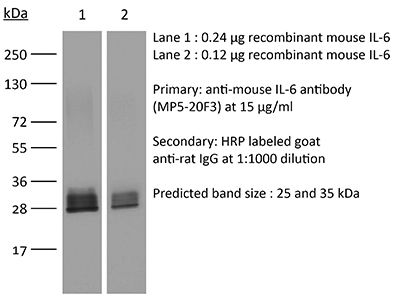
InVivoMAb anti-mouse IL-6
Product Description
Specifications
| Isotype | Rat IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG1 isotype control, anti-horseradish peroxidase |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Recombinant mouse IL-6 |
| Reported Applications |
in vivo IL-6 neutralization in vitro IL-6 neutralization |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107709 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo IL-6 neutralization
Benevides, L., et al (2015). "IL17 Promotes Mammary Tumor Progression by Changing the Behavior of Tumor Cells and Eliciting Tumorigenic Neutrophils Recruitment" Cancer Res 75(18): 3788-3799.
PubMed
The aggressiveness of invasive ductal carcinoma (IDC) of the breast is associated with increased IL17 levels. Studying the role of IL17 in invasive breast tumor pathogenesis, we found that metastatic primary tumor-infiltrating T lymphocytes produced elevated levels of IL17, whereas IL17 neutralization inhibited tumor growth and prevented the migration of neutrophils and tumor cells to secondary disease sites. Tumorigenic neutrophils promote disease progression, producing CXCL1, MMP9, VEGF, and TNFalpha, and their depletion suppressed tumor growth. IL17A also induced IL6 and CCL20 production in metastatic tumor cells, favoring the recruitment and differentiation of Th17. In addition, IL17A changed the gene-expression profile and the behavior of nonmetastatic tumor cells, causing tumor growth in vivo, confirming the protumor role of IL17. Furthermore, high IL17 expression was associated with lower disease-free survival and worse prognosis in IDC patients. Thus, IL17 blockade represents an attractive approach for the control of invasive breast tumors. Cancer Res; 75(18); 3788-99. (c)2015 AACR.
in vivo IL-6 neutralization
Tsukamoto, H., et al (2015). "IL-6-mediated environmental conditioning of defective Th1 differentiation dampens antitumour immune responses in old age" Nat Commun 6: 6702.
PubMed
Decline in immune function and inflammation concomitantly develop with ageing. Here we focus on the impact of this inflammatory environment on T cells, and demonstrate that in contrast to successful tumour elimination in young mice, replenishment of tumour-specific CD4(+) T cells fails to induce tumour regression in aged hosts. The impaired antitumour effect of CD4(+) T cells with their defective Th1 differentiation in an aged environment is restored by interleukin (IL)-6 blockade or IL-6 deficiency. IL-6 blockade also restores the impaired ability of CD4(+) T cells to promote CD8(+) T-cell-dependent tumour elimination in aged mice, which requires IFN-gamma. Furthermore, IL-6-stimulated production of IL-4/IL-21 through c-Maf induction is responsible for impaired Th1 differentiation. IL-6 also contributes to IL-10 production from CD4(+) T cells in aged mice, causing attenuated responses of CD8(+) T cells. These findings suggest that IL-6 serves as an extrinsic factor counteracting CD4(+) T-cell-mediated immunity against tumour in old age.
in vivo IL-6 neutralization
Liang, Y., et al (2015). "Innate lymphotoxin receptor mediated signaling promotes HSV-1 associated neuroinflammation and viral replication" Sci Rep 5: 10406.
PubMed
Host anti-viral innate immunity plays important roles in the defense against HSV-1 infection. In this study, we find an unexpected role for innate LT/LIGHT signaling in promoting HSV-1 replication and virus induced inflammation in immunocompromised mice. Using a model of footpad HSV-1 infection in Rag1(-/-) mice, we observed that blocking LT/LIGHT signaling with LTbetaR-Ig could significantly delay disease progression and extend the survival of infected mice. LTbetaR-Ig treatment reduced late proinflammatory cytokine release in the serum and nervous tissue, and inhibited chemokine expression and inflammatory cells infiltration in the dorsal root ganglia (DRG). Intriguingly, LTbetaR-Ig treatment restricted HSV-1 replication in the DRG but not the footpad. These findings demonstrate a critical role for LT/LIGHT signaling in modulating innate inflammation and promoting HSV-1 replication in the nervous system, and suggest a new target for treatment of virus-induced adverse immune response and control of severe HSV-1 infection.
in vivo IL-6 neutralization
Liang, Y., et al (2015). "Innate lymphotoxin receptor mediated signaling promotes HSV-1 associated neuroinflammation and viral replication" Sci Rep 5: 10406.
PubMed
Host anti-viral innate immunity plays important roles in the defense against HSV-1 infection. In this study, we find an unexpected role for innate LT/LIGHT signaling in promoting HSV-1 replication and virus induced inflammation in immunocompromised mice. Using a model of footpad HSV-1 infection in Rag1(-/-) mice, we observed that blocking LT/LIGHT signaling with LTbetaR-Ig could significantly delay disease progression and extend the survival of infected mice. LTbetaR-Ig treatment reduced late proinflammatory cytokine release in the serum and nervous tissue, and inhibited chemokine expression and inflammatory cells infiltration in the dorsal root ganglia (DRG). Intriguingly, LTbetaR-Ig treatment restricted HSV-1 replication in the DRG but not the footpad. These findings demonstrate a critical role for LT/LIGHT signaling in modulating innate inflammation and promoting HSV-1 replication in the nervous system, and suggest a new target for treatment of virus-induced adverse immune response and control of severe HSV-1 infection.
in vivo IL-6 neutralization
Tsukamoto, H., et al (2015). "IL-6-mediated environmental conditioning of defective Th1 differentiation dampens antitumour immune responses in old age" Nat Commun 6: 6702.
PubMed
Decline in immune function and inflammation concomitantly develop with ageing. Here we focus on the impact of this inflammatory environment on T cells, and demonstrate that in contrast to successful tumour elimination in young mice, replenishment of tumour-specific CD4(+) T cells fails to induce tumour regression in aged hosts. The impaired antitumour effect of CD4(+) T cells with their defective Th1 differentiation in an aged environment is restored by interleukin (IL)-6 blockade or IL-6 deficiency. IL-6 blockade also restores the impaired ability of CD4(+) T cells to promote CD8(+) T-cell-dependent tumour elimination in aged mice, which requires IFN-gamma. Furthermore, IL-6-stimulated production of IL-4/IL-21 through c-Maf induction is responsible for impaired Th1 differentiation. IL-6 also contributes to IL-10 production from CD4(+) T cells in aged mice, causing attenuated responses of CD8(+) T cells. These findings suggest that IL-6 serves as an extrinsic factor counteracting CD4(+) T-cell-mediated immunity against tumour in old age.
in vivo IL-6 neutralization
Benevides, L., et al (2015). "IL17 Promotes Mammary Tumor Progression by Changing the Behavior of Tumor Cells and Eliciting Tumorigenic Neutrophils Recruitment" Cancer Res 75(18): 3788-3799.
PubMed
The aggressiveness of invasive ductal carcinoma (IDC) of the breast is associated with increased IL17 levels. Studying the role of IL17 in invasive breast tumor pathogenesis, we found that metastatic primary tumor-infiltrating T lymphocytes produced elevated levels of IL17, whereas IL17 neutralization inhibited tumor growth and prevented the migration of neutrophils and tumor cells to secondary disease sites. Tumorigenic neutrophils promote disease progression, producing CXCL1, MMP9, VEGF, and TNFalpha, and their depletion suppressed tumor growth. IL17A also induced IL6 and CCL20 production in metastatic tumor cells, favoring the recruitment and differentiation of Th17. In addition, IL17A changed the gene-expression profile and the behavior of nonmetastatic tumor cells, causing tumor growth in vivo, confirming the protumor role of IL17. Furthermore, high IL17 expression was associated with lower disease-free survival and worse prognosis in IDC patients. Thus, IL17 blockade represents an attractive approach for the control of invasive breast tumors. Cancer Res; 75(18); 3788-99. (c)2015 AACR.
in vivo IL-6 neutralization
Khmaladze, I., et al (2014). "Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice" Proc Natl Acad Sci U S A 111(35): E3669-3678.
PubMed
Psoriasis (Ps) and psoriasis arthritis (PsA) are poorly understood common diseases, induced by unknown environmental factors, affecting skin and articular joints. A single i.p. exposure to mannan from Saccharomyces cerevisiae induced an acute inflammation in inbred mouse strains resembling human Ps and PsA-like disease, whereas multiple injections induced a relapsing disease. Exacerbation of disease severity was observed in mice deficient for generation of reactive oxygen species (ROS). Interestingly, restoration of ROS production, specifically in macrophages, ameliorated both skin and joint disease. Neutralization of IL-17A, mainly produced by gammadelta T cells, completely blocked disease symptoms. Furthermore, mice depleted of granulocytes were resistant to disease development. In contrast, certain acute inflammatory mediators (C5, Fcgamma receptor III, mast cells, and histamine) and adaptive immune players (alphabeta T and B cells) were redundant in disease induction. Hence, we propose that mannan-induced activation of macrophages leads to TNF-alpha secretion and stimulation of local gammadelta T cells secreting IL-17A. The combined action of activated macrophages and IL-17A produced in situ drives neutrophil infiltration in the epidermis and dermis of the skin, leading to disease manifestations. Thus, our finding suggests a new mechanism triggered by exposure to exogenous microbial components, such as mannan, that can induce and exacerbate Ps and PsA.
in vitro IL-6 neutralization
Jose, S., et al (2014). "Mesenchymal stem cells exert anti-proliferative effect on lipopolysaccharide-stimulated BV2 microglia by reducing tumour necrosis factor-alpha levels" J Neuroinflammation 11: 149.
PubMed
BACKGROUND: Progression of neurodegenerative diseases occurs when microglia, upon persistent activation, perpetuate a cycle of damage in the central nervous system. Use of mesenchymal stem cells (MSC) has been suggested as an approach to manage microglia activation based on their immunomodulatory functions. In the present study, we describe the mechanism through which bone marrow-derived MSC modulate the proliferative responses of lipopolysaccharide-stimulated BV2 microglia. METHODS: BV2 microglia were cultured with MSC and stimulated with 1 mug/ml lipopolysaccharide. Using an inducible nitric oxide synthase inhibitor, tritiated thymidine (3H-TdR) incorporation assay was performed to determine the role of nitric oxide in the anti-proliferative effect of MSC. We also studied apoptosis and the cell cycle of both cell types using flow cytometry and explored their cytokine profile using protein and cytometric arrays. Moreover, the role of IL-6 and TNF-alpha in immunomodulation was deduced using specific blocking antibodies and recombinant proteins. RESULTS: MSC reduces microglia proliferation upon lipopolysaccharide stimulation by 21 to 28% and modulates the levels of nitric oxide, IL-6 and TNF-alpha. The role of nitric oxide in conferring the anti-proliferative effect of MSC was ruled out. Furthermore, we found that MSC exert their anti-proliferative effect by restoring the percentage of BV2 cells at S and G2/M phase to levels similar to unstimulated cells. MSC undergo a G0/G1 arrest while exerting this effect. We have also identified that MSC-mediated modulation of microglia is independent of IL-6, whilst reduction of TNF-alpha in co-culture is critical for inhibition of microglia proliferation. CONCLUSIONS: Our study demonstrates that MSC inhibit microglia proliferation independent of nitric oxide and IL-6, although reduction of TNF-alpha is critical for this effect. The inhibition of proliferation is through cell cycle modulation. These findings shed light on the mechanisms of microglial immunomodulation by MSC.
in vivo IL-6 neutralization
Hock, K., et al (2014). "Donor CD4 T cells trigger costimulation blockade-resistant donor bone marrow rejection through bystander activation requiring IL-6" Am J Transplant 14(9): 2011-2022.
PubMed
Bone marrow (BM) transplantation under costimulation blockade induces chimerism and tolerance. Cotransplantation of donor T cells (contained in substantial numbers in mobilized peripheral blood stem cells and donor lymphocyte infusions) together with donor BM paradoxically triggers rejection of donor BM through undefined mechanisms. Here, nonmyeloablatively irradiated C57BL/6 recipients simultaneously received donor BM (BALB/c) and donor T cells under costimulation blockade (anti-CD154 and CTLA4Ig). Donor CD4, but not CD8 cells, triggered natural killer-independent donor BM rejection which was associated with increased production of IL-6, interferon gamma (IFN-gamma) and IL-17A. BM rejection was prevented through neutralization of IL-6, but not of IFN-gamma or IL-17A. IL-6 counteracted the antiproliferative effect of anti-CD154 in vitro. Rapamycin and anti-lymphocyte function-associated antigen 1 negated this effect of IL-6 in vitro and prevented BM rejection in vivo. Simultaneous cotransplantation of (BALB/cxB6)F1, recipient or irradiated donor CD4 cells, or late transfer of donor CD4 cells did not lead to BM rejection, whereas cotransplantation of third party CD4 cells did. Transferred donor CD4 cells became activated, rapidly underwent apoptosis and triggered activation and proliferation of recipient T cells. Collectively, these results provide evidence that donor T cells recognizing the recipient as allogeneic lead to the release of IL-6, which abolishes the effect of anti-CD154, triggering donor BM rejection through bystander activation.
in vivo IL-6 neutralization
Barber, D. L., et al (2014). "Role of IL-6 in Mycobacterium avium–associated immune reconstitution inflammatory syndrome" J Immunol 192(2): 676-682.
PubMed
Immune reconstitution inflammatory syndrome (IRIS) is a major adverse event of antiretroviral therapy in HIV infection, and paradoxically occurs as HIV viremia is suppressed and CD4 T cell numbers recover. IRIS reflects pathogenic immune responses against opportunistic infections acquired during the period of immunodeficiency, but little is understood about the mechanisms of inflammatory pathology. In this study, we show that IL-6 and C-reactive protein levels transiently rise at the time of the IRIS event in HIV-infected patients, unmasking Mycobacterium avium complex infection after starting antiretroviral therapy. To directly test the role of IL-6 in IRIS pathology, we used a model of experimentally inducible IRIS in which M. avium-infected T cell-deficient mice undergo a fatal inflammatory disease after reconstitution with CD4 T cells. We find that IL-6 neutralization reduces C-reactive protein levels, alleviates wasting disease, and extends host survival during experimental IRIS. Moreover, we show that combined blockade of IL-6 and IFN-gamma further reduces IRIS pathology, even after the onset of wasting disease. The combination of these clinical and experimental-model data show that the IL-6 pathway is not only a biomarker of mycobacterial IRIS but also a major mediator of pathology distinct from IFN-gamma and may be a useful target for therapeutic intervention.
in vivo IL-6 neutralization
Hock, K., et al (2014). "Donor CD4 T cells trigger costimulation blockade-resistant donor bone marrow rejection through bystander activation requiring IL-6" Am J Transplant 14(9): 2011-2022.
PubMed
Bone marrow (BM) transplantation under costimulation blockade induces chimerism and tolerance. Cotransplantation of donor T cells (contained in substantial numbers in mobilized peripheral blood stem cells and donor lymphocyte infusions) together with donor BM paradoxically triggers rejection of donor BM through undefined mechanisms. Here, nonmyeloablatively irradiated C57BL/6 recipients simultaneously received donor BM (BALB/c) and donor T cells under costimulation blockade (anti-CD154 and CTLA4Ig). Donor CD4, but not CD8 cells, triggered natural killer-independent donor BM rejection which was associated with increased production of IL-6, interferon gamma (IFN-gamma) and IL-17A. BM rejection was prevented through neutralization of IL-6, but not of IFN-gamma or IL-17A. IL-6 counteracted the antiproliferative effect of anti-CD154 in vitro. Rapamycin and anti-lymphocyte function-associated antigen 1 negated this effect of IL-6 in vitro and prevented BM rejection in vivo. Simultaneous cotransplantation of (BALB/cxB6)F1, recipient or irradiated donor CD4 cells, or late transfer of donor CD4 cells did not lead to BM rejection, whereas cotransplantation of third party CD4 cells did. Transferred donor CD4 cells became activated, rapidly underwent apoptosis and triggered activation and proliferation of recipient T cells. Collectively, these results provide evidence that donor T cells recognizing the recipient as allogeneic lead to the release of IL-6, which abolishes the effect of anti-CD154, triggering donor BM rejection through bystander activation.
in vitro IL-6 neutralization
Jose, S., et al (2014). "Mesenchymal stem cells exert anti-proliferative effect on lipopolysaccharide-stimulated BV2 microglia by reducing tumour necrosis factor-alpha levels" J Neuroinflammation 11: 149.
PubMed
BACKGROUND: Progression of neurodegenerative diseases occurs when microglia, upon persistent activation, perpetuate a cycle of damage in the central nervous system. Use of mesenchymal stem cells (MSC) has been suggested as an approach to manage microglia activation based on their immunomodulatory functions. In the present study, we describe the mechanism through which bone marrow-derived MSC modulate the proliferative responses of lipopolysaccharide-stimulated BV2 microglia. METHODS: BV2 microglia were cultured with MSC and stimulated with 1 mug/ml lipopolysaccharide. Using an inducible nitric oxide synthase inhibitor, tritiated thymidine (3H-TdR) incorporation assay was performed to determine the role of nitric oxide in the anti-proliferative effect of MSC. We also studied apoptosis and the cell cycle of both cell types using flow cytometry and explored their cytokine profile using protein and cytometric arrays. Moreover, the role of IL-6 and TNF-alpha in immunomodulation was deduced using specific blocking antibodies and recombinant proteins. RESULTS: MSC reduces microglia proliferation upon lipopolysaccharide stimulation by 21 to 28% and modulates the levels of nitric oxide, IL-6 and TNF-alpha. The role of nitric oxide in conferring the anti-proliferative effect of MSC was ruled out. Furthermore, we found that MSC exert their anti-proliferative effect by restoring the percentage of BV2 cells at S and G2/M phase to levels similar to unstimulated cells. MSC undergo a G0/G1 arrest while exerting this effect. We have also identified that MSC-mediated modulation of microglia is independent of IL-6, whilst reduction of TNF-alpha in co-culture is critical for inhibition of microglia proliferation. CONCLUSIONS: Our study demonstrates that MSC inhibit microglia proliferation independent of nitric oxide and IL-6, although reduction of TNF-alpha is critical for this effect. The inhibition of proliferation is through cell cycle modulation. These findings shed light on the mechanisms of microglial immunomodulation by MSC.
in vivo IL-6 neutralization
Barber, D. L., et al (2014). "Role of IL-6 in Mycobacterium avium–associated immune reconstitution inflammatory syndrome" J Immunol 192(2): 676-682.
PubMed
Immune reconstitution inflammatory syndrome (IRIS) is a major adverse event of antiretroviral therapy in HIV infection, and paradoxically occurs as HIV viremia is suppressed and CD4 T cell numbers recover. IRIS reflects pathogenic immune responses against opportunistic infections acquired during the period of immunodeficiency, but little is understood about the mechanisms of inflammatory pathology. In this study, we show that IL-6 and C-reactive protein levels transiently rise at the time of the IRIS event in HIV-infected patients, unmasking Mycobacterium avium complex infection after starting antiretroviral therapy. To directly test the role of IL-6 in IRIS pathology, we used a model of experimentally inducible IRIS in which M. avium-infected T cell-deficient mice undergo a fatal inflammatory disease after reconstitution with CD4 T cells. We find that IL-6 neutralization reduces C-reactive protein levels, alleviates wasting disease, and extends host survival during experimental IRIS. Moreover, we show that combined blockade of IL-6 and IFN-gamma further reduces IRIS pathology, even after the onset of wasting disease. The combination of these clinical and experimental-model data show that the IL-6 pathway is not only a biomarker of mycobacterial IRIS but also a major mediator of pathology distinct from IFN-gamma and may be a useful target for therapeutic intervention.
in vivo IL-6 neutralization
Khmaladze, I., et al (2014). "Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice" Proc Natl Acad Sci U S A 111(35): E3669-3678.
PubMed
Psoriasis (Ps) and psoriasis arthritis (PsA) are poorly understood common diseases, induced by unknown environmental factors, affecting skin and articular joints. A single i.p. exposure to mannan from Saccharomyces cerevisiae induced an acute inflammation in inbred mouse strains resembling human Ps and PsA-like disease, whereas multiple injections induced a relapsing disease. Exacerbation of disease severity was observed in mice deficient for generation of reactive oxygen species (ROS). Interestingly, restoration of ROS production, specifically in macrophages, ameliorated both skin and joint disease. Neutralization of IL-17A, mainly produced by gammadelta T cells, completely blocked disease symptoms. Furthermore, mice depleted of granulocytes were resistant to disease development. In contrast, certain acute inflammatory mediators (C5, Fcgamma receptor III, mast cells, and histamine) and adaptive immune players (alphabeta T and B cells) were redundant in disease induction. Hence, we propose that mannan-induced activation of macrophages leads to TNF-alpha secretion and stimulation of local gammadelta T cells secreting IL-17A. The combined action of activated macrophages and IL-17A produced in situ drives neutrophil infiltration in the epidermis and dermis of the skin, leading to disease manifestations. Thus, our finding suggests a new mechanism triggered by exposure to exogenous microbial components, such as mannan, that can induce and exacerbate Ps and PsA.
in vivo IL-6 neutralization
Berger, H., et al (2013). "SOCS3 transactivation by PPARgamma prevents IL-17-driven cancer growth" Cancer Res 73(12): 3578-3590.
PubMed
Activation of the transcription factor PPARgamma by the n-3 fatty acid docosahexaenoic acid (DHA) is implicated in controlling proinflammatory cytokine secretion, but the intracellular signaling pathways engaged by PPARgamma are incompletely characterized. Here, we identify the adapter-encoding gene SOCS3 as a critical transcriptional target of PPARgamma. SOCS3 promoter binding and gene transactivation by PPARgamma was associated with a repression in differentiation of proinflammatory T-helper (TH)17 cells. Accordingly, TH17 cells induced in vitro displayed increased SOCS3 expression and diminished capacity to produce interleukin (IL)-17 following activation of PPARgamma by DHA. Furthermore, naive CD4 T cells derived from mice fed a DHA-enriched diet displayed less capability to differentiate into TH17 cells. In two different mouse models of cancer, DHA prevented tumor outgrowth and angiogenesis in an IL-17-dependent manner. Altogether, our results uncover a novel molecular pathway by which PPARgamma-induced SOCS3 expression prevents IL-17-mediated cancer growth.
in vivo IL-6 neutralization
Kugler, D. G., et al (2013). "CD4+ T cells are trigger and target of the glucocorticoid response that prevents lethal immunopathology in toxoplasma infection" J Exp Med 210(10): 1919-1927.
PubMed
Synthetic glucocorticoids (GCs) are commonly used in the treatment of inflammatory diseases, but the role of endogenous GCs in the regulation of host-protective immune responses is poorly understood. Here we show that GCs are induced during acute Toxoplasma gondii infection and directly control the T cell response to the parasite. When infected with toxoplasma, mice that selectively lack GC receptor (GR) expression in T cells (GR(lck-Cre)) rapidly succumb to infection despite displaying parasite burdens indistinguishable from control animals and unaltered levels of the innate cytokines IL-12 and IL-27. Mortality in the GR(lck-Cre) mice was associated with immunopathology and hyperactive Th1 cell function as revealed by enhanced IFN-gamma and TNF production in vivo. Unexpectedly, these CD4(+) T lymphocytes also overexpressed IL-10. Importantly, CD4(+) T cell depletion in wild-type or GR(lck-Cre) mice led to ablation of the GC response to infection. Moreover, in toxoplasma-infected RAG(-/-) animals, adoptive transfer of CD4(+) T lymphocytes was required for GC induction. These findings establish a novel IL-10-independent immunomodulatory circuit in which CD4(+) T cells trigger a GC response that in turn dampens their own effector function. In the case of T. gondii infection, this self-regulatory pathway is critical for preventing collateral tissue damage and promoting host survival.
in vivo IL-6 neutralization
Berger, H., et al (2013). "SOCS3 transactivation by PPARgamma prevents IL-17-driven cancer growth" Cancer Res 73(12): 3578-3590.
PubMed
Activation of the transcription factor PPARgamma by the n-3 fatty acid docosahexaenoic acid (DHA) is implicated in controlling proinflammatory cytokine secretion, but the intracellular signaling pathways engaged by PPARgamma are incompletely characterized. Here, we identify the adapter-encoding gene SOCS3 as a critical transcriptional target of PPARgamma. SOCS3 promoter binding and gene transactivation by PPARgamma was associated with a repression in differentiation of proinflammatory T-helper (TH)17 cells. Accordingly, TH17 cells induced in vitro displayed increased SOCS3 expression and diminished capacity to produce interleukin (IL)-17 following activation of PPARgamma by DHA. Furthermore, naive CD4 T cells derived from mice fed a DHA-enriched diet displayed less capability to differentiate into TH17 cells. In two different mouse models of cancer, DHA prevented tumor outgrowth and angiogenesis in an IL-17-dependent manner. Altogether, our results uncover a novel molecular pathway by which PPARgamma-induced SOCS3 expression prevents IL-17-mediated cancer growth.
in vivo IL-6 neutralization
Kugler, D. G., et al (2013). "CD4+ T cells are trigger and target of the glucocorticoid response that prevents lethal immunopathology in toxoplasma infection" J Exp Med 210(10): 1919-1927.
PubMed
Synthetic glucocorticoids (GCs) are commonly used in the treatment of inflammatory diseases, but the role of endogenous GCs in the regulation of host-protective immune responses is poorly understood. Here we show that GCs are induced during acute Toxoplasma gondii infection and directly control the T cell response to the parasite. When infected with toxoplasma, mice that selectively lack GC receptor (GR) expression in T cells (GR(lck-Cre)) rapidly succumb to infection despite displaying parasite burdens indistinguishable from control animals and unaltered levels of the innate cytokines IL-12 and IL-27. Mortality in the GR(lck-Cre) mice was associated with immunopathology and hyperactive Th1 cell function as revealed by enhanced IFN-gamma and TNF production in vivo. Unexpectedly, these CD4(+) T lymphocytes also overexpressed IL-10. Importantly, CD4(+) T cell depletion in wild-type or GR(lck-Cre) mice led to ablation of the GC response to infection. Moreover, in toxoplasma-infected RAG(-/-) animals, adoptive transfer of CD4(+) T lymphocytes was required for GC induction. These findings establish a novel IL-10-independent immunomodulatory circuit in which CD4(+) T cells trigger a GC response that in turn dampens their own effector function. In the case of T. gondii infection, this self-regulatory pathway is critical for preventing collateral tissue damage and promoting host survival.
in vivo IL-6 neutralization
Debock, I., et al (2012). "Th17 alloimmunity prevents neonatal establishment of lymphoid chimerism in IL-4-deprived mice" Am J Transplant 12(1): 81-89.
PubMed
Immune responses in newborn mice are known to be biased toward the helper type 2 phenotype. This may account for their propensity to develop tolerance. Herein, we evaluated the effects of IL-4 deprivation on CD4(+) T-cell activities elicited by neonatal exposure to allogeneic spleen cells. We showed that chimerism, Th2-type polarization and pathology, as well as skin allograft acceptance were inhibited in BALB/c mice immunized at birth with (A/J x BALB/c) F(1) spleen cells upon in vivo IL-4 neutralization. While IL-4 neutralization inhibited the development of Th2 cells in this model, it led to the accumulation of IL-17A, IL-17F, IL-22, IL-6 and RORgammat mRNA in the spleen or graft tissues. Moreover, IL-4 deprivation led to the differentiation of donor-specific Th17 cells with a concomitant Th1 response characterized by IFN-gamma production. The Th17-type response emerging in IL-4-deprived mice was found to mediate both intragraft neutrophil infiltration and the abrogation of B-cell chimerism. Neutralization of this Th17 response failed however to restore functional skin graft acceptance. Collectively, our observations indicate that the neonatal Th2 response opposes the development of Th17 cells, and that Th17 cells are responsible for controlling lymphoid chimerism in mice neonatally injected with semiallogeneic cells.
in vivo IL-6 neutralization
Debock, I., et al (2012). "Th17 alloimmunity prevents neonatal establishment of lymphoid chimerism in IL-4-deprived mice" Am J Transplant 12(1): 81-89.
PubMed
Immune responses in newborn mice are known to be biased toward the helper type 2 phenotype. This may account for their propensity to develop tolerance. Herein, we evaluated the effects of IL-4 deprivation on CD4(+) T-cell activities elicited by neonatal exposure to allogeneic spleen cells. We showed that chimerism, Th2-type polarization and pathology, as well as skin allograft acceptance were inhibited in BALB/c mice immunized at birth with (A/J x BALB/c) F(1) spleen cells upon in vivo IL-4 neutralization. While IL-4 neutralization inhibited the development of Th2 cells in this model, it led to the accumulation of IL-17A, IL-17F, IL-22, IL-6 and RORgammat mRNA in the spleen or graft tissues. Moreover, IL-4 deprivation led to the differentiation of donor-specific Th17 cells with a concomitant Th1 response characterized by IFN-gamma production. The Th17-type response emerging in IL-4-deprived mice was found to mediate both intragraft neutrophil infiltration and the abrogation of B-cell chimerism. Neutralization of this Th17 response failed however to restore functional skin graft acceptance. Collectively, our observations indicate that the neonatal Th2 response opposes the development of Th17 cells, and that Th17 cells are responsible for controlling lymphoid chimerism in mice neonatally injected with semiallogeneic cells.
in vivo IL-6 neutralization
Prabhakara, R., et al (2011). "Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus" Infect Immun 79(12): 5010-5018.
PubMed
Staphylococcus aureus is a common cause of prosthetic implant infections, which can become chronic due to the ability of S. aureus to grow as a biofilm. Little is known about adaptive immune responses to these infections in vivo. We hypothesized that S. aureus elicits inflammatory Th1/Th17 responses, associated with biofilm formation, instead of protective Th2/Treg responses. We used an adapted mouse model of biofilm-mediated prosthetic implant infection to determine chronic infection rates, Treg cell frequencies, and local cytokine levels in Th1-biased C57BL/6 and Th2-biased BALB/c mice. All C57BL/6 mice developed chronic S. aureus implant infection at all time points tested. However, over 75% of BALB/c mice spontaneously cleared the infection without adjunctive therapy and demonstrated higher levels of Th2 cytokines and anti-inflammatory Treg cells. When chronic infection rates in mice deficient in the Th2 cytokine interleukin-4 (IL-4) via STAT6 mutation in a BALB/c background were assessed, the mice were unable to clear the S. aureus implant infection. Additionally, BALB/c mice depleted of Treg cells via an anti-CD25 monoclonal antibody (MAb) were also unable to clear the infection. In contrast, the C57BL/6 mice that were susceptible to infection were able to eliminate S. aureus biofilm populations on infected intramedullary pins once the Th1 and Th17 responses were diminished by MAb treatment with anti-IL-12 p40. Together, these results indicate that Th2/Treg responses are mechanisms of protection against chronic S. aureus implant infection, as opposed to Th1/Th17 responses, which may play a role in the development of chronic infection.
in vitro IL-6 neutralization
Molinero, L. L., et al (2011). "High TCR stimuli prevent induced regulatory T cell differentiation in a NF-kappaB-dependent manner" J Immunol 186(8): 4609-4617.
PubMed
The concentration of Ag or mitogenic stimuli is known to play an important role in controlling the differentiation of naive CD4(+) T cells into different effector phenotypes. In particular, whereas TCR engagement at low Ag doses in the presence of TGF-beta and IL-2 can promote differentiation of Foxp3-expressing induced regulatory T cells (iTregs), high levels of Ag have been shown in vitro and in vivo to prevent Foxp3 upregulation. This tight control of iTreg differentiation dictated by Ag dose most likely determines the quality and duration of an immune response. However, the molecular mechanism by which this high-dose inhibition of Foxp3 induction occurs is not well understood. In this study, we demonstrate that when cells are in the presence of CD28 costimulation, TCR-dependent NF-kappaB signaling is essential for Foxp3 inhibition at high doses of TCR engagement in mouse T cells. Prevention of Foxp3 induction depends on the production of NF-kappaB-dependent cytokines by the T cells themselves. Moreover, T cells that fail to upregulate Foxp3 under iTreg-differentiating conditions and high TCR stimulation acquire the capacity to make TNF and IFN-gamma, as well as IL-17 and IL-9. Thus, NF-kappaB helps T cells control their differentiation fate in a cell-intrinsic manner and prevents peripheral iTreg development under conditions of high Ag load that may require more vigorous effector T cell responses.
in vivo IL-6 neutralization
Prabhakara, R., et al (2011). "Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus" Infect Immun 79(12): 5010-5018.
PubMed
Staphylococcus aureus is a common cause of prosthetic implant infections, which can become chronic due to the ability of S. aureus to grow as a biofilm. Little is known about adaptive immune responses to these infections in vivo. We hypothesized that S. aureus elicits inflammatory Th1/Th17 responses, associated with biofilm formation, instead of protective Th2/Treg responses. We used an adapted mouse model of biofilm-mediated prosthetic implant infection to determine chronic infection rates, Treg cell frequencies, and local cytokine levels in Th1-biased C57BL/6 and Th2-biased BALB/c mice. All C57BL/6 mice developed chronic S. aureus implant infection at all time points tested. However, over 75% of BALB/c mice spontaneously cleared the infection without adjunctive therapy and demonstrated higher levels of Th2 cytokines and anti-inflammatory Treg cells. When chronic infection rates in mice deficient in the Th2 cytokine interleukin-4 (IL-4) via STAT6 mutation in a BALB/c background were assessed, the mice were unable to clear the S. aureus implant infection. Additionally, BALB/c mice depleted of Treg cells via an anti-CD25 monoclonal antibody (MAb) were also unable to clear the infection. In contrast, the C57BL/6 mice that were susceptible to infection were able to eliminate S. aureus biofilm populations on infected intramedullary pins once the Th1 and Th17 responses were diminished by MAb treatment with anti-IL-12 p40. Together, these results indicate that Th2/Treg responses are mechanisms of protection against chronic S. aureus implant infection, as opposed to Th1/Th17 responses, which may play a role in the development of chronic infection.
in vitro IL-6 neutralization
Molinero, L. L., et al (2011). "High TCR stimuli prevent induced regulatory T cell differentiation in a NF-kappaB-dependent manner" J Immunol 186(8): 4609-4617.
PubMed
The concentration of Ag or mitogenic stimuli is known to play an important role in controlling the differentiation of naive CD4(+) T cells into different effector phenotypes. In particular, whereas TCR engagement at low Ag doses in the presence of TGF-beta and IL-2 can promote differentiation of Foxp3-expressing induced regulatory T cells (iTregs), high levels of Ag have been shown in vitro and in vivo to prevent Foxp3 upregulation. This tight control of iTreg differentiation dictated by Ag dose most likely determines the quality and duration of an immune response. However, the molecular mechanism by which this high-dose inhibition of Foxp3 induction occurs is not well understood. In this study, we demonstrate that when cells are in the presence of CD28 costimulation, TCR-dependent NF-kappaB signaling is essential for Foxp3 inhibition at high doses of TCR engagement in mouse T cells. Prevention of Foxp3 induction depends on the production of NF-kappaB-dependent cytokines by the T cells themselves. Moreover, T cells that fail to upregulate Foxp3 under iTreg-differentiating conditions and high TCR stimulation acquire the capacity to make TNF and IFN-gamma, as well as IL-17 and IL-9. Thus, NF-kappaB helps T cells control their differentiation fate in a cell-intrinsic manner and prevents peripheral iTreg development under conditions of high Ag load that may require more vigorous effector T cell responses.
Product Citations
-
-
Immunology and Microbiology
-
Neuroscience
IL-6 Inhibition Partially Ameliorates Maternal Immune Activation-Induced Autism-Like Behavioral Abnormalities in Mice.
In Curr Issues Mol Biol on 16 October 2025 by Zhang, X., Luo, W., et al.
PubMed
Prenatal maternal immune activation (MIA) has been implicated in autism spectrum disorder (ASD) pathogenesis, with interleukin-6 (IL-6) identified as a key inflammatory mediator. We investigated the therapeutic potential of IL-6 inhibition in an MIA mouse model induced by Toxoplasma gondii soluble tachyzoite antigen (STAg). Adult MIA offspring received systemic administration of the IL-6-neutralizing antibody (MP5-20F3) or isotype control, followed by behavioral assessments one week later. Open field and elevated plus maze tests revealed heightened anxiety-like behaviors in the STAg offspring, which were largely reversed by IL-6 inhibition. Reciprocal social interaction tests showed diminished sociability in the STAg offspring, which was partially restored by IL-6 inhibition. However, core ASD-like features, including impaired social preference and recognition in the three-chamber test, as well as increased repetitive behaviors, remained resistant to IL-6 inhibition. These findings demonstrate that STAg-induced MIA elicits anxiety-like and ASD-like phenotypes in adult offspring, with IL-6 playing an important role in anxiety-like behaviors and social interaction deficits. Systemic IL-6 inhibition partially ameliorates behavioral abnormalities. This study suggests that IL-6-targeted therapies may address a subset of ASD-related symptoms, and comprehensive strategies are needed for broader efficacy.
-
-
-
Cancer Research
-
Neuroscience
Cancer-induced nerve injury promotes resistance to anti-PD-1 therapy.
In Nature on 1 October 2025 by Baruch, E. N., Gleber-Netto, F. O., et al.
PubMed
Perineural invasion (PNI) is a well-established factor of poor prognosis in multiple cancer types1, yet its mechanism remains unclear. Here we provide clinical and mechanistic insights into the role of PNI and cancer-induced nerve injury (CINI) in resistance to anti-PD-1 therapy. Our study demonstrates that PNI and CINI of tumour-associated nerves are associated with poor response to anti-PD-1 therapy among patients with cutaneous squamous cell carcinoma, melanoma and gastric cancer. Electron microscopy and electrical conduction analyses reveal that cancer cells degrade the nerve fibre myelin sheets. The injured neurons respond by autonomously initiating IL-6- and type I interferon-mediated inflammation to promote nerve healing and regeneration. As the tumour grows, the CINI burden increases, and its associated inflammation becomes chronic and skews the general immune tone within the tumour microenvironment into a suppressive and exhaustive state. The CINI-driven anti-PD-1 resistance can be reversed by targeting multiple steps in the CINI signalling process: denervating the tumour, conditional knockout of the transcription factor mediating the injury signal within neurons (Atf3), knockout of interferon-α receptor signalling (Ifnar1-/-) or by combining anti-PD-1 and anti-IL-6-receptor blockade. Our findings demonstrate the direct immunoregulatory roles of CINI and its therapeutic potential.
-
-
-
Cancer Research
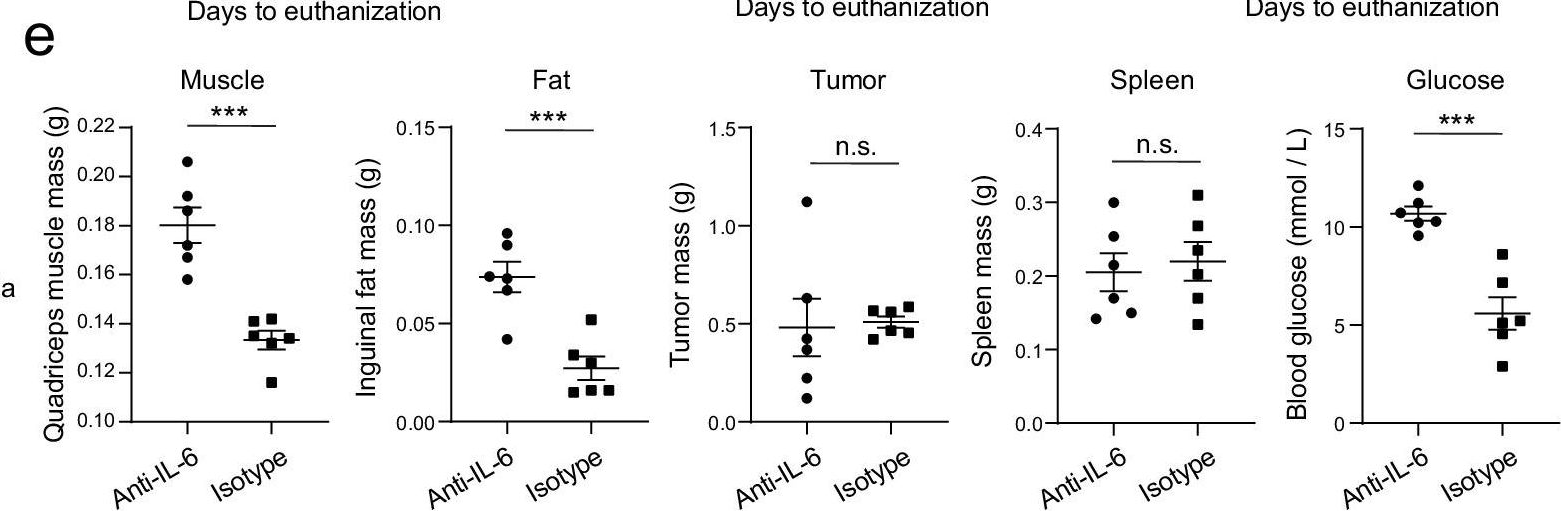
Early adipose tissue wasting in a preclinical model of human lung cancer cachexia.
In Cell Rep on 23 September 2025 by Snoke, D. B., van der Velden, J. L., et al.
PubMed
Cancer cachexia (CC), a syndrome of skeletal muscle and adipose wasting, reduces responsiveness to therapies and increases mortality. There are no approved treatments for CC, which may relate to discordance between preclinical models and human CC. To address the need for clinically relevant models of lung CC, we generated inducible, lung epithelial cell-specific KrasG12D/+ (G12D) mice. G12D mice develop CC over a protracted time course and phenocopy tissue and tumor, cellular, mutational, transcriptomic, and metabolic characteristics of human lung CC. G12D mice demonstrate early loss of adipose, a phenotype that was apparent across numerous models of CC and translates to patients with lung cancer. Tumor-released factors promote adipocyte lipolysis, a driver of adipose wasting in CC, and adipose wasting was inversely related to tumor burden. Thus, G12D mice model key features of human lung CC and highlight a role for early tumor metabolic reprogramming of adipose tissue in CC.
-
-
-
Cancer Research
-
Immunology and Microbiology
Immature monocytic cells within tumors differentiate into immunosuppressive cells in resistant tumors to immunotherapy.
In iScience on 15 August 2025 by Levin, S., Benguigui, M., et al.
PubMed
Immune checkpoint inhibitors (ICIs) have improved outcomes in advanced cancers, yet resistance remains a major obstacle. Here, we investigated the role of myeloid cells in shaping the immunosuppressive tumor microenvironment that contributes to ICI resistance. Using mutagenized ICI-sensitive and resistant 4T1 breast cancer clones, we performed single-cell RNA sequencing to characterize immune cell populations post-ICI therapy. We identified monocytic dendritic progenitors (MDPs) and common monocytic progenitors (cMOPs) enriched in sensitive tumors, which may differentiate into immunosuppressive cells in resistant tumors. Analysis of public datasets confirmed the presence of MDP-cMOPs in tumors and blood of patients with breast, lung, and colorectal cancer. We found high expression of CXCR4 and IL6R in MDP-cMOPs, and inhibiting these pathways blocked their recruitment and differentiation. Combined targeting of CXCR4 and IL6 pathway with ICI improved responses in resistant tumors, highlighting MDP-cMOPs as contributors to immunotherapy resistance and potential therapeutic targets.
-
-
-
Cardiovascular biology
Direct Interleukin-6 Inhibition Blunts Arterial Thrombosis by Reducing Collagen-Mediated Platelet Activation.
In Arterioscler Thromb Vasc Biol on 1 August 2025 by Ministrini, S., Liberale, L., et al.
PubMed
Recent clinical trials demonstrated a reduction in biomarkers of thrombosis and inflammation in patients with very high cardiovascular risk treated with the anti-IL-6 (interleukin 6) monoclonal antibody ziltivekimab. However, if and how direct IL-6 inhibition exerts antithrombotic effects remains unknown. This translational project aimed to investigate the effect of direct IL-6 inhibition on experimental arterial thrombus formation and its underlying cellular mechanisms.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
Intrapleural dual blockade of IL-6 and PD-L1 reprograms CAF dynamics and the tumor microenvironment in lung cancer-associated malignant pleural effusion.
In Respir Res on 10 May 2025 by Cheng, Q., Zuo, X., et al.
PubMed
Malignant pleural effusion (MPE) is a severe complication in lung cancer, characterized by an immunosuppressive tumor microenvironment (TME) and limited therapeutic options. This study investigates the role of IL-6 in regulating immune suppression and tumor progression in MPE and evaluates the efficacy of dual IL-6 and PD-L1 blockade.
-
-
IL-6 underlies microenvironment immunosuppression and resistance to therapy in glioblastoma
In bioRxiv on 14 March 2025 by Young, J. S., Cho, N. W., et al.
-
-
Mus musculus (Mouse)
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Hepatic TM6SF2 activates antitumour immunity to suppress metabolic dysfunction-associated steatotic liver disease-related hepatocellular carcinoma and boosts immunotherapy.
In Gut on 6 March 2025 by Zhang, Y., Xie, M., et al.
PubMed
Transmembrane 6 superfamily member 2 (TM6SF2) has a protective role against metabolic dysfunction-associated steatotic liver disease (MASLD).
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
IL-6 and PD-1 antibody blockade combination therapy regulate inflammation and T lymphocyte apoptosis in murine model of sepsis.
In BMC Immunol on 14 January 2025 by Lee, S. I., Kim, N. Y., et al.
PubMed
Interleukin-6 (IL-6) plays a central role in sepsis-induced cytokine storm involving immune hyperactivation and early neutrophil activation. Programmed death protein-1 (PD-1) is associated with sepsis-induced immunosuppression and lymphocyte apoptosis. However, the effects of simultaneous blockade of IL-6 and PD-1 in a murine sepsis model are not well understood.
-
-
-
Cancer Research
Acquired resistance to PD-L1 inhibition enhances a type I IFN-regulated secretory program in tumors.
In EMBO Rep on 1 January 2025 by Shi, Y., McKenery, A., et al.
PubMed
Therapeutic inhibition of programmed cell death ligand (PD-L1) is linked to alterations in interferon (IFN) signaling. Since IFN-regulated intracellular signaling can control extracellular secretory programs in tumors to modulate immunity, we examined IFN-related secretory changes in tumor cells following resistance to PD-L1 inhibition. Here we report an anti-PD-L1 treatment-induced secretome (PTIS) in tumor models of acquired resistance that is regulated by type I IFNs. These secretory changes can suppress activation of T cells ex vivo while diminishing tumor cell cytotoxicity, revealing that tumor-intrinsic treatment adaptations can exert broad tumor-extrinsic effects. When reimplanted in vivo, resistant tumor growth can slow or stop when PTIS components are disrupted individually, or when type I IFN signaling machinery is blocked. Interestingly, genetic and therapeutic disruption of PD-L1 in vitro can only partially recapitulate the PTIS phenotype highlighting the importance of developing in vivo-based resistance models to more faithfully mimic clinically-relevant treatment failure. Together, this study shows acquired resistance to immune-checkpoint inhibitors 'rewires' tumor secretory programs controlled by type I IFNs that, in turn, can protect from immune cell attack.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Pathobiont-induced suppressive immune imprints thwart T cell vaccine responses.
In Nat Commun on 16 December 2024 by Hajam, I. A., Tsai, C. M., et al.
PubMed
Pathobionts have evolved many strategies to coexist with the host, but how immune evasion mechanisms contribute to the difficulty of developing vaccines against pathobionts is unclear. Meanwhile, Staphylococcus aureus (SA) has resisted human vaccine development to date. Here we show that prior SA exposure induces non-protective CD4+ T cell imprints, leading to the blunting of protective IsdB vaccine responses. Mechanistically, these SA-experienced CD4+ T cells express IL-10, which is further amplified by vaccination and impedes vaccine protection by binding with IL-10Rα on CD4+ T cell and inhibit IL-17A production. IL-10 also mediates cross-suppression of IsdB and sdrE multi-antigen vaccine. By contrast, the inefficiency of SA IsdB, IsdA and MntC vaccines can be overcome by co-treatment with adjuvants that promote IL-17A and IFN-γ responses. We thus propose that IL-10 secreting, SA-experienced CD4+ T cell imprints represent a staphylococcal immune escaping mechanism that needs to be taken into consideration for future vaccine development.
-
-
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
RNA Shielding of p65 Is Required to Potentiate Oncogenic Inflammation in TET2-Mutated Clonal Hematopoiesis.
In Cancer Discov on 2 December 2024 by Ben-Crentsil, N. A., Mohammed Ismail, W., et al.
PubMed
This work identifies MALAT1 as a requisite downstream effector of oncogenic feedforward inflammatory circuits necessary for the development of TET2-mutated CH and fulminant myeloid malignancy. We elucidate a novel mechanism by which MALAT1 "shields" p65 from dephosphorylation to potentiate this circuit and nominate MALAT1 inhibition as a future therapeutic strategy.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
PD-L1 restrains PD-1+Nrp1lo Treg cells to suppress inflammation-driven colorectal tumorigenesis.
In Cell Rep on 22 October 2024 by Poschel, D. B., Klement, J. D., et al.
PubMed
T cells function not only as an essential component of host cancer immunosurveillance but also as a regulator of colonic inflammation, a process that promotes colorectal cancer. Programmed death-ligand 1 (PD-L1) is a T cell-negative regulator, but its role in regulation of T cell functions in the context of colorectal cancer is unknown. We report that global deletion of Cd274 results in increased colonic inflammation, PD-1+ T cells, and inflammation-driven colorectal tumorigenesis in mice. Single-cell RNA sequencing (scRNA-seq) analysis revealed that PD-L1 suppresses subpopulations of programmed cell death protein 1 (PD-1)+Nrp1lo regulatory T (Treg) cells and interleukin (IL) 6+ neutrophils in colorectal tumor. Treg cells produce transforming growth factor (TGF) β to recruit IL6+ neutrophils. Neutrophils produce IL6 to inhibit activation of tumor-specific cytotoxic T lymphocytes (CTLs) and primary CTLs. Accordingly, IL6 blockade immunotherapy increases CTL activation and suppresses colon tumor growth in vivo. Our findings determine that PD-L1 restrains PD-1+Nrp1loTGFβ+ Treg cells to suppress IL6+ neutrophil tumor recruitment to sustain CTL activation to control inflammation-driven colorectal tumorigenesis.
-
-
-
Immunology and Microbiology
IL-6 induces Treg dysfunction in desiccating stress-induced dry eye disease.
In Exp Eye Res on 1 September 2024 by Ortiz, G., Blanco, T., et al.
PubMed
Regulatory T cells (Tregs) play a critical role in maintaining immune homeostasis, and their dysfunction is implicated in the pathogenesis of various autoimmune disorders, including dry eye disease (DED). Treg dysfunction in DED allows T-helper cell 17 (Th17) mediated chronic inflammation at the ocular surface. In this study, the factors causing Treg dysfunction in DED were investigated. We observed reduced expression of Treg functional markers - FoxP3, CD25, and CTLA-4 in the cells isolated from DED mice (DED Tregs). Additionally, DED Tregs showed increased expression levels of receptors for pro-inflammatory cytokine receptors, namely IL-6R, IL-17RA, and IL-23R. An increased expression level of pro-inflammatory cytokine receptors was observed on exposing Tregs isolated from naïve mice (NTregs) to IL-6 or IL-17, but not IL-23, with a concomitant downregulation of FoxP3, CD25, and CTLA-4 in these cells. Furthermore, among these cytokines, IL-6 induced the most pronounced loss of Treg mediated suppression of Th17 proliferation and IL-10 secretion. In vitro and in vivo blockade of IL-6 effectively restored function in DED Tregs, leading to enhanced suppressive function against proliferating Th17 cells and ameliorating disease severity. In conclusion, this study provides insights into mechanisms of Treg dysregulation in DED, specifically delineating the effect of Th17-associated cytokines, with IL-6 emerging as the critical factor inducing Treg dysfunctionality. These findings highlight the potential for developing novel therapeutic interventions for DED through restoration of immunosuppressive function of Tregs.
-
-
-
Cancer Research
Mutant TP53 switches therapeutic vulnerability during gastric cancer progression within interleukin-6 family cytokines.
In Cell Rep on 27 August 2024 by Huber, A., Allam, A. H., et al.
PubMed
Although aberrant activation of the KRAS and PI3K pathway alongside TP53 mutations account for frequent aberrations in human gastric cancers, neither the sequence nor the individual contributions of these mutations have been clarified. Here, we establish an allelic series of mice to afford conditional expression in the glandular epithelium of KrasG12D;Pik3caH1047R or Trp53R172H and/or ablation of Pten or Trp53. We find that KrasG12D;Pik3caH1047R is sufficient to induce adenomas and that lesions progress to carcinoma when also harboring Pten deletions. An additional challenge with either Trp53 loss- or gain-of-function alleles further accelerated tumor progression and triggered metastatic disease. While tumor-intrinsic STAT3 signaling in response to gp130 family cytokines remained as a gatekeeper for all stages of tumor development, metastatic progression required a mutant Trp53-induced interleukin (IL)-11 to IL-6 dependency switch. Consistent with the poorer survival of patients with high IL-6 expression, we identify IL-6/STAT3 signaling as a therapeutic vulnerability for TP53-mutant gastric cancer.
-
-
-
Cancer Research
-
Neuroscience
-
Flow cytometry/Cell sorting
Area postrema neurons mediate interleukin-6 function in cancer cachexia.
In Nat Commun on 1 June 2024 by Sun, Q., van de Lisdonk, D., et al.
PubMed
Interleukin-6 (IL-6) has been long considered a key player in cancer cachexia. It is believed that sustained elevation of IL-6 production during cancer progression causes brain dysfunctions, which ultimately result in cachexia. However, how peripheral IL-6 influences the brain remains poorly understood. Here we show that neurons in the area postrema (AP), a circumventricular structure in the hindbrain, is a critical mediator of IL-6 function in cancer cachexia in male mice. We find that circulating IL-6 can rapidly enter the AP and activate neurons in the AP and its associated network. Peripheral tumor, known to increase circulating IL-6, leads to elevated IL-6 in the AP, and causes potentiated excitatory synaptic transmission onto AP neurons and AP network hyperactivity. Remarkably, neutralization of IL-6 in the brain of tumor-bearing mice with an anti-IL-6 antibody attenuates cachexia and the hyperactivity in the AP network, and markedly prolongs lifespan. Furthermore, suppression of Il6ra, the gene encoding IL-6 receptor, specifically in AP neurons with CRISPR/dCas9 interference achieves similar effects. Silencing Gfral-expressing AP neurons also attenuates cancer cachectic phenotypes and AP network hyperactivity. Our study identifies a central mechanism underlying the function of peripheral IL-6, which may serve as a target for treating cancer cachexia.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Inhibition of IL-25/IL-17RA improves immune-related adverse events of checkpoint inhibitors and reveals antitumor activity.
In J Immunother Cancer on 21 March 2024 by Hu, X., Bukhari, S. M., et al.
PubMed
Immune checkpoint inhibitors (ICIs) have improved outcomes and extended patient survival in several tumor types. However, ICIs often induce immune-related adverse events (irAEs) that warrant therapy cessation, thereby limiting the overall effectiveness of this class of therapeutic agents. Currently, available therapies used to treat irAEs might also blunt the antitumor activity of the ICI themselves. Therefore, there is an urgent need to identify treatments that have the potential to be administered alongside ICI to optimize their use.
-
-
-
Mus musculus (Mouse)
-
Cardiovascular biology
-
Immunology and Microbiology
Malaria blood stage infection suppresses liver stage infection via host-induced interferons but not hepcidin.
In Nat Commun on 7 March 2024 by Patel, H., Minkah, N. K., et al.
PubMed
Malaria-causing Plasmodium parasites first replicate as liver stages (LS), which then seed symptomatic blood stage (BS) infection. Emerging evidence suggests that these stages impact each other via perturbation of host responses, and this influences the outcome of natural infection. We sought to understand whether the parasite stage interplay would affect live-attenuated whole parasite vaccination, since the efficacy of whole parasite vaccines strongly correlates with their extend of development in the liver. We thus investigated the impact of BS infection on LS development of genetically attenuated and wildtype parasites in female rodent malaria models and observed that for both, LS infection suffered severe suppression during concurrent BS infection. Strikingly and in contrast to previously published studies, we find that the BS-induced iron-regulating hormone hepcidin is not mediating suppression of LS development. Instead, we demonstrate that BS-induced host interferons are the main mediators of LS developmental suppression. The type of interferon involved depended on the BS-causing parasite species. Our study provides important mechanistic insights into the BS-mediated suppression of LS development. This has direct implications for understanding the outcomes of live-attenuated Plasmodium parasite vaccination in malaria-endemic areas and might impact the epidemiology of natural malaria infection.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Cell Biology
-
Biochemistry and Molecular biology
Tumor cell senescence-induced macrophage CD73 expression is a critical metabolic immune checkpoint in the aging tumor microenvironment.
In Theranostics on 7 February 2024 by Deng, Y., Chen, Q., et al.
PubMed
Background: The role of senescent cells in the tumor microenvironment (TME) is usually bilateral, and diverse therapeutic approaches, such as radiotherapy and chemotherapy, can induce cellular senescence. Cellular interactions are widespread in the TME, and tumor cells reprogram immune cells metabolically by producing metabolites. However, how senescent cells remodel the metabolism of TME remains unclear. This study aimed to explore precise targets to enhance senescent cells-induced anti-tumor immunity from a metabolic perspective. Methods: The in vivo senescence model was induced by 8 Gy×3 radiotherapy or cisplatin chemotherapy, and the in vitro model was induced by 10 Gy-irradiation or cisplatin treatment. Metabonomic analysis and ELISA assay on tumor interstitial fluid were performed for metabolites screening. Marker expression and immune cell infiltration in the TME were analyzed by flow cytometry. Cell co-culture system and senescence-conditioned medium were used for crosstalk validation in vitro. RNA sequencing and rescue experiments were conducted for mechanism excavation. Immunofluorescence staining and single-cell transcriptome profiling analysis were performed for clinical validation. Results: We innovatively reveal the metabolic landscape of the senescent TME, characterized with the elevation of adenosine. It is attributed to the senescent tumor cell-induced CD73 upregulation of tumor-associated macrophages (TAMs). CD73 expression in TAMs is evoked by SASP-related pro-inflammatory cytokines, especially IL-6, and regulated by JAK/STAT3 pathway. Consistently, a positive correlation between tumor cells senescence and TAMs CD73 expression is identified in lung cancer clinical specimens and databases. Lastly, blocking CD73 in a senescent background suppresses tumors and activates CD8+ T cell-mediated antitumor immunity. Conclusions: TAMs expressed CD73 contributes significantly to the adenosine accumulation in the senescent TME, suggesting targeting CD73 is a novel synergistic anti-tumor strategy in the aging microenvironment.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
-
Cell Biology
Resveratrol-βcd inhibited premature ovarian insufficiency progression by regulating granulosa cell autophagy.
In J Ovarian Res on 15 January 2024 by Hu, B., Zheng, X., et al.
PubMed
The ovarian environment of premature ovarian insufficiency (POI) patients exhibits immune dysregulation, which leads to excessive secretion of numerous proinflammatory cytokines that affect ovarian function. An abnormal level of macrophage polarization directly or indirectly inhibits the differentiation of ovarian granulosa cells and steroid hormone production, ultimately leading to POI. Resveratrol, as a health supplement, has been widely recognized for its safety. There is a substantial amount of evidence indicating that resveratrol and its analogs possess significant immune-regulatory functions. It has also been reported that resveratrol can effectively inhibit the progression of POI. However, the underlying immunological and molecular mechanisms through which resveratrol inhibits the progression of POI are still unclear.
-