 blockade or IL-6 deficiency. IL-6 blockade also restores the impaired ability of CD4(+) T cells to promote CD8(+) T-cell-dependent tumour elimination in aged mice, which requires IFN-gamma. Furthermore, IL-6-stimulated production of IL-4/IL-21 through c-Maf induction is responsible for impaired Th1 differentiation. IL-6 also contributes to IL-10 production from CD4(+) T cells in aged mice, causing attenuated responses of CD8(+) T cells. These findings suggest that IL-6 serves as an extrinsic factor counteracting CD4(+) T-cell-mediated immunity against tumour in old age.
in vivo blocking of IL-6/IL-6R signaling
Barber, D. L., et al (2014). "Role of IL-6 in Mycobacterium avium–associated immune reconstitution inflammatory syndrome" J Immunol 192(2): 676-682.
PubMed
Immune reconstitution inflammatory syndrome (IRIS) is a major adverse event of antiretroviral therapy in HIV infection, and paradoxically occurs as HIV viremia is suppressed and CD4 T cell numbers recover. IRIS reflects pathogenic immune responses against opportunistic infections acquired during the period of immunodeficiency, but little is understood about the mechanisms of inflammatory pathology. In this study, we show that IL-6 and C-reactive protein levels transiently rise at the time of the IRIS event in HIV-infected patients, unmasking Mycobacterium avium complex infection after starting antiretroviral therapy. To directly test the role of IL-6 in IRIS pathology, we used a model of experimentally inducible IRIS in which M. avium-infected T cell-deficient mice undergo a fatal inflammatory disease after reconstitution with CD4 T cells. We find that IL-6 neutralization reduces C-reactive protein levels, alleviates wasting disease, and extends host survival during experimental IRIS. Moreover, we show that combined blockade of IL-6 and IFN-gamma further reduces IRIS pathology, even after the onset of wasting disease. The combination of these clinical and experimental-model data show that the IL-6 pathway is not only a biomarker of mycobacterial IRIS but also a major mediator of pathology distinct from IFN-gamma and may be a useful target for therapeutic intervention.
in vivo blocking of IL-6/IL-6R signaling
in vitro blocking of IL-6R signaling
Pham, D., et al (2013). "The transcription factor Twist1 limits T helper 17 and T follicular helper cell development by repressing the gene encoding the interleukin-6 receptor alpha chain" J Biol Chem 288(38): 27423-27433.
PubMed
Cytokine responsiveness is a critical component of the ability of cells to respond to the extracellular milieu. Transcription factor-mediated regulation of cytokine receptor expression is a common mode of altering responses to the external environment. We identify the transcription factor Twist1 as a component of a STAT3-induced feedback loop that controls IL-6 signals by directly repressing Il6ra. Human and mouse T cells lacking Twist1 have an increased ability to differentiate into Th17 cells. Mice with a T cell-specific deletion of Twist1 demonstrate increased Th17 and T follicular helper cell development, early onset experimental autoimmune encephalomyelitis, and increased antigen-specific antibody responses. Thus, Twist1 has a critical role in limiting both cell-mediated and humoral immunity.
in vivo blocking of IL-6/IL-6R signaling
Markey, K. A., et al (2012). "Immune insufficiency during GVHD is due to defective antigen presentation within dendritic cell subsets" Blood 119(24): 5918-5930.
PubMed
Alloreactivity after transplantation is associated with profound immune suppression, and consequent opportunistic infection results in high morbidity and mortality. This immune suppression is most profound during GVHD after bone marrow transplantation where an inflammatory cytokine storm dominates. Contrary to current dogma, which avers that this is a T-cell defect, we demonstrate that the impairment lies within conventional dendritic cells (cDCs). Significantly, exogenous antigens can only be presented by the CD8(-) cDC subset after bone marrow transplantation, and inflammation during GVHD specifically renders the MHC class II presentation pathway in this population incompetent. In contrast, both classic and cross-presentation within MHC class I remain largely intact. Importantly, this defect in antigen processing can be partially reversed by TNF inhibition or the adoptive transfer of donor cDCs generated in the absence of inflammation.
in vitro blocking of IL-6R signaling
Piconese, S., et al (2009). "Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation" Blood 114(13): 2639-2648.
PubMed
The development of inflammatory diseases implies inactivation of regulatory T (Treg) cells through mechanisms that still are largely unknown. Here we showed that mast cells (MCs), an early source of inflammatory mediators, are able to counteract Treg inhibition over effector T cells. To gain insight into the molecules involved in their interplay, we set up an in vitro system in which all 3 cellular components were put in contact. Reversal of Treg suppression required T cell-derived interleukin-6 (IL-6) and the OX40/OX40L axis. In the presence of activated MCs, concomitant abundance of IL-6 and paucity of Th1/Th2 cytokines skewed Tregs and effector T cells into IL-17-producing T cells (Th17). In vivo analysis of lymph nodes hosting T-cell priming in experimental autoimmune encephalomyelitis revealed activated MCs, Tregs, and Th17 cells displaying tight spatial interactions, further supporting the occurrence of an MC-mediated inhibition of Treg suppression in the establishment of Th17-mediated inflammatory responses.
Product Citations
-
-
Stem Cells and Developmental Biology
-
Genetics
Epigenetic dysregulation in aged muscle stem cells drives mesenchymal progenitor expansion via IL-6 and Spp1 signaling.
In Nat Aging on 29 October 2025 by Riparini, G., Mackenzie, M., et al.
PubMed
Sarcopenia, the age-related decline in muscle mass, strength and function, is characterized by impaired muscle homeostasis, reduced regenerative potential of muscle stem cells (MuSCs) and increased fibrosis. Here we report that aged MuSCs can autonomously instruct fibro-adipogenic progenitors (FAPs) to proliferate and acquire a fibrogenic phenotype, independent of other cell types. Both the polycomb-deficient Ezh2-/- mouse model and aged mice exhibited defective regeneration, FAP expansion, fibrosis and elevated secretion of interleukin 6 (IL-6) and secreted phosphoprotein 1 (Spp1; osteopontin) by MuSCs. In aged MuSCs, reduction of the histone H3K27me3 repressive mark at the Nfbk1 gene correlated with its increased expression and enhanced chromatin recruitment to the IL6 and Spp1 genes, leading to their activation. Pharmacological inhibition of IL-6 and Spp1 signaling in co-culture systems or in aged mice reduced FAP proliferation and muscle fibrosis. These findings indicate that epigenetic dysregulation of aged MuSCs contributes to aged-related muscle fibrosis.
-
-
-
Immunology and Microbiology
-
Biochemistry and Molecular biology
A novel modulator of IL-6R prevents inflammation-induced preterm birth and improves newborn outcome.
In EMBO Mol Med on 1 August 2025 by Côté, F., Prairie, E., et al.
PubMed
Preterm birth (PTB) is a major cause of neonatal mortality and morbidity. Evidence supports a determinant role for interleukin-6 (IL-6) in the pathophysiology of PTB. Our group developed a small peptide, HSJ633, that antagonizes the interleukin-6 receptor (IL-6R). Binding assays performed on HEK-Blue IL-6 cells reveal that HSJ633 appears to bind to IL-6R on a site remote from the IL-6 binding domain. Concordantly, HSJ633 selectively inhibits STAT3 phosphorylation while preserving the activation of cytoprotective AKT, p38, and ERK 1/2. In vivo, in a murine model of LPS-induced PTB, HSJ633 reduces inflammation in gestational and fetal tissues, preserves the integrity of fetal organs, and improves the survival of neonatal progeny when administered before and after the induction of labor by an inflammatory stimulus. Relevantly, the pharmacological inhibition of STAT3 in mice is sufficient to prevent PTB. Findings reveal first-in-class efficacy of a small peptide inhibitor of IL-6R, namely HSJ633, in impeding the inflammatory cascade associated with PTB and mitigating adverse neonatal outcomes.
-
-
-
Cancer Research
Evolution of tumor stress response during cytoreductive surgery for ovarian cancer.
In iScience on 16 May 2025 by Praiss, A. M., Moukarzel, L. A., et al.
PubMed
Upfront treatment for patients with advanced high-grade serous ovarian cancer (HGSOC) includes a multi-hour cytoreductive surgery. Although the procedure is necessary for maximal tumor cytoreduction, understanding of the biology of systemic and intratumoral responses induced by surgical cytoreduction is limited. Through analysis of matched tumor and normal tissues and peripheral blood collected at multiple time points during cytoreductive surgery in patients with HGSOC, we demonstrate that surgery leads to rapid induction of systemic inflammatory response and activation of inflammatory signaling in the tumor and normal tissue, with interleukin-6 emerging as a dominant inflammatory pathway. A parallel study in a syngeneic murine HGSOC model recapitulated these findings and demonstrated accelerated tumor growth in response to surgery. This study highlights the previously unappreciated impact of specimen collection timing on the tumor signaling networks and provides insights into stress pathways activated by surgery, generating rationale for perioperative therapeutic interventions to reduce protumorigenic effects.
-
-
-
Cancer Research
-
Immunology and Microbiology
Establishment and validation of an immune-related nomogram for the prognosis of pancreatic adenocarcinoma.
In Sci Rep on 18 April 2025 by Wang, K., Lu, Y., et al.
PubMed
Pancreatic adenocarcinoma (PDAC) is a highly aggressive neoplasm characterized by limited therapeutic options, particularly in the realm of immunotherapy. This study aims to improve prognosis prediction to guide therapeutic decision-making, and to identify novel targets for immunotherapy of PDAC. We conducted Cox and LASSO regression analyses to develop immune-related gene signature and corresponding nomogram, and the robustness of these signatures was demonstrated using multiple approaches. Additionally, CIBERSORT, ESTIMATE, and xCell algorithms were utilized to assess immune cell infiltration, with experimental validation performed though qPCR. An immune-related gene signature consisting of 18 genes, and the prognostic nomogram was established with superior performance compared to the conventional staging system. Key parameters incorporated into the nomogram included the gene signature, tumor stage, and postoperative treatment. Patients identified as high-risk exhibited an anti-inflammatory tumor microenvironment, characterized by an increase in M2-like tumor-associated macrophages and heightened tumor purity. Notably, the expression of interleukin 6 receptor (IL6R) in PDAC was predominantly derived from macrophages and was significantly associated with patient survival outcomes. Furthermore, attenuated IL-6/IL-6R signaling was found to promote M2-like macrophage differentiation. This study successfully established an immune-related gene signature and a robust nomogram for predicting clinical outcomes in patients with PDAC. Furthermore, we identified IL6R as a promising target for future immunotherapeutic strategies.
-
-
Enhanced Interleukin 6 Trans-Signaling Modulates Disease Process in Amyotrophic Lateral Sclerosis Mouse Models.
In Brain Sci on 17 January 2025 by Milligan, C., Cowley, D. O., et al.
PubMed
Background/Objectives: Charcot first described ALS in 1869, but the specific mechanisms that mediate the disease pathology are still not clear. Intense research efforts have provided insight into unique neuroanatomical regions, specific neuronal populations and genetic associations for ALS and other neurodegenerative diseases; however, the experimental results also suggest a convergence of these events to common toxic pathways. We propose that common toxic pathways can be therapeutically targeted, and this intervention will be effective in slowing progression and improving patient quality of life. Here, we focus on understanding the role of IL6 trans-signaling in ALS disease processes. Methods: We leveraged unique mouse models of IL6 trans-signaling that we developed that recapitulate the production of active sIL6R in a genotypic and quantitative fashion observed in humans. Given that the SOD1 transgenic mouse is one of the most highly studied and characterized models of ALS, we bred SOD1G93A mice with IL6R trans-signaling mice to determine how enhanced trans-signaling influenced symptom onset and pathological processes, including neuromuscular junction (NMJ) denervation, glial activation and motoneuron (MN) survival. Results: The results indicate that in animals with enhanced trans-signaling, symptom onset and pathological processes were accelerated, suggesting a role in disease modification. Administration of an IL6R functional blocking antibody failed to alter accelerated symptom onset and disease progression. Conclusions: Future work to investigate the site-specific influence of enhanced IL6 trans-signaling and the tissue-specific bioavailability of potential therapeutics will be necessary to identify targets for precise therapeutic interventions that may limit disease progression in the 60% of ALS patients who inherit the common Il6R Asp358Ala variant.
-
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Targeting cancer-associated fibroblast autophagy renders pancreatic cancer eradicable with immunochemotherapy by inhibiting adaptive immune resistance.
In Autophagy on 1 June 2024 by Zhang, X., Lao, M., et al.
PubMed
Accumulating evidence suggests that cancer-associated fibroblast (CAF) macroautophagy/autophagy is crucial in tumor development and may be a therapeutic target for pancreatic ductal adenocarcinoma (PDAC). However, the role of CAF autophagy during immune surveillance and cancer immunotherapy is unclear. The present study revealed that the inhibition of CAF autophagy suppresses in vivo tumor development in immune-deficient xenografts. This deletion compromises anti-tumor immunity and anti-tumor efficacy both in vitro and in vivo by upregulating CD274/PDL1 levels in an immune-competent mouse model. A block in CAF autophagy reduced the production of IL6 (interleukin 6), disrupting high desmoplastic TME and decreasing USP14 expression at the transcription level in pancreatic cancer cells. We further identify USP14 as the post-translational factor responsible for downregulating CD274 expression by removing K63 linked-ubiquitination at the K280 residue. Finally, chloroquine diphosphate-loaded mesenchymal stem cell (MSC)-liposomes, by accurately targeting CAFs, inhibited CAF autophagy, improving the efficacy of immunochemotherapy to combat pancreatic cancer.Abbreviation: AIR: adaptive immune resistance; ATRA: all-trans-retinoicacid; CAF: cancer-associated fibroblast; CD274/PDL1: CD274 molecule; CM: conditioned medium; CQ: chloroquine diphosphate; CyTOF: Mass cytometry; FGF2/bFGF: fibroblast growth factor 2; ICB: immune checkpoint blockade; IF: immunofluorescence; IHC: immunohistochemistry; IP: immunoprecipitation; MS: mass spectrometer; MSC: mesenchymal stem cell; PDAC: pancreatic ductal adenocarcinoma; TEM: transmission electron microscopy; TILs: tumor infiltrating lymphocytes; TME: tumor microenvironment; USP14: ubiquitin specific peptidase 14.
-
-
-
Mus musculus (Mouse)
-
COVID-19
-
Immunology and Microbiology
-
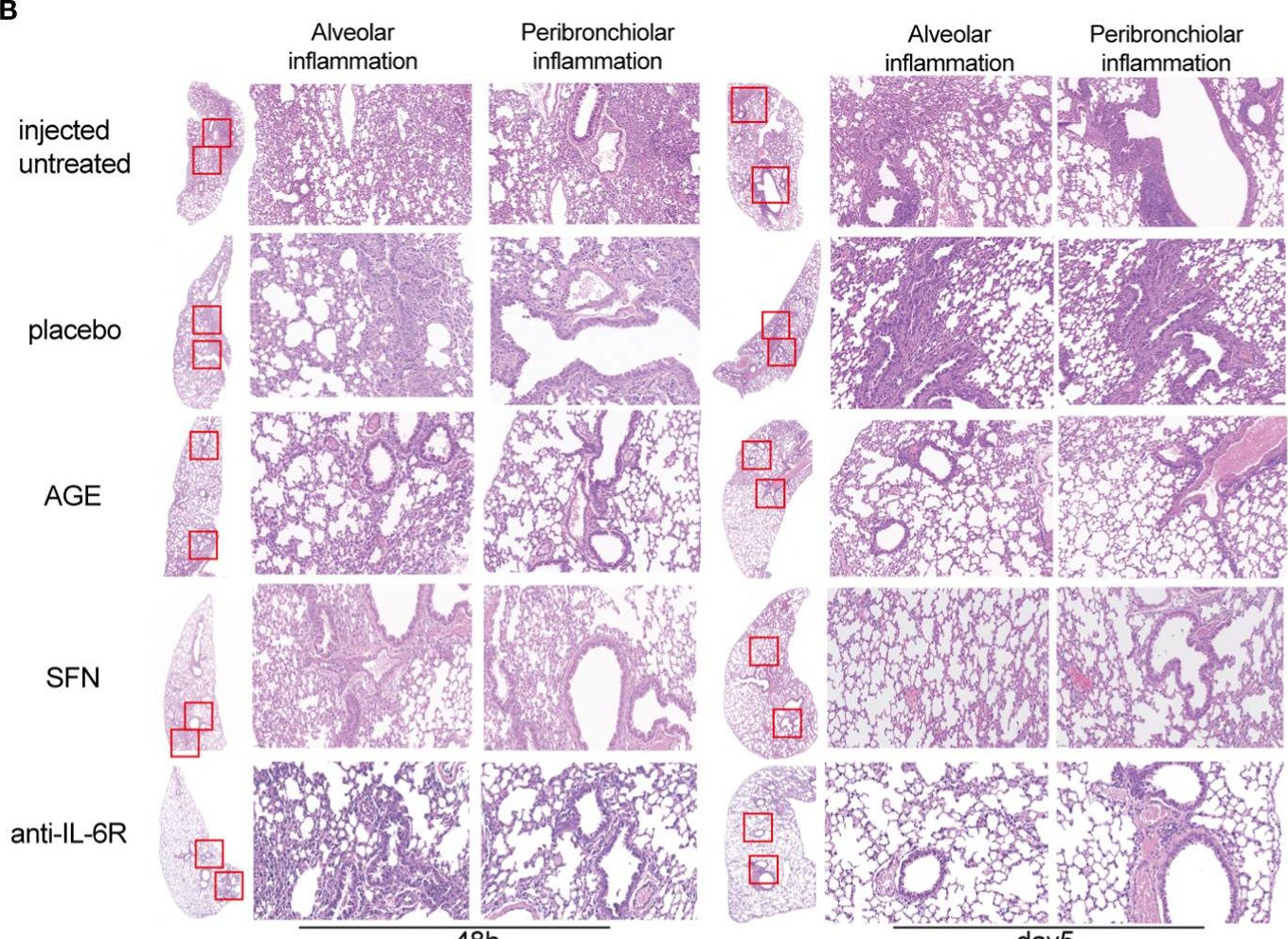
Two new and effective food-extracted immunomodulatory agents exhibit anti-inflammatory response activity in the hACE2 acute lung injury murine model of COVID-19.
In Front Immunol on 29 May 2024 by Liu, S., Wang, B., et al.
PubMed
The coronavirus disease 2019 (COVID-19) spread rapidly and claimed millions of lives worldwide. Acute respiratory distress syndrome (ARDS) is the major cause of COVID-19-associated deaths. Due to the limitations of current drugs, developing effective therapeutic options that can be used rapidly and safely in clinics for treating severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infections is necessary. This study aims to investigate the effects of two food-extracted immunomodulatory agents, ajoene-enriched garlic extract (AGE) and cruciferous vegetables-extracted sulforaphane (SFN), on anti-inflammatory and immune responses in a SARS-CoV-2 acute lung injury mouse model.
-
-
-
Immunology and Microbiology
-
Genetics
-
Endocrinology and Physiology
Small-molecule CBP/p300 histone acetyltransferase inhibition mobilizes leukocytes from the bone marrow via the endocrine stress response.
In Immunity on 13 February 2024 by Jaschke, N. P., Breining, D., et al.
PubMed
Mutations of the CBP/p300 histone acetyltransferase (HAT) domain can be linked to leukemic transformation in humans, suggestive of a checkpoint of leukocyte compartment sizes. Here, we examined the impact of reversible inhibition of this domain by the small-molecule A485. We found that A485 triggered acute and transient mobilization of leukocytes from the bone marrow into the blood. Leukocyte mobilization by A485 was equally potent as, but mechanistically distinct from, granulocyte colony-stimulating factor (G-CSF), which allowed for additive neutrophil mobilization when both compounds were combined. These effects were maintained in models of leukopenia and conferred augmented host defenses. Mechanistically, activation of the hypothalamus-pituitary-adrenal gland (HPA) axis by A485 relayed shifts in leukocyte distribution through corticotropin-releasing hormone receptor 1 (CRHR1) and adrenocorticotropic hormone (ACTH), but independently of glucocorticoids. Our findings identify a strategy for rapid expansion of the blood leukocyte compartment via a neuroendocrine loop, with implications for the treatment of human pathologies.
-
-
-
Mus musculus (Mouse)
Blockade of IL-6R prevents preterm birth and adverse neonatal outcomes.
In EBioMedicine on 1 December 2023 by Farías-Jofré, M., Romero, R., et al.
PubMed
Preterm birth preceded by spontaneous preterm labour often occurs in the clinical setting of sterile intra-amniotic inflammation (SIAI), a condition that currently lacks treatment.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
-
Biochemistry and Molecular biology
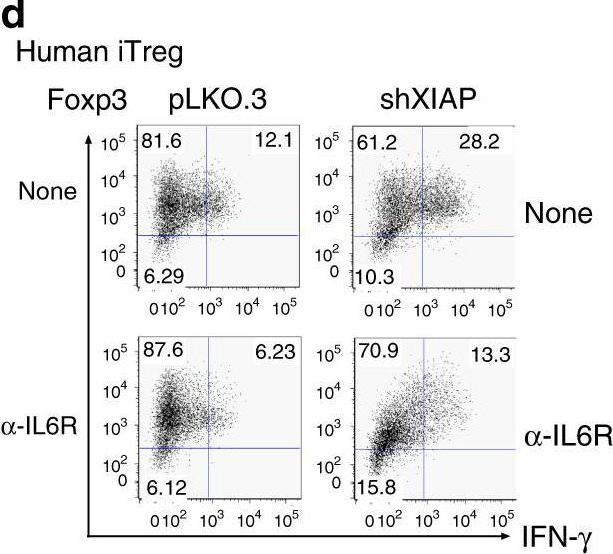
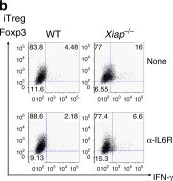
Regulatory T cells require IL6 receptor alpha signaling to control skeletal muscle function and regeneration.
In Cell Metab on 3 October 2023 by Becker, M., Joseph, S. S., et al.
PubMed
Muscle-residing regulatory T cells (Tregs) control local tissue integrity and function. However, the molecular interface connecting Treg-based regulation with muscle function and regeneration remains largely unexplored. Here, we show that exercise fosters a stable induction of highly functional muscle-residing Tregs with increased expression of amphiregulin (Areg), EGFR, and ST2. Mechanistically, we find that mice lacking IL6Rα on T cells (TKO) harbor significant reductions in muscle Treg functionality and satellite and fibro-adipogenic progenitor cells, which are required for muscle regeneration. Using exercise and sarcopenia models, IL6Rα TKO mice demonstrate deficits in Tregs, their functional maturation, and a more pronounced decline in muscle mass. Muscle injury models indicate that IL6Rα TKO mice have significant disabilities in muscle regeneration. Treg gain of function restores impaired muscle repair in IL6Rα TKO mice. Of note, pharmacological IL6R blockade in WT mice phenocopies deficits in muscle function identified in IL6Rα TKO mice, thereby highlighting the clinical implications of the findings.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
TLR7 activation at epithelial barriers promotes emergency myelopoiesis and lung antiviral immunity.
In Elife on 11 August 2023 by Jackson, W. D., Giacomassi, C., et al.
PubMed
Monocytes are heterogeneous innate effector leukocytes generated in the bone marrow and released into circulation in a CCR2-dependent manner. During infection or inflammation, myelopoiesis is modulated to rapidly meet the demand for more effector cells. Danger signals from peripheral tissues can influence this process. Herein we demonstrate that repetitive TLR7 stimulation via the epithelial barriers drove a potent emergency bone marrow monocyte response in mice. This process was unique to TLR7 activation and occurred independently of the canonical CCR2 and CX3CR1 axes or prototypical cytokines. The monocytes egressing the bone marrow had an immature Ly6C-high profile and differentiated into vascular Ly6C-low monocytes and tissue macrophages in multiple organs. They displayed a blunted cytokine response to further TLR7 stimulation and reduced lung viral load after RSV and influenza virus infection. These data provide insights into the emergency myelopoiesis likely to occur in response to the encounter of single-stranded RNA viruses at barrier sites.
-
-
Blockade of IL-6 signaling alleviates atherosclerosis in Tet2-deficient clonal hematopoiesis.
In Nat Cardiovasc Res on 1 June 2023 by Liu, W., Yalcinkaya, M., et al.
PubMed
Clonal hematopoiesis (CH) increases the risk of atherosclerotic cardiovascular disease possibly due to increased plaque inflammation. Human studies suggest that limitation of interleukin-6 (IL-6) signaling could be beneficial in people with large CH clones, particularly in TET2 CH. Here we show that IL-6 receptor antibody treatment reverses the atherosclerosis promoted by Tet2 CH, with reduction of monocytosis, lesional macrophage burden and macrophage colony-stimulating factor 1 receptor (CSF1R) expression. IL-6 induces expression of Csf1r in Tet2-deficient macrophages through enhanced STAT3 binding to its promoter. In mouse and human Tet2-deficient macrophages, IL-6 increases CSF1R expression and enhances macrophage survival. Treatment with the CSF1R inhibitor PLX3397 reversed accelerated atherosclerosis in Tet2 CH mice. Our study demonstrates the causality of IL-6 signaling in Tet2 CH accelerated atherosclerosis, identifies IL-6-induced CSF1R expression as a critical mechanism and supports blockade of IL-6 signaling as a potential therapy for CH-driven cardiovascular disease.
-
-
Immunology and Microbiology
-
Cancer Research
STAT3 gain-of-function mutations connect leukemia with autoimmune disease by pathological NKG2Dhi CD8+ T cell dysregulation and accumulation.
In Immunity on 13 December 2022 by Masle-Farquhar, E., Jackson, K. J. L., et al.
PubMed
The association between cancer and autoimmune disease is unexplained, exemplified by T cell large granular lymphocytic leukemia (T-LGL) where gain-of-function (GOF) somatic STAT3 mutations correlate with co-existing autoimmunity. To investigate whether these mutations are the cause or consequence of CD8+ T cell clonal expansions and autoimmunity, we analyzed patients and mice with germline STAT3 GOF mutations. STAT3 GOF mutations drove the accumulation of effector CD8+ T cell clones highly expressing NKG2D, the receptor for stress-induced MHC-class-I-related molecules. This subset also expressed genes for granzymes, perforin, interferon-γ, and Ccl5/Rantes and required NKG2D and the IL-15/IL-2 receptor IL2RB for maximal accumulation. Leukocyte-restricted STAT3 GOF was sufficient and CD8+ T cells were essential for lethal pathology in mice. These results demonstrate that STAT3 GOF mutations cause effector CD8+ T cell oligoclonal accumulation and that these rogue cells contribute to autoimmune pathology, supporting the hypothesis that somatic mutations in leukemia/lymphoma driver genes contribute to autoimmune disease.
-
-
-
Mus musculus (Mouse)
-
Neuroscience
Interleukin-6 trans-signalling in hippocampal CA1 neurones mediates perioperative neurocognitive disorders in mice.
In Br J Anaesth on 1 December 2022 by Hu, J., Zhang, Y., et al.
PubMed
Interleukin-6 (IL-6), a pleiotropic cytokine with both degenerative and regenerative properties, is necessary and sufficient to provoke perioperative neurocognitive disorders after aseptic trauma in mice. IL-6 initiates its actions after binding to either membrane-bound IL-6 receptor α (mIL-6Rα) through classical signalling, or soluble IL-6 receptor (IL-6R) through trans-signalling; both signalling pathways require the transducer gp130. We investigated the site and type of IL-6 signalling that pertains in a tibial fracture aseptic trauma model of perioperative neurocognitive disorder.
-
-
-
Cancer Research
-
Neuroscience
Astrocyte immunometabolic regulation of the tumour microenvironment drives glioblastoma pathogenicity.
In Brain on 14 September 2022 by Perelroizen, R., Philosof, B., et al.
PubMed
Malignant brain tumours are the cause of a disproportionate level of morbidity and mortality among cancer patients, an unfortunate statistic that has remained constant for decades. Despite considerable advances in the molecular characterization of these tumours, targeting the cancer cells has yet to produce significant advances in treatment. An alternative strategy is to target cells in the glioblastoma microenvironment, such as tumour-associated astrocytes. Astrocytes control multiple processes in health and disease, ranging from maintaining the brain's metabolic homeostasis, to modulating neuroinflammation. However, their role in glioblastoma pathogenicity is not well understood. Here we report that depletion of reactive astrocytes regresses glioblastoma and prolongs mouse survival. Analysis of the tumour-associated astrocyte translatome revealed astrocytes initiate transcriptional programmes that shape the immune and metabolic compartments in the glioma microenvironment. Specifically, their expression of CCL2 and CSF1 governs the recruitment of tumour-associated macrophages and promotes a pro-tumourigenic macrophage phenotype. Concomitantly, we demonstrate that astrocyte-derived cholesterol is key to glioma cell survival, and that targeting astrocytic cholesterol efflux, via ABCA1, halts tumour progression. In summary, astrocytes control glioblastoma pathogenicity by reprogramming the immunological properties of the tumour microenvironment and supporting the non-oncogenic metabolic dependency of glioblastoma on cholesterol. These findings suggest that targeting astrocyte immunometabolic signalling may be useful in treating this uniformly lethal brain tumour.
-
-
-
Cancer Research
-
Immunology and Microbiology
Selective suppression of melanoma lacking IFN-γ pathway by JAK inhibition depends on T cells and host TNF signaling.
In Nat Commun on 25 August 2022 by Shen, H., Huang, F., et al.
PubMed
Therapeutic resistance to immune checkpoint blockers (ICBs) in melanoma patients is a pressing issue, of which tumor loss of IFN-γ signaling genes is a major underlying mechanism. However, strategies of overcoming this resistance mechanism have been largely elusive. Moreover, given the indispensable role of tumor-infiltrating T cells (TILs) in ICBs, little is known about how tumor-intrinsic loss of IFN-γ signaling (IFNγR1KO) impacts TILs. Here, we report that IFNγR1KO melanomas have reduced infiltration and function of TILs. IFNγR1KO melanomas harbor a network of constitutively active protein tyrosine kinases centered on activated JAK1/2. Mechanistically, JAK1/2 activation is mediated by augmented mTOR. Importantly, JAK1/2 inhibition with Ruxolitinib selectively suppresses the growth of IFNγR1KO but not scrambled control melanomas, depending on T cells and host TNF. Together, our results reveal an important role of tumor-intrinsic IFN-γ signaling in shaping TILs and manifest a targeted therapy to bypass ICB resistance of melanomas defective of IFN-γ signaling.
-
-
Interleukin-6-dependent epithelial fluidization initiates fibrotic lung remodeling.
In Sci Transl Med on 20 July 2022 by Stancil, I. T., Michalski, J. E., et al.
PubMed
Chronic disease results from the failure of tissues to maintain homeostasis. In the lung, coordinated repair of the epithelium is essential for preserving homeostasis. In animal models and human lung disease, airway epithelial cells mobilize in response to lung injury, resulting in the formation of airway-like cysts with persistent loss of functional cell types and parenchymal architecture. Using live-cell imaging of human lung epithelial cultures and mouse precision-cut lung slices, we demonstrated that distal airway epithelia are aberrantly fluidized both after injury and in fibrotic lung disease. Through transcriptomic profiling and pharmacologic stimulation of epithelial cultures, we identified interleukin-6 (IL-6) signaling as a driver of tissue fluidization. This signaling cascade occurred independently of canonical Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling but instead was dependent on a downstream SRC family kinase (SFK)-yes-associated protein (YAP) axis. Airway epithelial-fibroblast cocultures revealed that the fibrotic mesenchyme acts as a source of IL-6 family cytokines, which drive airway fluidization. Inhibition of the IL-6-SFK-YAP cascade was sufficient to prevent fluidization in both in vitro and ex vivo models. Last, we demonstrated a reduction in fibrotic lung remodeling in mice through genetic or pharmacologic targeting of IL-6-related signaling. Together, our findings illustrate the critical role of airway epithelial fluidization in coordinating the balance between homeostatic lung repair and fibrotic airspace remodeling.
-
-
Immunology and Microbiology
Single-cell analysis identifies the interaction of altered renal tubules with basophils orchestrating kidney fibrosis.
In Nat Immunol on 1 June 2022 by Doke, T., Abedini, A., et al.
PubMed
Inflammation is an important component of fibrosis but immune processes that orchestrate kidney fibrosis are not well understood. Here we apply single-cell sequencing to a mouse model of kidney fibrosis. We identify a subset of kidney tubule cells with a profibrotic-inflammatory phenotype characterized by the expression of cytokines and chemokines associated with immune cell recruitment. Receptor-ligand interaction analysis and experimental validation indicate that CXCL1 secreted by profibrotic tubules recruits CXCR2+ basophils. In mice, these basophils are an important source of interleukin-6 and recruitment of the TH17 subset of helper T cells. Genetic deletion or antibody-based depletion of basophils results in reduced renal fibrosis. Human kidney single-cell, bulk gene expression and immunostaining validate a function for basophils in patients with kidney fibrosis. Collectively, these studies identify basophils as contributors to the development of renal fibrosis and suggest that targeting these cells might be a useful clinical strategy to manage chronic kidney disease.
-
-
-
Neuroscience
-
Mus musculus (Mouse)
An Intercellular Flow of Glutathione Regulated by Interleukin 6 Links Astrocytes and the Liver in the Pathophysiology of Amyotrophic Lateral Sclerosis.
In Antioxidants (Basel) on 16 December 2021 by López-Blanch, R., Salvador, R., et al.
PubMed
Oxidative stress has been proposed as a major mechanism of damage to motor neurons associated with the progression of amyotrophic lateral sclerosis (ALS). Astrocytes are the most numerous glial cells in the central nervous system and, under physiological conditions, protect neurons from oxidative damage. However, it is uncertain how their reactive phenotype may affect motor neurons during ALS progression. In two different ALS mouse models (SOD1G93A and FUS-R521C), we found that increased levels of proinflammatory interleukin 6 facilitate glutathione (GSH) release from the liver to blood circulation, which can reach the astrocytes and be channeled towards motor neurons as a mechanism of antioxidant protection. Nevertheless, although ALS progression is associated with an increase in GSH efflux from astrocytes, generation of reactive oxygen species also increases, suggesting that as the disease progresses, astrocyte-derived oxidative stress could be key to motor-neuron damage.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Microenvironmental IL-6 inhibits anti-cancer immune responses generated by cytotoxic chemotherapy.
In Nat Commun on 28 October 2021 by Bent, E. H., Millán-Barea, L. R., et al.
PubMed
Cytotoxic chemotherapeutics primarily function through DNA damage-induced tumor cell apoptosis, although the inflammation provoked by these agents can stimulate anti-cancer immune responses. The mechanisms that control these distinct effects and limit immunogenic responses to DNA-damage mediated cell death in vivo are currently unclear. Using a mouse model of BCR-ABL+ B-cell acute lymphoblastic leukemia, we show that chemotherapy-induced anti-cancer immunity is suppressed by the tumor microenvironment through production of the cytokine IL-6. The chemotherapeutic doxorubicin is curative in IL-6-deficient mice through the induction of CD8+ T-cell-mediated anti-cancer responses, while moderately extending lifespan in wild type tumor-bearing mice. We also show that IL-6 suppresses the effectiveness of immune-checkpoint inhibition with anti-PD-L1 blockade. Our results suggest that IL-6 is a key regulator of anti-cancer immune responses induced by genotoxic stress and that its inhibition can switch cancer cell clearance from primarily apoptotic to immunogenic, promoting and maintaining durable anti-tumor immune responses.
-