InVivoMAb anti-mouse/human/rat CD47 (IAP)
Product Description
Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | purified human placental CD47 |
| Reported Applications |
in vivo CD47 blockade in vitro CD47 blocking Immunofluorescence |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
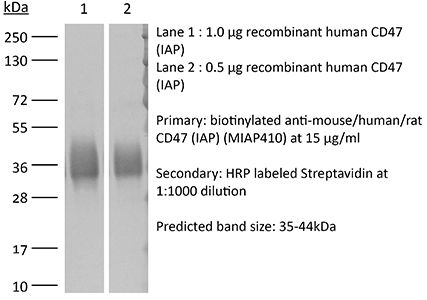
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687806 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo CD47 blockade
in vitro CD47 blockade
Kojima, Y., et al (2016). "CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis" Nature. DOI : 10.1038/nature18935.
PubMed
Atherosclerosis is the disease process that underlies heart attack and stroke. Advanced lesions at risk of rupture are characterized by the pathological accumulation of diseased vascular cells and apoptotic cellular debris. Why these cells are not cleared remains unknown. Here we show that atherogenesis is associated with upregulation of CD47, a key anti-phagocytic molecule that is known to render malignant cells resistant to programmed cell removal, or ‘efferocytosis’. We find that administration of CD47-blocking antibodies reverses this defect in efferocytosis, normalizes the clearance of diseased vascular tissue, and ameliorates atherosclerosis in multiple mouse models. Mechanistic studies implicate the pro-atherosclerotic factor TNF-alpha as a fundamental driver of impaired programmed cell removal, explaining why this process is compromised in vascular disease. Similar to recent observations in cancer, impaired efferocytosis appears to play a pathogenic role in cardiovascular disease, but is not a fixed defect and may represent a novel therapeutic target.
in vivo CD47 blockade
in vitro CD47 blockade
Immunofluorescence
Willingham, S. B., et al (2012). "The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors" Proc Natl Acad Sci U S A 109(17): 6662-6667.
PubMed
CD47, a “don’t eat me” signal for phagocytic cells, is expressed on the surface of all human solid tumor cells. Analysis of patient tumor and matched adjacent normal (nontumor) tissue revealed that CD47 is overexpressed on cancer cells. CD47 mRNA expression levels correlated with a decreased probability of survival for multiple types of cancer. CD47 is a ligand for SIRPalpha, a protein expressed on macrophages and dendritic cells. In vitro, blockade of CD47 signaling using targeted monoclonal antibodies enabled macrophage phagocytosis of tumor cells that were otherwise protected. Administration of anti-CD47 antibodies inhibited tumor growth in orthotopic immunodeficient mouse xenotransplantation models established with patient tumor cells and increased the survival of the mice over time. Anti-CD47 antibody therapy initiated on larger tumors inhibited tumor growth and prevented or treated metastasis, but initiation of the therapy on smaller tumors was potentially curative. The safety and efficacy of targeting CD47 was further tested and validated in immune competent hosts using an orthotopic mouse breast cancer model. These results suggest all human solid tumor cells require CD47 expression to suppress phagocytic innate immune surveillance and elimination. These data, taken together with similar findings with other human neoplasms, show that CD47 is a commonly expressed molecule on all cancers, its function to block phagocytosis is known, and blockade of its function leads to tumor cell phagocytosis and elimination. CD47 is therefore a validated target for cancer therapies.
Immunofluorescence
Han, X., et al (2000). "CD47, a ligand for the macrophage fusion receptor, participates in macrophage multinucleation" J Biol Chem 275(48): 37984-37992.
PubMed
The macrophage fusion receptor (MFR), also called P84/BIT/SIRPalpha/SHPS-1, is a transmembrane glycoprotein that belongs to the superfamily of immunoglobulins. Previously, we showed that MFR expression is highly induced at the onset of fusion in macrophages, and that MFR appears to play a role in macrophage-macrophage adhesion/fusion leading to multinucleation. The recent finding that IAP/CD47 acts as a ligand for MFR led us to hypothesize that it interacts with CD47 at the onset of cell-cell fusion. CD47 is a transmembrane glycoprotein, which, like MFR, belongs to the superfamily of immunoglobulins. We show that macrophages express the hemopoietic form of CD47, the expression of which is induced at the onset of fusion, but to a lower level than MFR. A glutathione S-transferase CD47 fusion protein engineered to contain the extracellular domain of CD47, binds macrophages, associates with MFR, and prevents multinucleation. CD47 and MFR associate via their amino-terminal immunoglobulin variable domain. Of the nine monoclonal antibodies raised against the extracellular domain of CD47, three block fusion, as well as MFR-CD47 interaction, whereas the others have no effect. Together, these data suggest that CD47 is involved in macrophage multinucleation by virtue of interacting with MFR during adhesion/fusion.
Product Citations
-
-
Immunology and Microbiology
Inhibiting KRAS with CD47 and immune checkpoint overcomes intrinsic resistance to combined KRAS and immune checkpoint inhibitor therapy.
In Cell Rep Med on 16 September 2025 by Hirade, K., Tanaka, N., et al.
PubMed
Although Kirsten rat sarcoma virus (KRAS) G12C inhibitors alter the treatment strategy for patients with KRAS G12C-mutant lung cancer, their efficacy remains insufficient to eliminate tumors. Here, we identify that inhibition of mutant KRAS promotes escape from macrophage phagocytosis by upregulating the expression of cluster of differentiation 47 (CD47) and CD24. These proteins are induced by the binding of FOXA1 to the super-enhancer of CD47 and grainyhead-like transcription factor 2 (GRHL2) to the promoter of CD24, respectively. Whereas the addition of an anti-CD47 antibody restores macrophage phagocytosis, phagocytic macrophages induce programmed death-ligand 1 (PD-L1) expression, resulting in the suppression of CD8 T cell activation. Combination of a KRAS inhibitor with anti-CD47 and anti-PD-L1 antibodies achieves long-term survival in an orthotopic murine model recalcitrant to KRAS inhibition with immune checkpoint therapy. These results suggest that targeting KRAS with an anti-CD47 antibody and immune checkpoint blockade is a promising strategy, especially in immune-cold lung tumors.
-
-
-
Immunocytochemistry-immunofluorescence
-
Immunology and Microbiology
-
Cancer Research
Ex vivo engineering of phagocytic signals in breast cancer cells for a whole tumor cell-based vaccine.
In BMC Cancer on 1 July 2025 by Martí-Díaz, R., Sánchez-del-Campo, L., et al.
PubMed
Today, cell therapies are constantly evolving and providing new options for cancer patients. These therapies are mostly based on the inoculation of immune cells extracted from a person's own tumor; however, some studies using whole tumor cell-based vaccines are approaching the level of maturity required for clinical use. Although these latest therapies will have to be developed further and adapted to overcome many ethical barriers, there is no doubt that therapeutic cancer vaccines are the next frontier of immunotherapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Mus musculus (Mouse)
-
Immunocytochemistry-immunofluorescence
Ex vivo engineering of phagocytic signals in breast cancer cells for a whole tumor cell-based vaccine.
In BMC Cancer on 1 July 2025 by Martí-Díaz, R., Sánchez-del-Campo, L., et al.
PubMed
Today, cell therapies are constantly evolving and providing new options for cancer patients. These therapies are mostly based on the inoculation of immune cells extracted from a person's own tumor; however, some studies using whole tumor cell-based vaccines are approaching the level of maturity required for clinical use. Although these latest therapies will have to be developed further and adapted to overcome many ethical barriers, there is no doubt that therapeutic cancer vaccines are the next frontier of immunotherapy.
-
-
-
Cell Biology
-
Biochemistry and Molecular biology
CD47-blocking antibody confers metabolic benefits against obesity.
In Cell Rep Med on 20 May 2025 by Su, Y., Sun, J., et al.
PubMed
CD47-blocking antibody is a well-known potential antibody drug for tumor immunotherapy. However, it is unclear whether CD47-blocking antibody can protect against metabolic disorders. We report that high-fat diet (HFD)-induced obesity increases CD47 expression, while exercise downregulates it in skeletal muscle. Administration of CD47-blocking antibody in mice prevents HFD-induced weight gain and glucose intolerance, enhances exercise capacity, and improves body composition and skeletal muscle mitochondrial function. Mechanistically, the protective effects conferred by CD47-blocking antibody are mediated through activation of AMP-activated protein kinase (AMPK) in skeletal muscle. Consistently, muscle-specific CD47-knockout mice show similar metabolic improvements, indicating a direct muscle-specific role of CD47 in regulating AMPK activation in vivo. Furthermore, the CD47-blocking antibody reduces the phosphorylation of heat shock protein 90α (HSP90α) to activate AMPK in skeletal muscle. In conclusion, CD47-blocking antibody confers metabolic benefits by activating the AMPK pathway in skeletal muscle.
-
-
Effective extracellular payload release and immunomodulatory interactions govern the therapeutic effect of trastuzumab deruxtecan (T-DXd).
In Nat Commun on 2 April 2025 by Tsao, L. C., Wang, J. S., et al.
PubMed
Trastuzumab deruxtecan (T-DXd) is an antibody-drug conjugate (ADC) targeting HER2, exhibiting significant clinical efficacy in breast cancer (BC) with varying HER2 expression, including HER2-low and HER2-ultralow. However, the precise mechanism underlying its efficacy and the contribution of immune activation in these settings remain unclear. Here, we demonstrate that T-DXd efficacy in HER2-low and HER2-negative BC is independent of HER2 engagement and ADC internalization. Instead, its activity relies on extracellular proteases, such as cathepsin L (CTSL), within the tumor microenvironment. Irrespective of their HER2 status, tumor and stromal compartments of invasive BC abundantly express CTSL, which efficiently cleaves the specialized linker of T-DXd, facilitating payload release and inducing cytotoxicity against HER2-low/negative tumors. In HER2-positive BC, the antibody backbone of T-DXd engages Fcγ-receptors and drives antibody-dependent cellular phagocytosis (ADCP). Concurrently, its cytotoxic payload (DXd) induces immunogenic cell death, further activating myeloid cells via TLR4 and STING pathways to enhance tumor antigen presentation to CD8+ T cells. Notably, T-DXd cytotoxicity also upregulates tumor CD47 expression, dampening immune activation. Combining T-DXd with CD47 checkpoint blockade significantly enhances anti-tumor immune responses in a HER2-transgenic BC mouse model, while also inducing durable CD8+ T cell memory to prevent tumor recurrence after therapy cessation.
-
-
Immunology and Microbiology
-
Cancer Research
An antibody-toxin conjugate targeting CD47 linked to the bacterial toxin listeriolysin O for cancer immunotherapy.
In Nat Cancer on 1 March 2025 by Schrank, B. R., Wang, Y., et al.
PubMed
Antigen-presenting cells phagocytose tumor cells and subsequently cross-present tumor-derived antigens. However, these processes are impeded by phagocytosis checkpoints and inefficient cytosolic transport of antigenic peptides from phagolysosomes. Here, using a microbial-inspired strategy, we engineered an antibody-toxin conjugate (ATC) that targets the 'don't eat me' signal CD47 linked to the bacterial toxin listeriolysin O from the intracellular bacterium Listeria monocytogenes via a cleavable linker (CD47-LLO). CD47-LLO promotes cancer cell phagocytosis by macrophages followed by LLO release and activation to form pores on phagolysosomal membranes that enhance antigen cross-presentation of tumor-derived peptides and activate cytosolic immune sensors. CD47-LLO treatment in vivo significantly inhibited the growth of both localized and metastatic breast and melanoma tumors and improved animal survival as a monotherapy or in combination with checkpoint blockade. Together, these results demonstrate that designing ATCs to promote immune recognition of tumor cells represents a promising therapeutic strategy for treating multiple cancers.
-
-
-
Cancer Research
-
Immunology and Microbiology
AFP shields hepatocellular carcinoma from macrophage phagocytosis by regulating HuR-mediated CD47 translocation in cellular membrane.
In Transl Oncol on 1 February 2025 by Pan, Y., Yin, Q., et al.
PubMed
Alpha fetoprotein(AFP) overexpression connecting with macrophage dysfunction remain poorly defined. In this study, explore AFP regulates macrophage immunomodulation in hepatocellular carcinoma(HCC) through comprehensive in vitro and in vivo studies.
-
-
-
Cancer Research
-
Immunology and Microbiology
CD47 predominates over CD24 as a macrophage immune checkpoint in cancer
In bioRxiv on 26 November 2024 by Allen, J., Meglan, A., et al.
-
-
-
Immunology and Microbiology
An Engineered Self-biomineralized Oncolytic Adenovirus Induces Effective Antitumor Immunity and Synergizes With Immune Checkpoint Blockade.
In Cancer Immunol Res on 4 November 2024 by Wang, S., Yang, X., et al.
PubMed
Oncolytic adenoviruses (oADV) are promising cancer treatment agents. However, in vivo hepatic sequestration and the host immunologic response against the agents limit the therapeutic potential of oADVs. In this study, we present a combined method with a rational design for improving oADV infection efficiency, immunogenicity, and treatment efficacy by self-biomineralization. We integrated the biomimetic nucleopeptide W6p into the capsid of oADV using reverse genetics, allowing calcium phosphate mineralization to be biologically induced on the surface of oADV under physiologic conditions, resulting in a mineral exterior. This self-biomineralized, modified oADV (oADV-W6-CaP) enhanced infection efficiency and therapeutic efficacy in coxsackievirus and adenovirus receptor (CAR)-negative cancer cells wherein protecting them against neutralization by preexisting neutralizing antibodies. In subcutaneous mouse tumor models, systemic injection of oADV-W6-CaP demonstrated improved antitumor effectiveness, which was associated with increased T-cell infiltration and CD8+ T-cell activation. In addition, the anticancer immune response elicited by oADV-W6-CaP was dependent on CD8+ T cells, which mediated long-term immunologic memory and systemic antitumor immunity against the same tumor. Finally, the addition of PD1 or CD47 inhibition boosted the anticancer effects of oADV-W6-CaP and increased the rate of complete tumor clearance in tumor-bearing animals. The self-biomineralized oADV shifted the suppressive tumor microenvironment from a "cold" to "hot" state and synergized with immune checkpoint blockade to exert outstanding tumoricidal effects, demonstrating promising potential for cancer immunotherapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
Antibody nanoparticle conjugate-based targeted immunotherapy for non-small cell lung cancer.
In Sci Adv on 14 June 2024 by Saha, T., Fojtu, M., et al.
PubMed
The use of immune checkpoint inhibitors, which activate T cells, is a paradigm shift in the treatment of non-small cell lung cancer. However, the overall response remains low. To address this limitation, here we describe a novel platform, termed antibody-conjugated drug-loaded nanotherapeutics (ADN), which combines immunotherapy and molecularly targeted therapy. An ADN was designed with an anti-CD47 and anti-programmed death ligand 1 (PDL1) antibody pair on the surface of the nanoparticle and a molecularly targeted inhibitor of the PI3K (phosphatidylinositol 3-kinase)/AKT/mTOR (mammalian target of rapamycin) pathway, PI103, entrapped in the nanoparticle. The anti-CD47-PDL1-ADN exhibited greater antitumor efficacy than current treatment options with a PDL1 inhibitor in vivo in an aggressive lung cancer immunocompetent mouse model. Dual antibody-drug-loaded nanotherapeutics can emerge as an attractive platform to improve outcomes with cancer immunotherapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Flow cytometry/Cell sorting
An oncolytic vaccinia virus encoding hyaluronidase reshapes the extracellular matrix to enhance cancer chemotherapy and immunotherapy.
In J Immunother Cancer on 7 March 2024 by Wang, S., Li, Y., et al.
PubMed
The redundant extracellular matrix (ECM) within tumor microenvironment (TME) such as hyaluronic acid (HA) often impairs intratumoral dissemination of antitumor drugs. Oncolytic viruses (OVs) are being studied extensively for cancer therapy either alone or in conjunction with chemotherapy and immunotherapy. Here, we designed a novel recombinant vaccinia virus encoding a soluble version of hyaluronidase Hyal1 (OVV-Hyal1) to degrade the HA and investigated its antitumor effects in combination with chemo drugs, polypeptide, immune cells, and antibodies.
-
-
-
Immunology and Microbiology
Glioblastoma extracellular vesicles modulate immune PD-L1 expression in accessory macrophages upon radiotherapy.
In iScience on 16 February 2024 by Schweiger, M. W., Amoozgar, Z., et al.
PubMed
Glioblastoma (GBM) is the most aggressive brain tumor, presenting major challenges due to limited treatment options. Standard care includes radiation therapy (RT) to curb tumor growth and alleviate symptoms, but its impact on GBM is limited. In this study, we investigated the effect of RT on immune suppression and whether extracellular vesicles (EVs) originating from GBM and taken up by the tumor microenvironment (TME) contribute to the induced therapeutic resistance. We observed that (1) ionizing radiation increases immune-suppressive markers on GBM cells, (2) macrophages exacerbate immune suppression in the TME by increasing PD-L1 in response to EVs derived from GBM cells which is further modulated by RT, and (3) RT increases CD206-positive macrophages which have the most potential in inducing a pro-oncogenic environment due to their increased uptake of tumor-derived EVs. In conclusion, RT affects GBM resistance by immuno-modulating EVs taken up by myeloid cells in the TME.
-
-
-
Cancer Research
CXCR2 inhibition in G-MDSCs enhances CD47 blockade for melanoma tumor cell clearance.
In Proc Natl Acad Sci U S A on 30 January 2024 by Banuelos, A., Zhang, A., et al.
PubMed
The use of colony-stimulating factor-1 receptor (CSF1R) inhibitors has been widely explored as a strategy for cancer immunotherapy due to their robust depletion of tumor-associated macrophages (TAMs). While CSF1R blockade effectively eliminates TAMs from the solid tumor microenvironment, its clinical efficacy is limited. Here, we use an inducible CSF1R knockout model to investigate the persistence of tumor progression in the absence of TAMs. We find increased frequencies of granulocytic myeloid-derived suppressor cells (G-MDSCs) in the bone marrow, throughout circulation, and in the tumor following CSF1R deletion and loss of TAMs. We find that G-MDSCs are capable of suppressing macrophage phagocytosis, and the elimination of G-MDSCs through CXCR2 inhibition increases macrophage capacity for tumor cell clearance. Further, we find that combination therapy of CXCR2 inhibition and CD47 blockade synergize to elicit a significant anti-tumor response. These findings reveal G-MDSCs as key drivers of tumor immunosuppression and demonstrate their inhibition as a potent strategy to increase macrophage phagocytosis and enhance the anti-tumor efficacy of CD47 blockade in B16-F10 melanoma.
-
-
-
Immunology and Microbiology
Practical Mouse Model to Investigate Therapeutics for Staphylococcusaureus Contaminated Surgical Mesh Implants.
In J Surg Res on 1 March 2023 by Collins, M. M., Race, B., et al.
PubMed
The use of prosthetic mesh in hernia repair provides a powerful tool to increase repair longevity, decrease recurrence rates, and facilitate complex abdominal wall reconstruction. Overall infection rates with mesh are low, but for those affected there is high morbidity and economic cost. The availability of a practicable small animal model would be advantageous for the preclinical testing of prophylactics, therapeutics, and new biomaterials. To this end, we have developed a novel mouse model for implantation of methicillin-resistant Staphylococcus aureus-infected surgical mesh and provide results from antibiotic and immunotherapeutic testing.
-
-
-
Cancer Research
-
Immunology and Microbiology
Immunological conversion of solid tumours using a bispecific nanobioconjugate for cancer immunotherapy.
In Nat Nanotechnol on 1 December 2022 by Lu, Y., Huntoon, K., et al.
PubMed
Solid tumours display a limited response to immunotherapies. By contrast, haematological malignancies exhibit significantly higher response rates to immunotherapies as compared with solid tumours. Among several microenvironmental and biological disparities, the differential expression of unique immune regulatory molecules contributes significantly to the interaction of blood cancer cells with immune cells. The self-ligand receptor of the signalling lymphocytic activation molecule family member 7 (SLAMF7), a molecule that is critical in promoting the body's innate immune cells to detect and engulf cancer cells, is expressed nearly exclusively on the cell surface of haematologic tumours, but not on solid ones. Here we show that a bispecific nanobioconjugate that enables the decoration of SLAMF7 on the surface of solid tumours induces robust phagocytosis and activates the phagocyte cyclic guanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon genes (cGAS-STING) pathway, sensitizing the tumours to immune checkpoint blockade. Our findings support an immunological conversion strategy that uses nano-adjuvants to improve the effectiveness of immunotherapies for solid tumours.
-
-
CD47-SIRPα axis blockade in NASH promotes necroptotic hepatocyte clearance by liver macrophages and decreases hepatic fibrosis.
In Sci Transl Med on 23 November 2022 by Shi, H., Wang, X., et al.
PubMed
Necroptosis contributes to hepatocyte death in nonalcoholic steatohepatitis (NASH), but the fate and roles of necroptotic hepatocytes (necHCs) in NASH remain unknown. We show here that the accumulation of necHCs in human and mouse NASH liver is associated with an up-regulation of the "don't-eat-me" ligand CD47 on necHCs, but not on apoptotic hepatocytes, and an increase in the CD47 receptor SIRPα on liver macrophages, consistent with impaired macrophage-mediated clearance of necHCs. In vitro, necHC clearance by primary liver macrophages was enhanced by treatment with either anti-CD47 or anti-SIRPα. In a proof-of-concept mouse model of inducible hepatocyte necroptosis, anti-CD47 antibody treatment increased necHC uptake by liver macrophages and inhibited markers of hepatic stellate cell (HSC) activation, which is responsible for liver fibrogenesis. Treatment of two mouse models of diet-induced NASH with anti-CD47, anti-SIRPα, or AAV8-H1-shCD47 to silence CD47 in hepatocytes increased the uptake of necHC by liver macrophages and decreased markers of HSC activation and liver fibrosis. Anti-SIRPα treatment avoided the adverse effect of anemia found in anti-CD47-treated mice. These findings provide evidence that impaired clearance of necHCs by liver macrophages due to CD47-SIRPα up-regulation contributes to fibrotic NASH, and suggest therapeutic blockade of the CD47-SIRPα axis as a strategy to decrease the accumulation of necHCs in NASH liver and dampen the progression of hepatic fibrosis.
-
-
Cardiovascular biology
Comparative efficacy and mechanism of action of cardiac progenitor cells after cardiac injury.
In iScience on 19 August 2022 by Gunasekaran, M., Mishra, R., et al.
PubMed
Successful cell therapy requires cells to resist the hostile ischemic myocardium, be retained to continue secreting cardioprotective growth factors/exosomes, and resist immunological host responses. Clinically relevant stem/progenitor cells in a rodent model of acute myocardial infarction (MI) demonstrated that neonatal cardiac mesenchymal stromal cells (nMSCs) provide the most robust cardiac functional recovery. Transplanted nMSCs significantly increased the number of tissue reparative macrophages and regulatory T-cells and decreased monocyte-derived inflammatory macrophages and neutrophils in the host myocardium. mRNA microarray and single-cell analyses combined with targeted depletion studies established CD47 in nMSCs as a key molecule responsible for cell retention in the myocardium through an antiphagocytic mechanism regulated by miR34a-5p. Gain and loss-of-function studies demonstrated that miR34a-5p also regulated the production of exosomes and cardioprotective paracrine factors in the nMSC secretome. In conclusion, miR34a-5p and CD47 play an important role in determining the composition of nMSCs' secretome and immune evasion, respectively.
-
-
-
Cancer Research
-
Immunology and Microbiology
Alpha-emitter radium-223 mediates STING-dependent pyroptosis to trigger robust tumor immunogenicity
In Research Square on 5 May 2022 by Yang, M., Cheng, C., et al.
-
-
-
Immunology and Microbiology
SIRPα - CD47 axis regulates dendritic cell-T cell interactions and TCR activation during T cell priming in spleen.
In PLoS One on 13 April 2022 by Autio, A., Wang, H., et al.
PubMed
The SIRPα-CD47 axis plays an important role in T cell recruitment to sites of immune reaction and inflammation but its role in T cell antigen priming is incompletely understood. Employing OTII TCR transgenic mice bred to Cd47-/- (Cd47KO) or SKI mice, a knock-in transgenic animal expressing non-signaling cytoplasmic-truncated SIRPα, we investigated how the SIRPα-CD47 axis contributes to antigen priming. Here we show that adoptive transfer of Cd47KO or SKI Ova-specific CD4+ T cells (OTII) into Cd47KO and SKI recipients, followed by Ova immunization, elicited reduced T cell division and proliferation indices, increased apoptosis, and reduced expansion compared to transfer into WT mice. We confirmed prior reports that splenic T cell zone, CD4+ conventional dendritic cells (cDCs) and CD4+ T cell numbers were reduced in Cd47KO and SKI mice. We report that in vitro derived DCs from Cd47KO and SKI mice exhibited impaired migration in vivo and exhibited reduced CD11c+ DC proximity to OTII T cells in T cell zones after Ag immunization, which correlates with reduced TCR activation in transferred OTII T cells. These findings suggest that reduced numbers of CD4+ cDCs and their impaired migration contributes to reduced T cell-DC proximity in splenic T cell zone and reduced T cell TCR activation, cell division and proliferation, and indirectly increased T cell apoptosis.
-
-
-
Immunology and Microbiology
Anti-CD47 antibody treatment attenuates liver inflammation and fibrosis in experimental non-alcoholic steatohepatitis models.
In Liver Int on 1 April 2022 by Gwag, T., Ma, E., et al.
PubMed
With the epidemic burden of obesity and metabolic diseases, nonalcoholic fatty liver disease (NAFLD) including steatohepatitis (NASH) has become the most common chronic liver disease in the western world. NASH may progress to cirrhosis and hepatocellular carcinoma. Currently, no treatment is available for NASH. Therefore, finding a therapy for NAFLD/NASH is in urgent need. Previously we have demonstrated that mice lacking CD47 or its ligand thrombospondin1 (TSP1) are protected from obesity-associated NALFD. This suggests that CD47 blockade might be a novel treatment for obesity-associated metabolic disease. Thus, in this study, the therapeutic potential of an anti-CD47 antibody in NAFLD progression was determined.
-