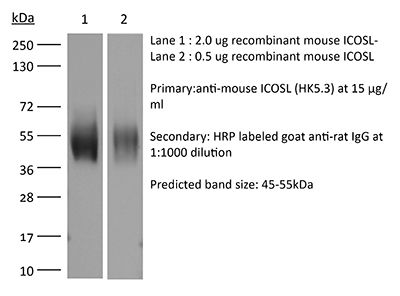
InVivoMAb anti-mouse ICOSL (CD275)
Product Description
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse B7-RP1 transfected cell line |
| Reported Applications | in vivo ICOSL neutralization |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107566 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo ICOSL neutralization
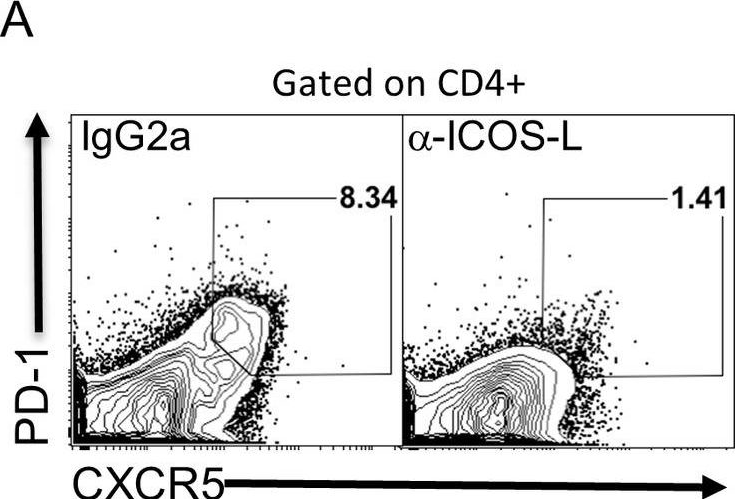
Stone, E. L., et al (2015). "ICOS coreceptor signaling inactivates the transcription factor FOXO1 to promote Tfh cell differentiation" Immunity 42(2): 239-251.
PubMed
T follicular helper (Tfh) cells are essential in the induction of high-affinity, class-switched antibodies. The differentiation of Tfh cells is a multi-step process that depends upon the co-receptor ICOS and the activation of phosphoinositide-3 kinase leading to the expression of key Tfh cell genes. We report that ICOS signaling inactivates the transcription factor FOXO1, and a Foxo1 genetic deletion allowed for generation of Tfh cells with reduced dependence on ICOS ligand. Conversely, enforced nuclear localization of FOXO1 inhibited Tfh cell development even though ICOS was overexpressed. FOXO1 regulated Tfh cell differentiation through a broad program of gene expression exemplified by its negative regulation of Bcl6. Final differentiation to germinal center Tfh cells (GC-Tfh) was instead FOXO1 dependent as the Foxo1(-/-) GC-Tfh cell population was substantially reduced. We propose that ICOS signaling transiently inactivates FOXO1 to initiate a Tfh cell contingency that is completed in a FOXO1-dependent manner.
in vivo ICOSL neutralization
Raynor, J., et al (2015). "IL-6 and ICOS Antagonize Bim and Promote Regulatory T Cell Accrual with Age" J Immunol 195(3): 944-952.
PubMed
Regulatory T cells (Tregs), a subset of CD4(+) T cells, dramatically accumulate with age in humans and mice and contribute to age-related immune suppression. Recently, we showed that a majority of accumulating Tregs in aged mice expressed low levels of CD25, and their accrual is associated with declining levels of IL-2 in aged mice. In this study, we further investigated the origin of CD25(lo) Tregs in aged mice. First, aged Tregs had high expression of neuropilin-1 and Helios, and had a broad Vbeta repertoire. Next, we analyzed the gene expression profile of Tregs, naive T cells, and memory T cells in aged mice. We found that the gene expression profile of aged CD25(lo) Tregs were more related to young CD25(lo) Tregs than to either naive or memory T cells. Further, the gene expression profile of aged Tregs was consistent with recently described “effector” Tregs (eTregs). Additional analysis revealed that nearly all Tregs in aged mice were of an effector phenotype (CD44(hi)CD62L(lo)) and could be further characterized by high levels of ICOS and CD69. ICOS contributed to Treg maintenance in aged mice, because in vivo Ab blockade of ICOSL led to a loss of eTregs, and this loss was rescued in Bim-deficient mice. Further, serum levels of IL-6 increased with age and contributed to elevated expression of ICOS on aged Tregs. Finally, Treg accrual was significantly blunted in aged IL-6-deficient mice. Together, our data show a role for IL-6 in promoting eTreg accrual with age likely through maintenance of ICOS expression.
in vivo ICOSL neutralization
Xin, L., et al (2014). "Commensal microbes drive intestinal inflammation by IL-17-producing CD4+ T cells through ICOSL and OX40L costimulation in the absence of B7-1 and B7-2" Proc Natl Acad Sci U S A 111(29): 10672-10677.
PubMed
The costimulatory B7-1 (CD80)/B7-2 (CD86) molecules, along with T-cell receptor stimulation, together facilitate T-cell activation. This explains why in vivo B7 costimulation neutralization efficiently silences a variety of human autoimmune disorders. Paradoxically, however, B7 blockade also potently moderates accumulation of immune-suppressive regulatory T cells (Tregs) essential for protection against multiorgan systemic autoimmunity. Here we show that B7 deprivation in mice overrides the necessity for Tregs in averting systemic autoimmunity and inflammation in extraintestinal tissues, whereas peripherally induced Tregs retained in the absence of B7 selectively mitigate intestinal inflammation caused by Th17 effector CD4(+) T cells. The need for additional immune suppression in the intestine reflects commensal microbe-driven T-cell activation through the accessory costimulation molecules ICOSL and OX40L. Eradication of commensal enteric bacteria mitigates intestinal inflammation and IL-17 production triggered by Treg depletion in B7-deficient mice, whereas re-establishing intestinal colonization with Candida albicans primes expansion of Th17 cells with commensal specificity. Thus, neutralizing B7 costimulation uncovers an essential role for Tregs in selectively averting intestinal inflammation by Th17 CD4(+) T cells with commensal microbe specificity.
in vivo ICOSL neutralization
Srivastava, S., et al (2014). "Type I interferons directly inhibit regulatory T cells to allow optimal antiviral T cell responses during acute LCMV infection" J Exp Med 211(5): 961-974.
PubMed
Regulatory T (T reg) cells play an essential role in preventing autoimmunity but can also impair clearance of foreign pathogens. Paradoxically, signals known to promote T reg cell function are abundant during infection and could inappropriately enhance T reg cell activity. How T reg cell function is restrained during infection to allow the generation of effective antiviral responses remains largely unclear. We demonstrate that the potent antiviral type I interferons (IFNs) directly inhibit co-stimulation-dependent T reg cell activation and proliferation, both in vitro and in vivo during acute infection with lymphocytic choriomeningitis virus (LCMV). Loss of the type I IFN receptor specifically in T reg cells results in functional impairment of virus-specific CD8(+) and CD4(+) T cells and inefficient viral clearance. Together, these data demonstrate that inhibition of T reg cells by IFNs is necessary for the generation of optimal antiviral T cell responses during acute LCMV infection.
in vivo ICOSL neutralization
Huang, W., et al (2014). "IL-2-inducible T cell kinase tunes T regulatory cell development and is required for suppressive function" J Immunol 193(5): 2267-2272.
PubMed
IL-2-inducible T cell kinase (ITK) is a key signaling mediator downstream of TCR, mediating T cell positive selection, as well as innate T cell and CD4(+) Th2/Th17 differentiation. In this article, we show that ITK also negatively tunes IL-2-induced expansion of conventional Foxp3-expressing regulatory T cells (Tregs). In vivo, Treg abundance is inversely correlated with ITK expression, and inducible Treg development is inversely dependent on ITK kinase activity. While Treg development normally requires both hematopoietic and thymic MHC class 2 (MHC2) expression, the absence of ITK allows Treg development with MHC2 expression in either compartment, with preference for selection by thymic MHC2, suggesting a gatekeeper role for ITK in ensuring that only Tregs selected by both thymic and hematopoietic MHC2 survive selection. Although ITK suppresses Treg development and is not required for maintenance of neuropilin-1-positive natural Tregs in the periphery, it is indispensable for Treg functional suppression of naive CD4(+) T cell-induced colitis in Rag(-/-) recipients. ITK thus regulates the development and function of Tregs.
in vivo ICOSL neutralization
Baumjohann, D., et al (2013). "Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype" Immunity 38(3): 596-605.
PubMed
T follicular helper (Tfh) cells provide help to B cells and are crucial for establishment of germinal center (GC) reactions, including production of high-affinity antibodies and generation of memory B cells and long-lived plasma cells. Here we report that the magnitude of the Tfh cell response was dictated by the amount of antigen and directly correlated with the magnitude of the GC B cell response. In addition, maintenance of the Tfh cell phenotype required sustained antigenic stimulation by GC B cells. In lymphopenic conditions, a strong and prolonged Tfh cell response led to bystander B cell activation, hypergammaglobulinemia, and production of poly- and self-reactive antibodies. These data demonstrate that antigen dose determines the size and duration of the Tfh cell response and GC reaction, highlight the transient nature of the Tfh cell phenotype, and suggest a link between overstimulation of Tfh cells and the development of dysregulated humoral immune responses.
in vivo ICOSL neutralization
Colino, J., et al (2013). "Noncovalent association of protein and capsular polysaccharide on bacteria-sized latex beads as a model for polysaccharide-specific humoral immunity to intact gram-positive extracellular bacteria" J Immunol 191(6): 3254-326
PubMed
Intact Streptococcus pneumoniae expressing type 14 capsular polysaccharide (PPS14) and type III S. agalactiae containing a PPS14 core capsule identical to PPS14 exhibit noncovalent associations of PPS14 and bacterial protein, in contrast to soluble covalent conjugates of these respective Ags. Both bacteria and conjugates induce murine PPS14-specific IgG responses dependent on CD4(+) T cells. Further, secondary immunization with conjugate and S. agalactiae, although not S. pneumoniae, results in a boosted response. However, in contrast to conjugate, PPS14-specific IgG responses to bacteria lack affinity maturation use the 44.1-idiotype and are dependent on marginal zone B cells. To better understand the mechanism underlying this dichotomy, we developed a minimal model of intact bacteria in which PPS14 and pneumococcal surface protein A (PspA) were stably attached to 1 mum (bacteria-sized) latex beads, but not directly linked to each other, in contrast to PPS14-PspA conjugate. Beads coated simultaneously with PPS14+, similar to conjugate, induced in mice boosted PPS14-specific IgG secondary responses, dependent on T cells and ICOS-dependent costimulation, and in which priming could be achieved with PspA alone. In contrast to conjugate, but similar to intact bacteria, the primary PPS14-specific IgG response to beads coated simultaneously with PPS14+ peaked rapidly, with the secondary response highly enriched for the 44.1-idiotype and lacking affinity maturation. These results demonstrate that noncovalent association in a particle, of polysaccharide and protein, recapitulates essential immunologic characteristics of intact bacteria that are distinct from soluble covalent conjugates of these respective Ags.
in vivo ICOSL neutralization
Arjunaraja, S., et al (2012). "Structurally identical capsular polysaccharide expressed by intact group B streptococcus versus Streptococcus pneumoniae elicits distinct murine polysaccharide-specific IgG responses in vivo" J Immunol 188(11): 5238-524
PubMed
We previously reported distinct differences in the murine in vivo Ig polysaccharide (PS)-specific responses to intact Streptococcus pneumoniae compared with responses to Neisseria meningitidis and that in each case, the bacterial subcapsular domain markedly influences the Ig response to the associated PS. In light of potentially unique contributions of biochemically distinct capsular PS and/or their characteristic attachments to the underlying bacterium, it remains unresolved whether different bacterial subcapsular domains can exert differential effects on PS-specific Ig responses to distinct bacterial pathogens. In this report, we used a mutant strain of group B Streptococcus (Streptococcus agalactiae) type III (GBS-III) that expresses desialylated capsular polysaccharide of GBS-III, biochemically identical to capsular pneumococcal polysaccharide type 14 (PPS14) of Streptococcus pneumoniae (intact inactivated Streptococcus pneumoniae, capsular type 14, Pn14), directly to compare the in vivo PPS14-specific IgG responses to two distinct gram-positive bacteria. Although both GBS-III and Pn14 elicited relatively rapid primary PPS14-specific IgG responses dependent on CD4(+) T cells, B7-dependent costimulation, and CD40-CD40L interactions, only GBS-III induced a highly boosted ICOS-dependent PPS14-specific IgG response after secondary immunization. Of note, priming with Pn14 and boosting with GBS-III, although not isolated PPS14, elicited a similar boosted PPS14-specific IgG response that was dependent on CD4(+) T cells during secondary immunization, indicating that Pn14 primes for memory but, unlike GBS-III, fails to elicit it. The inability of Pn14 to elicit a boosted PPS14-specific IgG response was overcome by coimmunization with unencapsulated GBS-III. Collectively, these data establish that structurally identical capsular PS expressed by two distinct gram-positive extracellular bacteria can indeed elicit distinct PS-specific IgG responses in vivo.
in vivo ICOSL neutralization
Arjunaraja, S., et al (2012). "The nature of an in vivo anti-capsular polysaccharide response is markedly influenced by the composition and/or architecture of the bacterial subcapsular domain" J Immunol 188(2): 569-577.
PubMed
In vivo anti-polysaccharide Ig responses to isolated polysaccharide (PS) are T cell independent, rapid, and fail to generate memory. However, little is known regarding PS-specific Ig responses to intact gram-positive and gram-negative extracellular bacteria. We previously demonstrated that intact heat-killed Streptococcus pneumoniae, a gram-positive bacterium, elicited a rapid primary pneumococcal capsular PS (PPS) response in mice that was dependent on CD4(+) T cells, B7-dependent costimulation, and CD40-CD40L interactions. However, this response was ICOS independent and failed to generate a boosted PPS-specific secondary IgG response. In the current study, we analyzed the murine meningococcal type C PS (MCPS)-specific Ig response to i.p.-injected intact, heat-killed Neisseria meningitidis, serogroup C (MenC), a gram-negative bacterium. In contrast to S. pneumoniae, the IgG anti-MCPS response to MenC exhibited delayed primary kinetics and was highly boosted after secondary immunization, whereas the IgG anti-MCPS response to isolated MCPS was rapid, without secondary boosting, and consisted of only IgG1 and IgG3, as opposed to all four IgG isotypes in response to intact MenC. The secondary, but not primary, IgG anti-MCPS response to MenC was dependent on CD4(+) T cells, CD40L, CD28, and ICOS. The primary and secondary IgG anti-MCPS responses were lower in TLR4-defective (C3H/HeJ) but not TLR2(-/-) or MyD88(-/-) mice, but secondary boosting was still observed. Of interest, coimmunization of S. pneumoniae and MenC resulted in a boosted secondary IgG anti-PPS response to S. pneumoniae. Our data demonstrate that the nature of the in vivo anti-PS response is markedly influenced by the composition and/or architecture of the bacterial subcapsular domain.
in vivo ICOSL neutralization
Choi, Y. S., et al (2011). "ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6" Immunity 34(6): 932-946.
PubMed
The nature of follicular helper CD4(+) T (Tfh) cell differentiation remains controversial, including the minimal signals required for Tfh cell differentiation and the time at which Tfh cell differentiation occurs. Here we determine that Tfh cell development initiates immediately during dendritic cell (DC) priming in vivo. We demonstrate that inducible costimulator (ICOS) provides a critical early signal to induce the transcription factor Bcl6, and Bcl6 then induces CXCR5, the canonical feature of Tfh cells. Strikingly, a bifurcation between Tfh and effector Th cells was measurable by the second cell division of CD4(+) T cells, at day 2 after an acute viral infection: IL2Ralpha(int) cells expressed Bcl6 and CXCR5 (Tfh cell program), whereas IL2Ralpha(hi) cells exhibited strong Blimp1 expression that repressed Bcl6 (effector Th cell program). Virtually complete polarization between Bcl6(+) Tfh cells and Blimp1(+) effector Th cell populations developed by 72 hr, even without B cells. Tfh cells were subsequently lost in the absence of B cells, demonstrating a B cell requirement for maintenance of Bcl6 and Tfh cell commitment via sequential ICOS signals.
Product Citations
-
-
Mus musculus (Mouse)
The protozoan commensal Tritrichomonas musculis is a natural adjuvant for mucosal IgA.
In J Exp Med on 2 December 2024 by Cao, E. Y., Burrows, K., et al.
PubMed
Immunoglobulin (Ig) A supports mucosal immune homeostasis and host-microbiota interactions. While commensal bacteria are known for their ability to promote IgA, the role of non-bacterial commensal microbes in the induction of IgA remains elusive. Here, we demonstrate that permanent colonization with the protozoan commensal Tritrichomonas musculis (T.mu) promotes T cell-dependent, IgA class-switch recombination, and intestinal accumulation of IgA-secreting plasma cells (PC). T.mu colonization specifically drives the expansion of T follicular helper cells and a unique ICOS+ non-Tfh cell population, accompanied by an increase in germinal center B cells. Blockade of ICOS:ICOSL co-stimulation or MHCII-expression on B cells is central for the induction of IgA following colonization by T.mu, implicating a previously underappreciated mode of IgA induction following protozoan commensal colonization. Finally, T.mu further improves the induction of IgA-secreting PC specific to orally ingested antigens and their peripheral dissemination, identifying T.mu as a "natural adjuvant" for IgA. Collectively, these findings propose a protozoa-driven mode of IgA induction to support intestinal immune homeostasis.
-
-
-
Immunology and Microbiology
-
Cancer Research
Interruption of the intratumor CD8+ T cell:Treg crosstalk improves the efficacy of PD-1 immunotherapy.
In Cancer Cell on 10 June 2024 by Geels, S. N., Moshensky, A., et al.
PubMed
PD-1 blockade unleashes potent antitumor activity in CD8+ T cells but can also promote immunosuppressive T regulatory (Treg) cells, which may worsen the response to immunotherapy. Tumor-Treg inhibition is a promising strategy to improve the efficacy of checkpoint blockade immunotherapy; however, our understanding of the mechanisms supporting tumor-Tregs during PD-1 immunotherapy is incomplete. Here, we show that PD-1 blockade increases tumor-Tregs in mouse models of melanoma and metastatic melanoma patients. Mechanistically, Treg accumulation is not caused by Treg-intrinsic inhibition of PD-1 signaling but depends on an indirect effect of activated CD8+ T cells. CD8+ T cells produce IL-2 and colocalize with Tregs in mouse and human melanomas. IL-2 upregulates the anti-apoptotic protein ICOS on tumor-Tregs, promoting their accumulation. Inhibition of ICOS signaling before PD-1 immunotherapy improves control over immunogenic melanoma. Thus, interrupting the intratumor CD8+ T cell:Treg crosstalk represents a strategy to enhance the therapeutic efficacy of PD-1 immunotherapy.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Kruppel-like factor 2+ CD4 T cells avert microbiota-induced intestinal inflammation.
In Cell Rep on 28 November 2023 by Shao, T. Y., Jiang, T. T., et al.
PubMed
Intestinal colonization by antigenically foreign microbes necessitates expanded peripheral immune tolerance. Here we show commensal microbiota prime expansion of CD4 T cells unified by the Kruppel-like factor 2 (KLF2) transcriptional regulator and an essential role for KLF2+ CD4 cells in averting microbiota-driven intestinal inflammation. CD4 cells with commensal specificity in secondary lymphoid organs and intestinal tissues are enriched for KLF2 expression, and distinct from FOXP3+ regulatory T cells or other differentiation lineages. Mice with conditional KLF2 deficiency in T cells develop spontaneous rectal prolapse and intestinal inflammation, phenotypes overturned by eliminating microbiota or reconstituting with donor KLF2+ cells. Activated KLF2+ cells selectively produce IL-10, and eliminating IL-10 overrides their suppressive function in vitro and protection against intestinal inflammation in vivo. Together with reduced KLF2+ CD4 cell accumulation in Crohn's disease, a necessity for the KLF2+ subpopulation of T regulatory type 1 (Tr1) cells in sustaining commensal tolerance is demonstrated.
-
-
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
-
Mus musculus (Mouse)
Senescence-induced endothelial phenotypes underpin immune-mediated senescence surveillance.
In Genes Dev on 1 May 2022 by Yin, K., Patten, D. A., et al.
PubMed
Senescence is a stress-responsive tumor suppressor mechanism associated with expression of the senescence-associated secretory phenotype (SASP). Through the SASP, senescent cells trigger their own immune-mediated elimination, which if evaded leads to tumorigenesis. Senescent parenchymal cells are separated from circulating immunocytes by the endothelium, which is targeted by microenvironmental signaling. Here we show that SASP induces endothelial cell NF-κB activity and that SASP-induced endothelial expression of the canonical NF-κB component Rela underpins senescence surveillance. Using human liver sinusoidal endothelial cells (LSECs), we show that SASP-induced endothelial NF-κB activity regulates a conserved transcriptional program supporting immunocyte recruitment. Furthermore, oncogenic hepatocyte senescence drives murine LSEC NF-κB activity in vivo. Critically, we show two distinct endothelial pathways in senescence surveillance. First, endothelial-specific loss of Rela prevents development of Stat1-expressing CD4+ T lymphocytes. Second, the SASP up-regulates ICOSLG on LSECs, with the ICOS-ICOSLG axis contributing to senescence cell clearance. Our results show that the endothelium is a nonautonomous SASP target and an organizing center for immune-mediated senescence surveillance.
-
-
-
Immunology and Microbiology
-
Neuroscience
-
Mus musculus (Mouse)
Engagement of the costimulatory molecule ICOS in tissues promotes establishment of CD8+ tissue-resident memory T cells.
In Immunity on 11 January 2022 by Peng, C., Huggins, M. A., et al.
PubMed
Elevated gene expression of the costimulatory receptor Icos is a hallmark of CD8+ tissue-resident memory (Trm) T cells. Here, we examined the contribution of ICOS in Trm cell differentiation. Upon transfer into WT mice, Icos-/- CD8+ T cells exhibited defective Trm generation but produced recirculating memory populations normally. ICOS deficiency or ICOS-L blockade compromised establishment of CD8+ Trm cells but not their maintenance. ICOS ligation during CD8+ T cell priming did not determine Trm induction; rather, effector CD8+ T cells showed reduced Trm differentiation after seeding into Icosl-/- mice. IcosYF/YF CD8+ T cells were compromised in Trm generation, indicating a critical role for PI3K signaling. Modest transcriptional changes in the few Icos-/- Trm cells suggest that ICOS-PI3K signaling primarily enhances the efficiency of CD8+ T cell tissue residency. Thus, local ICOS signaling promotes production of Trm cells, providing insight into the contribution of costimulatory signals in the generation of tissue-resident populations.
-
-
-
Immunology and Microbiology
ICOS expression is required for maintenance but not the formation of germinal centers in the spleen in response to P. yoelii infection
In bioRxiv on 25 August 2021 by O’Neal, K. A., Latham, L. E., et al.
-
-
-
Immunology and Microbiology
CTLA4-Ig-Based Bifunctional Costimulation Inhibitor Blocks CD28 and ICOS Signaling to Prevent T Cell Priming and Effector Function.
In J Immunol on 1 March 2021 by Goenka, R., Xu, Z., et al.
PubMed
CTLA4-Ig/abatacept dampens activation of naive T cells by blocking costimulation via CD28. It is an approved drug for rheumatoid arthritis but failed to deliver efficacy in a number of other autoimmune diseases. One explanation is that activated T cells rely less on CD28 signaling and use alternate coreceptors for effector function. ICOS is critical for activation of T-dependent humoral immune responses, which drives pathophysiology of IgG-mediated autoimmune diseases. In this study, we asked whether CD28 and ICOS play nonredundant roles for maintenance of T-dependent responses in mouse models. Using a hapten-protein immunization model, we show that during an ongoing germinal center response, combination treatment with CTLA4-Ig and ICOS ligand (ICOSL) blocking Ab completely dissolves ongoing germinal center responses, whereas single agents show only partial activity. Next, we took two approaches to engineer a therapeutic molecule that blocks both pathways. First, we engineered CTLA4-Ig to enhance binding to ICOSL while retaining affinity to CD80/CD86. Using a library approach, binding affinity of CTLA4-Ig to human ICOSL was increased significantly from undetectable to 15-42 nM; however, the affinity was still insufficient to completely block binding of ICOSL to ICOS. Second, we designed a bispecific costimulation inhibitor with high-affinity CTLA4 extracellular domains fused to anti-ICOSL Ab termed bifunctional costimulation inhibitor. With this bispecific approach, we achieved complete inhibition of CD80 and CD86 binding to CD28 as well as ICOS binding to ICOSL. Such bispecific molecules may provide greater therapeutic benefit in IgG-mediated inflammatory diseases compared with CTLA4-Ig alone.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Cytomegalovirus restricts ICOSL expression on antigen-presenting cells disabling T cell co-stimulation and contributing to immune evasion.
In Elife on 18 January 2021 by Angulo, G., Zeleznjak, J., et al.
PubMed
Viral infections are controlled, and very often cleared, by activated T lymphocytes. The inducible co-stimulator (ICOS) mediates its functions by binding to its ligand ICOSL, enhancing T-cell activation and optimal germinal center (GC) formation. Here, we show that ICOSL is heavily downmodulated during infection of antigen-presenting cells by different herpesviruses. We found that, in murine cytomegalovirus (MCMV), the immunoevasin m138/fcr-1 physically interacts with ICOSL, impeding its maturation and promoting its lysosomal degradation. This viral protein counteracts T-cell responses, in an ICOS-dependent manner, and limits virus control during the acute MCMV infection. Additionally, we report that blockade of ICOSL in MCMV-infected mice critically regulates the production of MCMV-specific antibodies due to a reduction of T follicular helper and GC B cells. Altogether, these findings reveal a novel mechanism evolved by MCMV to counteract adaptive immune surveillance, and demonstrates a role of the ICOS:ICOSL axis in the host defense against herpesviruses.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Host immunology and rational immunotherapy for carbapenem-resistant Klebsiella pneumoniae infection.
In JCI Insight on 23 April 2020 by Iwanaga, N., Sandquist, I., et al.
PubMed
Infections due to carbapenem-resistant Klebsiella pneumoniae have emerged as a global threat due to its widespread antimicrobial resistance. Transplant recipients and patients with hematologic malignancies have high mortality rate, suggesting host factors in susceptibility. We developed a model of pulmonary infection using ST258 strain C4, KPC-2 clone, which are predominant K. pneumoniae carbapenemase-producing (KPC-producing) bacteria, and demonstrated that Rag2-/- Il2rg-/- mice - but not WT C57BL/6 or Rag2-/- mice - were susceptible to this opportunistic infection. Using single cell RNA sequencing in infected Rag2-/- mice, we identified distinct clusters of Ifng+ NK cells and Il17a+, Il22+, and inducible T cell costimulatory molecule-positive (ICOS+) group 3 innate lymphoid cells (ILCs) that were critical for host resistance. As solid organ transplantation is a risk factor, we generated a more clinically relevant model using FK506 in WT C57BL/6 mice. We further demonstrated that immunotherapy with recombinant IL-22 treatment ameliorated the ST258 pulmonary infection in both FK506-treated WT mice and Rag2-/- Il2rg-/- mice via hepatic IL-22ra1 signaling. These data support the development of host-directed immunotherapy as an adjunct treatment to new antibiotics.
-
-
-
Blocking experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
ICOS signaling promotes a secondary humoral response after re-challenge with Plasmodium chabaudi chabaudi AS.
In PLoS Pathog on 1 April 2020 by Latham, L. E., Wikenheiser, D. J., et al.
PubMed
The co-stimulatory molecule ICOS is associated with the induction and regulation of T helper cell responses, including the differentiation of follicular helper T (Tfh) cells and the formation and maintenance of memory T cells. However, the role of ICOS signaling in secondary immune responses is largely unexplored. Here we show that memory T cell formation and maintenance are influenced by persistent infection with P. chabaudi chabaudi AS infection, as memory T cell numbers decline in wild-type and Icos-/- mice after drug-clearance. Following drug-clearance Icos-/- mice display a relapsing parasitemia that occurs more frequently and with higher peaks compared to wild-type mice after re-challenge. The secondary immune response in Icos-/- mice is characterized by significant impairment in the expansion of effector cells with a Tfh-like phenotype, which is associated with a diminished and delayed parasite-specific Ab response and the absence of germinal centers. Similarly, the administration of an anti-ICOSL antagonizing antibody to wild-type mice before and after reinfection with P. c. chabaudi AS leads to an early defect in Tfh cell expansion and parasite-specific antibody production, confirming a need for ICOS-ICOSL interactions to promote memory B cell responses. Furthermore, adoptive transfer of central memory T (TCM) cells from wild-type and Icos-/- mice into tcrb-/- mice to directly evaluate the ability of TCM cells to give rise to Tfh cells revealed that TCM cells from wild-type mice acquire a mixed Th1- and Tfh-like phenotype after P. c. chabaudi AS infection. While TCM cells from Icos-/- mice expand and display markers of activation to a similar degree as their WT counterparts, they displayed a reduced capacity to upregulate markers indicative of a Tfh cell phenotype, resulting in a diminished humoral response. Together these findings verify that ICOS signaling in memory T cells plays an integral role in promoting T cell effector responses during secondary infection with P. c. chabaudi AS.
-
-
-
Neutralization experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Complement Signals Determine Opposite Effects of B Cells in Chemotherapy-Induced Immunity.
In Cell on 19 March 2020 by Lu, Y., Zhao, Q., et al.
PubMed
Understanding molecular mechanisms that dictate B cell diversity is important for targeting B cells as anti-cancer treatment. Through the single-cell dissection of B cell heterogeneity in longitudinal samples of patients with breast cancer before and after neoadjuvant chemotherapy, we revealed that an ICOSL+ B cell subset emerges after chemotherapy. Using three immunocompetent mouse models, we recapitulated the subset switch of human tumor-infiltrating B cells during chemotherapy. By employing B-cell-specific deletion mice, we showed that ICOSL in B cells boosts anti-tumor immunity by enhancing the effector to regulatory T cell ratio. The signature of ICOSL+ B cells is imprinted by complement-CR2 signaling, which is triggered by immunogenic cell death. Moreover, we identified that CD55, a complement inhibitory protein, determines the opposite roles of B cells in chemotherapy. Collectively, we demonstrated a critical role of the B cell subset switch in chemotherapy response, which has implications in designing novel anti-cancer therapies. VIDEO ABSTRACT.
-
-
-
Immunology and Microbiology
B cells are sufficient to prime the dominant CD4+ Tfh response to Plasmodium infection.
In J Exp Med on 3 February 2020 by Arroyo, E. N., Pepper, M., et al.
PubMed
CD4+ T follicular helper (Tfh) cells dominate the acute response to a blood-stage Plasmodium infection and provide signals to direct B cell differentiation and protective antibody expression. We studied antigen-specific CD4+ Tfh cells responding to Plasmodium infection in order to understand the generation and maintenance of the Tfh response. We discovered that a dominant, phenotypically stable, CXCR5+ Tfh population emerges within the first 4 d of infection and results in a CXCR5+ CCR7+ Tfh/central memory T cell response that persists well after parasite clearance. We also found that CD4+ T cell priming by B cells was both necessary and sufficient to generate this Tfh-dominant response, whereas priming by conventional dendritic cells was dispensable. This study provides important insights into the development of CD4+ Tfh cells during Plasmodium infection and highlights the heterogeneity of antigen-presenting cells involved in CD4+ T cell priming.
-
-
-
Genetics
-
Immunology and Microbiology
Histone methyltransferase Nsd2 is required for follicular helper T cell differentiation.
In J Exp Med on 6 January 2020 by Long, X., Zhang, L., et al.
PubMed
Follicular helper T (Tfh) cells provide essential help for humoral immune response. Transcriptional factor Bcl6 is the master regulator for Tfh generation and is induced very early after T cell activation in a CD28-dependent manner, but how CD28 signal promotes Bcl6 early expression remains unknown. Here we found that CD28 signal quickly induces expression of the H3K36me2 methytransferase Nsd2, which is required for Bcl6 expression as early as the first cell division after T cell activation. Nsd2 deficiency in T cells leads to decreased Bcl6 expression, impaired Tfh generation, compromised germinal center response, and delayed virus clearance. Ectopic Bcl6 expression rescues the Tfh defect of Nsd2 KO cells. ICOS signal is dispensable for early Nsd2 induction but required for sustained Nsd2 expression, which is critical for Tfh maintenance. Overexpression of Nsd2 increases Bcl6 expression and enhances Tfh generation; 4-mo-old mice even develop spontaneous Tfh. Overall, our study reveals Nsd2 as a critical epigenetic regulator for Tfh differentiation.
-
-
-
Neuroscience
Protection of bona fide T follicular memory cells during tissue isolation reveals their persistence, plasticity and functional impact
In bioRxiv on 23 June 2019 by Künzli, M., Schreiner, D., et al.
-
-
Food antigens drive spontaneous IgE elevation in the absence of commensal microbiota.
In Sci Adv on 1 May 2019 by Hong, S. W., O, E., et al.
PubMed
Immunoglobulin E (IgE), a key mediator in allergic diseases, is spontaneously elevated in mice with disrupted commensal microbiota such as germ-free (GF) and antibiotics-treated mice. However, the underlying mechanisms for aberrant IgE elevation are still unclear. Here, we demonstrate that food antigens drive spontaneous IgE elevation in GF and antibiotics-treated mice by generating T helper 2 (TH2)-skewed T follicular helper (TFH) cells in gut-associated lymphoid tissues (GALTs). In these mice, depriving contact with food antigens results in defective IgE elevation as well as impaired generation of TFH cells and IgE-producing cells in GALT. Food antigen-driven TFH cells in GF mice are mostly generated in early life, especially during the weaning period. We also reveal that food antigen-driven TFH cells in GF mice are actively depleted by colonization with commensal microbiota. Thus, our findings provide a possible explanation for why the perturbation of commensal microbiota in early life increases the occurrence of allergic diseases.
-
-
Cancer Research
-
Immunology and Microbiology
-
Flow cytometry/Cell sorting
Acute Myeloid Leukemia Cells Express ICOS Ligand to Promote the Expansion of Regulatory T Cells.
In Front Immunol on 16 October 2018 by Han, Y., Dong, Y., et al.
PubMed
CD4+CD25+Foxp3+ regulatory T cells (Tregs) accumulate in bone marrow microenvironment in acute myeloid leukemia (AML). However, little is known about how the tumor environment including tumor cells themselves affects this process. Here we demonstrated that AML cells expressed inducible T-cell costimulator ligand (ICOSL) that can provide costimulation through ICOS for the conversion and expansion of Tregs sustaining high Foxp3 and CD25 expression as well as a suppressive function. TNF-a stimulation up-regulated the expression of ICOSL. Furthermore, both the conversion and expansion of CD4+CD25+Foxp3+ T cells and CD4+ICOS+Foxp3+ T cells were induced by co-culture with AML cells overexpressed ICOSL. CD4+CD25+ICOS+ T cells possessed stronger ability to secrete IL-10 than CD4+CD25+ICOS- T cells. The mechanism by which IL-10 promoted the proliferation of AML cells was dependent on the activation of the Akt, Erk1/2, p38, and Stat3 signaling pathways. Blockade of ICOS signaling using anti-ICOSL antibody impaired the generation of Tregs and retarded the progression of an AML mice model injected with C1498 cells. The expression of ICOSL of patient AML cells and ICOS+ Tregs were found to be predictors for overall survival and disease-free survival in patients with AML, with ICOS+ Treg cell subset being a stronger predictor than total Tregs. These results suggest that ICOSL expression by AML cells may directly drive Treg expansion as a mechanism of immune evasion and ICOS+ Treg cell frequency is a better prognostic predictor in patients with AML.
-
-
-
Immunology and Microbiology
-
In vivo experiments
-
Mus musculus (Mouse)
Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis.
In Nat Commun on 15 March 2018 by Gaddis, D. E., Padgett, L. E., et al.
PubMed
Regulatory T (Treg) cells contribute to the anti-inflammatory response during atherogenesis. Here we show that during atherogenesis Treg cells lose Foxp3 expression and their immunosuppressive function, leading to the conversion of a fraction of these cells into T follicular helper (Tfh) cells. We show that Tfh cells are pro-atherogenic and that their depletion reduces atherosclerosis. Mechanistically, the conversion of Treg cells to Tfh cells correlates with reduced expression of IL-2Rα and pSTAT5 levels and increased expression of IL-6Rα. In vitro, incubation of naive T cells with oxLDL prevents their differentiation into Treg cells. Furthermore, injection of lipid-free Apolipoprotein AI (ApoAI) into ApoE-/- mice reduces intracellular cholesterol levels in Treg cells and prevents their conversion into Tfh cells. Together our results suggest that ApoAI, the main protein in high-density lipoprotein particles, modulates the cellular fate of Treg cells and thus influences the immune response during atherosclerosis.
-
-
Therapeutic ICOS blockade reduces T follicular helper cells and improves allergic airway disease
In bioRxiv on 18 January 2018 by Uwadiae, F. I., Pyle, C., et al.
-
-
Immunology and Microbiology
Foxp1 Negatively Regulates T Follicular Helper Cell Differentiation and Germinal Center Responses by Controlling Cell Migration and CTLA-4.
In J Immunol on 15 January 2018 by Shi, B., Geng, J., et al.
PubMed
T follicular helper (Tfh) cells play an essential role in the formation of germinal centers (GC) and generation of high-affinity Abs. The homing of activated CD4+ T cells into B cell follicles and the involvement of key costimulatory and coinhibitory molecules are critical in controlling both the initiation and the magnitude of GC responses. Meanwhile, studies have shown that a high number of single clone B cells leads to intraclonal competition, which inhibits the generation of high-affinity Abs. Our previous work has shown that transcription factor Foxp1 is a critical negative regulator of Tfh cell differentiation. In this study, we report that the deletion of Foxp1 leads to a high proportion of activated CD4+ T cells homing into B cell follicles with faster kinetics, resulting in earlier GC formation. In addition, we show that Foxp1-deficient Tfh cells restore the generation of high-affinity Abs when cotransferred with high numbers of single clone B cells. We find that Foxp1 regulates the expression levels of cytotoxic T lymphocyte-associated Ag-4 (CTLA-4) in activated CD4+ T cells and that Ctla4 is a direct Foxp1 target. Finally, we demonstrate that CTLA-4 expression on conventional CD4+ T cells plays a cell-intrinsic role in Tfh cell differentiation in vivo, and CTLA-4 blockade helps abolish the intraclonal competition of B cells in generating high-affinity Abs.
-
-
-
Immunology and Microbiology
The strength of BCR signaling shapes terminal development of follicular helper T cells in mice.
In Eur J Immunol on 1 August 2017 by Sacquin, A., Gador, M., et al.
PubMed
Antibody production is key for effective immune response and relies on follicular helper T (Tfh) cells. B cell-Tfh cell interactions result either in an extra-follicular low affinity B-cell response or in germinal center reactions producing high-affinity memory B cells and long-lived plasma cells. As Tfh cells influence B-cell commitment, it also became clear that B cells influence these interactions in ways that still remain unresolved. We observed that strong BCR signals decreased Tfh-cell differentiation in vitro, which correlated with decreased expression of ICOS-L at the surface of stimulated B cells. Further, we comprehensively demonstrated that ICOS-L expression correlated with the level of Tfh differentiation irrespective of antigen presentation at the surface of activated B cells. Our in vivo experiments could show that immunization with a high-affinity antigen for B cells resulted in much less Tfh development than immunization with low-affinity antigen. Furthermore, blocking ICOS-L in vivo inhibited Tfh development when using low-affinity antigen. Altogether, these results indicate that BCR affinity shapes Tfh-cell development in part through ICOS/ICOS-L interactions. Ultimately, we reveal new depths in the B cell-Tfh cell crosstalk that could eventually result in better vaccine protocols.
-