InVivoMAb anti-mouse/human/rat CCL2 (MCP-1)
Product Description
Specifications
| Isotype | Armenian Hamster IgG, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb polyclonal Armenian hamster IgG |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | CHO-expressed mouse MCP-1 |
| Reported Applications |
in vivo CCL2 neutralization Immunohistochemistry (frozen) |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_10950302 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo CCL2 neutralization
Berg, N. K., et al (2021). "Hypoxia-inducible factor-dependent induction of myeloid-derived netrin-1 attenuates natural killer cell infiltration during endotoxin-induced lung injury" Faseb j 35(4): e21334.
PubMed
Sepsis and sepsis-associated lung inflammation significantly contribute to the morbidity and mortality of critical illness. Here, we examined the hypothesis that neuronal guidance proteins could orchestrate inflammatory events during endotoxin-induced lung injury. Through a targeted array, we identified netrin-1 as the top upregulated neuronal guidance protein in macrophages treated with lipopolysaccharide (LPS). Furthermore, we found that netrin-1 is highly enriched in infiltrating myeloid cells, particularly in macrophages during LPS-induced lung injury. Transcriptional studies implicate hypoxia-inducible factor HIF-1α in the transcriptional induction of netrin-1 during LPS treatment. Subsequently, the deletion of netrin-1 in the myeloid compartment (Ntn1(loxp/loxp) LysM Cre) resulted in exaggerated mortality and lung inflammation. Surprisingly, further studies revealed enhanced natural killer cells (NK cells) infiltration in Ntn1(loxp/loxp) LysM Cre mice, and neutralization of NK cell chemoattractant chemokine (C-C motif) ligand 2 (CCL2) reversed the exaggerated lung inflammation. Together, these studies provide functional insight into myeloid cell-derived netrin-1 in controlling lung inflammation through the modulation of CCL2-dependent infiltration of NK cells.
in vivo CCL2 neutralization
Brunner, P. M., et al (2015). "CCL7 contributes to the TNF-alpha-dependent inflammation of lesional psoriatic skin" Exp Dermatol 24(7): 522-528.
PubMed
Chemokines are small chemotactic proteins that have a crucial role in leukocyte recruitment into tissue. Targeting these mediators has been suggested as a potential therapeutic option in inflammatory skin diseases such as psoriasis. Using quantitative RT-PCR, we found CCL7, a chemokine ligand known to interact with multiple C-C chemokine receptors, to be markedly increased in lesional psoriasis as opposed to atopic dermatitis, lichen planus, non-lesional psoriatic and normal control skin. Surprisingly, this increase in CCL7 mRNA expression exceeded that of all other chemokines investigated, and keratinocytes and dermal blood endothelial cells were identified as its likely cellular sources. In an imiquimod-induced psoriasis-like mouse model, CCL7 had a profound impact on myeloid cell inflammation as well as on the upregulation of key pro-psoriatic cytokines such as CCL20, IL-12p40 and IL-17C, while its blockade led to an increase in the antipsoriatic cytokine IL-4. In humans receiving the TNF-alpha-blocker infliximab, CCL7 was downregulated in lesional psoriatic skin already within 16 hours after a single intravenous infusion. These data suggest that CCL7 acts as a driver of TNF-alpha-dependent Th1/Th17-mediated inflammation in lesional psoriatic skin.
in vivo CCL2 neutralization
Singh, M., et al (2014). "Effective innate and adaptive antimelanoma immunity through localized TLR7/8 activation" J Immunol 193(9): 4722-4731.
PubMed
Intratumoral immune activation can induce local and systemic antitumor immunity. Imiquimod is a cream-formulated, TLR7 agonist that is Food and Drug Administration approved for the treatment of nonmelanoma skin cancers, but it has limited activity against melanoma. We studied the antitumor activity and mechanism of action of a novel, injectable, tissue-retained TLR7/8 agonist, 3M-052, which avoids systemic distribution. Intratumoral administration of 3M-052 generated systemic antitumor immunity and suppressed both injected and distant, uninjected wild-type B16.F10 melanomas. Treated tumors showed that an increased level of CCL2 chemokines and infiltration of M1 phenotype-shifted macrophages, which could kill tumor cells directly through production of NO and CCL2, were essential for the antitumor activity of 3M-052. CD8(+) T cells, B cells, type I IFN, IFN-gamma, and plasmacytoid dendritic cells were contributed to efficient tumor suppression, whereas perforin, NK cells, and CD4 T cells were not required. Finally, 3M-052 therapy potentiated checkpoint blockade therapy with anti-CTLA-4 and anti-programmed death ligand 1 Abs, even when checkpoint blockade alone was ineffective. Our findings suggest that intratumoral treatment with 3M-052 is a promising approach for the treatment of cancer and establish a rational strategy and mechanistic understanding for combination therapy with intratumoral, tissue-retained TLR7/8 agonist and checkpoint blockade in metastatic cancer.
Immunohistochemistry (frozen)
Tominaga, T., et al (2009). "Blocking mast cell-mediated type I hypersensitivity in experimental allergic conjunctivitis by monocyte chemoattractant protein-1/CCR2" Invest Ophthalmol Vis Sci 50(11): 5181-5188.
PubMed
PURPOSE: To characterize the roles played by monocyte chemoattractant protein-1 and its preferential receptor CCR2 (MCP-1/CCL2) in acute allergic inflammation. METHODS: The direct effects of MCP-1 were evaluated histologically after a subconjunctival injection of recombinant MCP-1 into naive mice. The mice were sensitized to ragweed pollen, and allergic conjunctivitis was induced by an allergen challenge. The location of the induced MCP-1 was determined by immunohistochemistry. Anti-MCP-1 antibody and CCR2-specific antagonist, RS 504393, were used to determine whether an inhibition of MCP-1 or CCR2 signals would suppress the allergen-induced immediate hypersensitivity reaction. The effect of blocking CCR2 was tested in vitro with isolated mast cells from connective tissue, to evaluate the co-stimulatory signals mediated by CCR2 in mast cells directly. RESULTS: A subconjunctival injection of MCP-1 stimulated conjunctival mast cell degranulation and recruited monocytes/macrophages. In the allergic conjunctivitis model, the allergen-induced MCP-1 protein was located in the monocytes/macrophages in the substantia propria of the conjunctiva. Blocking MCP-1 significantly suppressed the allergen-induced clinical signs and mast cell degranulation without affecting the allergen-specific IgE, or the release of Th2 cytokine from the isolated draining lymph node cells. Inhibition of CCR2 similarly suppressed the acute inflammatory responses. Consistent with the outcome of the disease model, inhibition of CCR2 suppressed allergen-specific degranulation of IgE-primed, isolated conjunctival mast cells. CONCLUSIONS: Stimulation of the co-stimulatory axis of CCR2 by MCP-1 is essentially required for mast cell-mediated hypersensitivity reactions in mouse eyes.
Product Citations
-
-
Cancer Research
-
Genetics
Glutamate promotes CCL2 expression to recruit tumor-associated macrophages by restraining EZH2-mediated histone methylation in hepatocellular carcinoma.
In Oncoimmunology on 1 December 2025 by Chen, J., Sun, H. W., et al.
PubMed
Glutamate is well-known as metabolite for maintaining the energy and redox homeostasis in cancer, moreover it is also the primary excitatory neurotransmitter in the central nervous system. However, whether glutamatergic signaling can regulate hepatocellular carcinoma (HCC) progression and the specific regulatory mechanisms are unknown. In the present study, we found that glutamate and its receptor NMDAR2B were significantly elevated in HCC patients, which predicts poor prognosis. Glutamate could upregulate CCL2 expression on hepatoma cells and further enhance the capability of tumor cells to recruit tumor-associated macrophages (TAMs). Mechanistically, glutamate could facilitate CCL2 expression through NMDAR pathway by decreasing the expression of EZH2, which regulates the H3K27me3 levels on the CCL2 promoter, rather than affecting DNA methylation. Moreover, inhibiting glutamate pathway with MK801 could significantly delay tumor growth, with reduced TAMs in implanted Hepa1-6 mouse HCC models. Our work suggested that glutamate could induce CCL2 expression to promote TAM infiltration by negatively regulating EZH2 levels in hepatoma cells, which might serve as a potential prognostic marker and a therapeutic target for HCC patients.
-
-
-
Immunology and Microbiology
-
Cancer Research
TBK1 Targeting Is Identified as a Therapeutic Strategy to Enhance CAR T-Cell Efficacy Using Patient-Derived Organotypic Tumor Spheroids.
In Cancer Immunol Res on 3 February 2025 by Sun, Y., Maggs, L., et al.
PubMed
Novel therapeutic strategies are needed to improve the efficacy of chimeric antigen receptor (CAR) T cells as a treatment of solid tumors. Multiple tumor microenvironmental factors are thought to contribute to resistance to CAR T-cell therapy in solid tumors, and appropriate model systems to identify and examine these factors using clinically relevant biospecimens are limited. In this study, we examined the activity of B7-H3-directed CAR T cells (B7-H3.CAR-T) using 3D microfluidic cultures of patient-derived organotypic tumor spheroids (PDOTS) and then confirmed the activity of B7-H3.CAR T cells in PDOTS. Although B7-H3 expression in PDOTS was associated with B7-H3.CAR-T sensitivity, mechanistic studies revealed dynamic upregulation of co-inhibitory receptors on CAR T-cells following target cell encounter that led to CAR T-cell dysfunction and limited efficacy against B7-H3-expressing tumors. PD-1 blockade restored CAR T-cell activity in monotypic and organotypic tumor spheroids with improved tumor control and upregulation of effector cytokines. Given the emerging role of TANK-binding kinase 1 (TBK1) as an immune evasion gene, we examined the effect of TBK1 inhibition on CAR T-cell efficacy. Similar to PD-1 blockade, TBK1 inhibition restored CAR T-cell activity in monotypic and organotypic tumor spheroids, prevented CAR T-cell dysfunction, and enhanced CAR T-cell proliferation. Inhibition or deletion of TBK1 also enhanced the sensitivity of cancer cells to immune-mediated killing. Taken together, our results demonstrate the feasibility and utility of ex vivo profiling of CAR T cells using PDOTS and suggest that targeting TBK1 could be used to enhance CAR T-cell efficacy by overcoming tumor-intrinsic and -extrinsic resistance mechanisms.
-
-
-
Cancer Research
ASH1L in Hepatoma Cells and Hepatic Stellate Cells Promotes Fibrosis-Associated Hepatocellular Carcinoma by Modulating Tumor-Associated Macrophages.
In Adv Sci (Weinh) on 1 December 2024 by Du, Y., Wu, S., et al.
PubMed
Hepatocellular carcinoma (HCC) often occurs in the context of fibrosis or cirrhosis. Methylation of histone is an important epigenetic mechanism, but it is unclear whether histone methyltransferases are potent targets for fibrosis-associated HCC therapy. ASH1L, an H3K4 methyltransferase, is found at higher levels in activated hepatic stellate cells (HSCs) and hepatoma cells. To determine the role of ASH1L in vivo, transgenic mice with conditional Ash1l depletion in the hepatocyte cell lineage (Ash1lflox/floxAlbcre) or HSCs (Ash1lflox/floxGFAPcreERT2) are generated, and these mice are challenged in a diethylnitrosamine (DEN)/carbon tetrachloride (CCl4)-induced model of liver fibrosis and HCC. Depleting Ash1l in both hepatocytes and HSCs mitigates hepatic fibrosis and HCC development. Multicolor flow cytometry, bulk, and single-cell transcriptomic sequencing reveal that ASH1L creates an immunosuppressive microenvironment. Mechanically, ASH1L-mediated H3K4me3 modification increases the expression of CCL2 and CSF1, which recruites and polarizes M2-like pro-tumorigenic macrophages. The M2-like macrophages further enhance tumor cell proliferation and suppress CD8+ T cell activation. AS-99, a small molecule inhibitor of ASH1L, demonstrates similar anti-fibrosis and tumor-suppressive effects. Of pathophysiological significance, the increased expression levels of mesenchymal ASH1L and M2 marker CD68 are associated with poor prognosis of HCC. The findings reveal ASH1L as a potential small-molecule therapeutic target against fibrosis-related HCC.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Regulatory T cells require peripheral CCL2-CCR2 signaling to facilitate the resolution of medication overuse headache-related behavioral sensitization.
In J Headache Pain on 11 November 2024 by Ryu, S., Zhang, J., et al.
PubMed
Medication overuse headache (MOH) is the most common secondary headache disorder, resulting from chronic and excessive use of medication to treat headaches, for example, sumatriptan. In a recent study, we have shown that the peripheral C-C motif ligand 2 (CCL2), C-C motif chemokine receptor 2 (CCR2) and calcitonin-gene-related peptide (CGRP) signaling pathways interact with each other and play critical roles in the development of chronic migraine-related behavioral and cellular sensitization. In the present study, we investigated whether CCL2-CCR2 and CGRP signaling pathways play a role in the development of sumatriptan overuse-induced sensitization, and whether they are involved in its resolution by the low-dose interleukin-2 (LD-IL-2) treatment.
-
-
-
Mus musculus (Mouse)
-
Cardiovascular biology
-
Immunology and Microbiology
-
Neuroscience
Microglia LILRB4 upregulation reduces brain damage after acute ischemic stroke by limiting CD8+ T cell recruitment.
In J Neuroinflammation on 31 August 2024 by Ma, Y., Zheng, K., et al.
PubMed
Leukocyte immunoglobulin-like receptor B4 (LILRB4) plays a significant role in regulating immune responses. LILRB4 in microglia might influence the infiltration of peripheral T cells. However, whether and how LILRB4 expression aggravates brain damage after acute ischemic stroke remains unclear. This study investigates the role of LILRB4 in modulating the immune response and its potential protective effects against ischemic brain injury in mice.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Mural cell-derived chemokines provide a protective niche to safeguard vascular macrophages and limit chronic inflammation.
In Immunity on 10 October 2023 by Pekayvaz, K., Gold, C., et al.
PubMed
Maladaptive, non-resolving inflammation contributes to chronic inflammatory diseases such as atherosclerosis. Because macrophages remove necrotic cells, defective macrophage programs can promote chronic inflammation with persistent tissue injury. Here, we investigated the mechanisms sustaining vascular macrophages. Intravital imaging revealed a spatiotemporal macrophage niche across vascular beds alongside mural cells (MCs)-pericytes and smooth muscle cells. Single-cell transcriptomics, co-culture, and genetic deletion experiments revealed MC-derived expression of the chemokines CCL2 and MIF, which actively preserved macrophage survival and their homeostatic functions. In atherosclerosis, this positioned macrophages in viable plaque areas, away from the necrotic core, and maintained a homeostatic macrophage phenotype. Disruption of this MC-macrophage unit via MC-specific deletion of these chemokines triggered detrimental macrophage relocalizing, exacerbated plaque necrosis, inflammation, and atheroprogression. In line, CCL2 inhibition at advanced stages of atherosclerosis showed detrimental effects. This work presents a MC-driven safeguard toward maintaining the homeostatic vascular macrophage niche.
-
-
-
Immunology and Microbiology
The gut microbiota promotes distal tissue regeneration via RORγ+ regulatory T cell emissaries.
In Immunity on 11 April 2023 by Hanna, B. S., Wang, G., et al.
PubMed
Specific microbial signals induce the differentiation of a distinct pool of RORγ+ regulatory T (Treg) cells crucial for intestinal homeostasis. We discovered highly analogous populations of microbiota-dependent Treg cells that promoted tissue regeneration at extra-gut sites, notably acutely injured skeletal muscle and fatty liver. Inflammatory meditators elicited by tissue damage combined with MHC-class-II-dependent T cell activation to drive the accumulation of gut-derived RORγ+ Treg cells in injured muscle, wherein they regulated the dynamics and tenor of early inflammation and helped balance the proliferation vs. differentiation of local stem cells. Reining in IL-17A-producing T cells was a major mechanism underlying the rheostatic functions of RORγ+ Treg cells in compromised tissues. Our findings highlight the importance of gut-trained Treg cell emissaries in controlling the response to sterile injury of non-mucosal tissues.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Immune-interacting lymphatic endothelial subtype at capillary terminals drives lymphatic malformation.
In J Exp Med on 3 April 2023 by Petkova, M., Kraft, M., et al.
PubMed
Oncogenic mutations in PIK3CA, encoding p110α-PI3K, are a common cause of venous and lymphatic malformations. Vessel type-specific disease pathogenesis is poorly understood, hampering development of efficient therapies. Here, we reveal a new immune-interacting subtype of Ptx3-positive dermal lymphatic capillary endothelial cells (iLECs) that recruit pro-lymphangiogenic macrophages to promote progressive lymphatic overgrowth. Mouse model of Pik3caH1047R-driven vascular malformations showed that proliferation was induced in both venous and lymphatic ECs but sustained selectively in LECs of advanced lesions. Single-cell transcriptomics identified the iLEC population, residing at lymphatic capillary terminals of normal vasculature, that was expanded in Pik3caH1047R mice. Expression of pro-inflammatory genes, including monocyte/macrophage chemokine Ccl2, in Pik3caH1047R-iLECs was associated with recruitment of VEGF-C-producing macrophages. Macrophage depletion, CCL2 blockade, or anti-inflammatory COX-2 inhibition limited Pik3caH1047R-driven lymphangiogenesis. Thus, targeting the paracrine crosstalk involving iLECs and macrophages provides a new therapeutic opportunity for lymphatic malformations. Identification of iLECs further indicates that peripheral lymphatic vessels not only respond to but also actively orchestrate inflammatory processes.
-
-
-
Mus musculus (Mouse)
Shp2 Deficiency in Kupffer Cells and Hepatocytes Aggravates Hepatocarcinogenesis by Recruiting Non-Kupffer Macrophages.
In Cell Mol Gastroenterol Hepatol on 25 February 2023 by Du, L., Ji, Y., et al.
PubMed
Complex communications between hepatocytes and Kupffer cells (KCs) are known to drive or suppress hepatocarcinogenesis, with controversial data in the literature. In previous experiments that aimed to decipher hepatocyte/KC interactions, we unexpectedly unveiled a tumor-suppressing effect of polyinosinic-polycytidylic acid, a widely used inducer of MX dynamin like GTPase 1 (Mx1)-cre expression, which questioned a theory of interleukin 1a/6 cytokine circuit in hepatocyte/KC communication. The goal of this study was to clarify the controversy and decipher unique functions of KCs and non-KC macrophages in liver tumorigenesis.
-
-
-
Cell Biology
-
Immunology and Microbiology
Age-associated adipose tissue inflammation promotes monocyte chemotaxis and enhances atherosclerosis.
In Aging Cell on 1 February 2023 by Song, J., Farris, D., et al.
PubMed
Although aging enhances atherosclerosis, we do not know if this occurs via alterations in circulating immune cells, lipid metabolism, vasculature, or adipose tissue. Here, we examined whether aging exerts a direct pro-atherogenic effect on adipose tissue in mice. After demonstrating that aging augmented the inflammatory profile of visceral but not subcutaneous adipose tissue, we transplanted visceral fat from young or aged mice onto the right carotid artery of Ldlr-/- recipients. Aged fat transplants not only increased atherosclerotic plaque size with increased macrophage numbers in the adjacent carotid artery, but also in distal vascular territories, indicating that aging of the adipose tissue enhances atherosclerosis via secreted factors. By depleting macrophages from the visceral fat, we identified that adipose tissue macrophages are major contributors of the secreted factors. To identify these inflammatory factors, we found that aged fat transplants secreted increased levels of the inflammatory mediators TNFα, CXCL2, and CCL2, which synergized to promote monocyte chemotaxis. Importantly, the combined blockade of these inflammatory mediators impeded the ability of aged fat transplants to enhance atherosclerosis. In conclusion, our study reveals that aging enhances atherosclerosis via increased inflammation of visceral fat. Our study suggests that future therapies targeting the visceral fat may reduce atherosclerosis disease burden in the expanding older population.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
Long-term exposure to house dust mites accelerates lung cancer development in mice.
In J Exp Clin Cancer Res on 21 January 2023 by Wang, D., Li, W., et al.
PubMed
Individuals with certain chronic inflammatory lung diseases have a higher risk of developing lung cancer (LC). However, the underlying mechanisms remain largely unknown. Here, we hypothesized that chronic exposure to house dust mites (HDM), a common indoor aeroallergen associated with the development of asthma, accelerates LC development through the induction of chronic lung inflammation (CLI). METHODS: The effects of HDM and heat-inactivated HDM (HI-HDM) extracts were evaluated in two preclinical mouse models of LC (a chemically-induced model using the carcinogen urethane and a genetically-driven model with oncogenic KrasG12D activation in lung epithelial cells) and on murine macrophages in vitro. Pharmacological blockade or genetic deletion of the Nod-like receptor family pyrin domain-containing protein 3 (NLRP3) inflammasome, caspase-1, interleukin-1β (IL-1β), and C-C motif chemokine ligand 2 (CCL2) or treatment with an inhaled corticosteroid (ICS) was used to uncover the pro-tumorigenic effect of HDM. RESULTS: Chronic intranasal (i.n) instillation of HDM accelerated LC development in the two mouse models. Mechanistically, HDM caused a particular subtype of CLI, in which the NLRP3/IL-1β signaling pathway is chronically activated in macrophages, and made the lung microenvironment conducive to tumor development. The tumor-promoting effect of HDM was significantly decreased by heat treatment of the HDM extract and was inhibited by NLRP3, IL-1β, and CCL2 neutralization, or ICS treatment.
-
-
-
Cancer Research
-
Neuroscience
Astrocyte immunometabolic regulation of the tumour microenvironment drives glioblastoma pathogenicity.
In Brain on 14 September 2022 by Perelroizen, R., Philosof, B., et al.
PubMed
Malignant brain tumours are the cause of a disproportionate level of morbidity and mortality among cancer patients, an unfortunate statistic that has remained constant for decades. Despite considerable advances in the molecular characterization of these tumours, targeting the cancer cells has yet to produce significant advances in treatment. An alternative strategy is to target cells in the glioblastoma microenvironment, such as tumour-associated astrocytes. Astrocytes control multiple processes in health and disease, ranging from maintaining the brain's metabolic homeostasis, to modulating neuroinflammation. However, their role in glioblastoma pathogenicity is not well understood. Here we report that depletion of reactive astrocytes regresses glioblastoma and prolongs mouse survival. Analysis of the tumour-associated astrocyte translatome revealed astrocytes initiate transcriptional programmes that shape the immune and metabolic compartments in the glioma microenvironment. Specifically, their expression of CCL2 and CSF1 governs the recruitment of tumour-associated macrophages and promotes a pro-tumourigenic macrophage phenotype. Concomitantly, we demonstrate that astrocyte-derived cholesterol is key to glioma cell survival, and that targeting astrocytic cholesterol efflux, via ABCA1, halts tumour progression. In summary, astrocytes control glioblastoma pathogenicity by reprogramming the immunological properties of the tumour microenvironment and supporting the non-oncogenic metabolic dependency of glioblastoma on cholesterol. These findings suggest that targeting astrocyte immunometabolic signalling may be useful in treating this uniformly lethal brain tumour.
-
-
-
Immunology and Microbiology
-
Enzyme-linked immunosorbent assay
-
Homo sapiens (Human)
cGAS and DDX41-STING mediated intrinsic immunity spreads intercellularly to promote neuroinflammation in SOD1 ALS model.
In iScience on 17 June 2022 by Tan, H. Y., Yong, Y. K., et al.
PubMed
Neuroinflammation exacerbates the progression of SOD1-driven amyotrophic lateral sclerosis (ALS), although the underlying mechanisms remain largely unknown. Herein, we demonstrate that misfolded SOD1 (SOD1Mut)-causing ALS results in mitochondrial damage, thus triggering the release of mtDNA and an RNA:DNA hybrid into the cytosol in an mPTP-independent manner to activate IRF3- and IFNAR-dependent type I interferon (IFN-I) and interferon-stimulating genes. The neuronal hyper-IFN-I and pro-inflammatory responses triggered in ALS-SOD1Mut were sufficiently robust to cause a strong physiological outcome in vitro and in vivo. cGAS/DDX41-STING-signaling is amplified in bystander cells through inter-neuronal gap junctions. Our results highlight the importance of a common DNA-sensing pathway between SOD1 and TDP-43 in influencing the progression of ALS.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
-
In vivo experiments
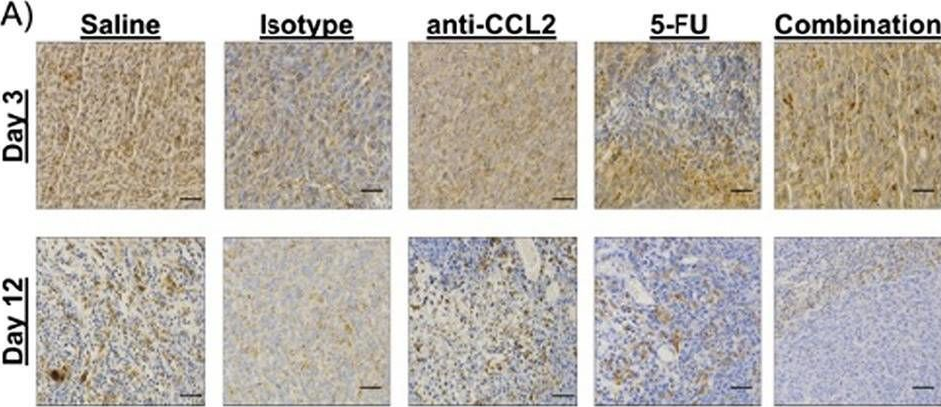
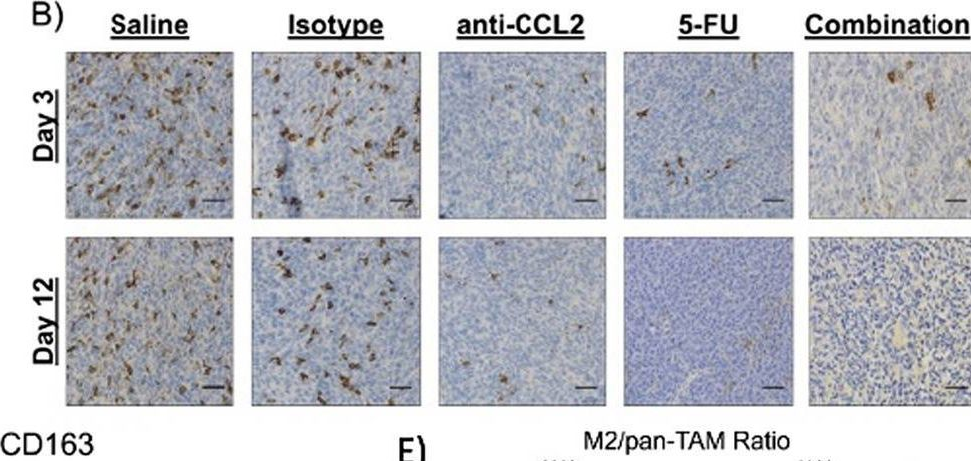
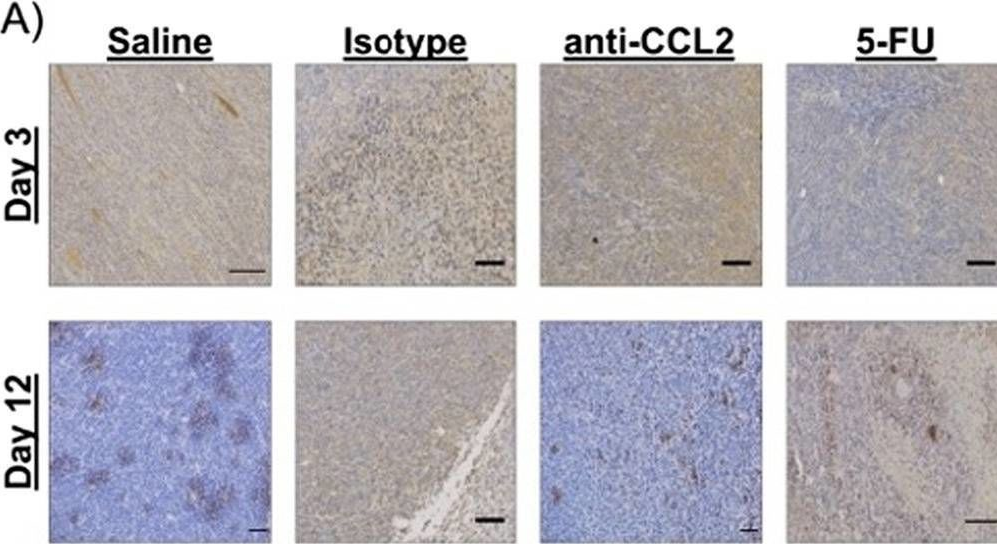
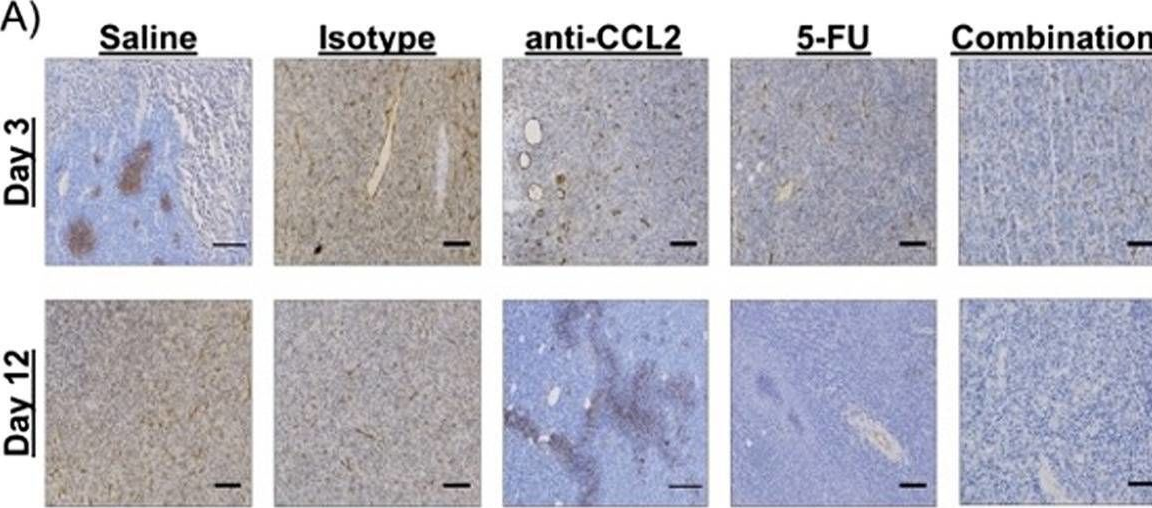
Macrophage-targeted anti-CCL2 immunotherapy enhances tumor sensitivity to 5-fluorouracil in a Balb/c-CT26 murine colon carcinoma model measured using diffuse reflectance spectroscopy.
In BMC Immunol on 23 April 2022 by Bess, S. N., Greening, G. J., et al.
PubMed
Immunotherapy in colorectal cancer (CRC) regulates specific immune checkpoints and, when used in combination with chemotherapy, can improve patient prognosis. One specific immune checkpoint is the recruitment of circulating monocytes that differentiate into tumor-associated macrophages (TAMs) and promote tumor angiogenesis. Changes in vascularization can be non-invasively assessed via diffuse reflectance spectroscopy using hemoglobin concentrations and oxygenation in a localized tumor volume. In this study, we examine whether blockade of monocyte recruitment via CCL2 (macrophage chemoattractant protein-1) leads to enhanced sensitivity of 5-fluorouracil (5-FU) in a CT26-Balb/c mouse model of CRC. It was hypothesized that the blockade of TAMs will alter tumor perfusion, increasing chemotherapy response. A subcutaneous tumor model using Balb/c mice injected with CT26 colon carcinoma cells received either a saline or isotype control, anti-CCL2, 5-FU, or a combination of anti-CCL2 and 5-FU.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
Immune deconvolution and temporal mapping identifies stromal targets and developmental intervals for abrogating murine low-grade optic glioma formation.
In Neurooncol Adv on 22 February 2022 by de Andrade Costa, A., Chatterjee, J., et al.
PubMed
Brain tumor formation and progression are dictated by cooperative interactions between neoplastic and non-neoplastic cells. This stromal dependence is nicely illustrated by tumors arising in the Neurofibromatosis type 1 (NF1) cancer predisposition syndrome, where children develop low-grade optic pathway gliomas (OPGs). Using several authenticated Nf1-OPG murine models, we previously demonstrated that murine Nf1-OPG growth is regulated by T cell function and microglia Ccl5 production, such that their inhibition reduces tumor proliferation in vivo. While these interactions are critical for established Nf1-OPG tumor growth, their importance in tumor formation has not been explored.
-
-
-
Cancer Research
-
Immunology and Microbiology
Loss of the intracellular enzyme QPCTL limits chemokine function and reshapes myeloid infiltration to augment tumor immunity
In bioRxiv on 28 January 2022 by Barreira da Silva, R., Leitao, R., et al.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
A GATA4-regulated secretory program suppresses tumors through recruitment of cytotoxic CD8 T cells.
In Nat Commun on 11 January 2022 by Patel, R. S., Romero, R., et al.
PubMed
The GATA4 transcription factor acts as a master regulator of development of multiple tissues. GATA4 also acts in a distinct capacity to control a stress-inducible pro-inflammatory secretory program that is associated with senescence, a potent tumor suppression mechanism, but also operates in non-senescent contexts such as tumorigenesis. This secretory pathway is composed of chemokines, cytokines, growth factors, and proteases. Since GATA4 is deleted or epigenetically silenced in cancer, here we examine the role of GATA4 in tumorigenesis in mouse models through both loss-of-function and overexpression experiments. We find that GATA4 promotes non-cell autonomous tumor suppression in multiple model systems. Mechanistically, we show that Gata4-dependent tumor suppression requires cytotoxic CD8 T cells and partially requires the secreted chemokine CCL2. Analysis of transcriptome data in human tumors reveals reduced lymphocyte infiltration in GATA4-deficient tumors, consistent with our murine data. Notably, activation of the GATA4-dependent secretory program combined with an anti-PD-1 antibody robustly abrogates tumor growth in vivo.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
GM-CSF production by non-classical monocytes controls antagonistic LPS-driven functions in allergic inflammation.
In Cell Rep on 28 December 2021 by Kaur, K., Bachus, H., et al.
PubMed
Lipopolysaccharide (LPS) can either promote or prevent T helper 2 (Th2) cell allergic responses. However, the underlying mechanism remains unknown. We show here that LPS activity switches from pro-pathogenic to protective depending on the production of granulocyte-macrophage colony-stimulating factor (GM-CSF) by non-classical monocytes. In the absence of GM-CSF, LPS can favor pathogenic Th2 cell responses by supporting the trafficking of lung-migratory dendritic cells (mDC2s) into the lung-draining lymph node. However, when non-classical monocytes produce GM-CSF, LPS and GM-CSF synergize to differentiate monocyte-derived DCs from classical Ly6Chi monocytes that instruct mDC2s for Th2 cell suppression. Importantly, only allergens with cysteine protease activity trigger GM-CSF production by non-classical monocytes. Hence, the therapeutic effect of LPS is restricted to allergens with this enzymatic activity. Treatment with GM-CSF, however, restores the protective effects of LPS. Thus, GM-CSF produced by non-classical monocytes acts as a rheostat that fine-tunes the pathogenic and therapeutic functions of LPS.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
A novel CXCR4 antagonist counteracts paradoxical generation of cisplatin-induced pro-metastatic niches in lung cancer.
In Mol Ther on 6 October 2021 by Bertolini, G., Cancila, V., et al.
PubMed
Platinum-based chemotherapy remains widely used in advanced non-small cell lung cancer (NSCLC) despite experimental evidence of its potential to induce long-term detrimental effects, including the promotion of pro-metastatic microenvironments. In this study, we investigated the interconnected pathways underlying the promotion of cisplatin-induced metastases. In tumor-free mice, cisplatin treatment resulted in an expansion in the bone marrow of CCR2+CXCR4+Ly6Chigh inflammatory monocytes (IMs) and an increase in lung levels of stromal SDF-1, the CXCR4 ligand. In experimental lung metastasis assays, cisplatin-induced IMs promoted the extravasation of tumor cells and the expansion of CD133+CXCR4+ metastasis-initiating cells (MICs). Peptide R, a novel CXCR4 inhibitor designed as an SDF-1 mimetic peptide, prevented cisplatin-induced IM expansion, the recruitment of IMs into the lungs, and the promotion of metastasis. At the primary tumor site, cisplatin treatment reduced tumor size while simultaneously inducing tumor release of SDF-1, MIC expansion, and recruitment of pro-invasive CXCR4+ macrophages. Co-recruitment of MICs and CCR2+CXCR4+ IMs to distant SDF-1-enriched sites also promoted spontaneous metastases that were prevented by CXCR4 blockade. In clinical specimens from NSCLC patients SDF-1 levels were found to be higher in platinum-treated samples and related to a worse clinical outcome. Our findings reveal that activation of the CXCR4/SDF-1 axis specifically mediates the pro-metastatic effects of cisplatin and suggest CXCR4 blockade as a possible novel combination strategy to control metastatic disease.
-
-
-
Blocking experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
-
Neuroscience
High-parameter cytometry unmasks microglial cell spatio-temporal response kinetics in severe neuroinflammatory disease.
In J Neuroinflammation on 26 July 2021 by Spiteri, A. G., Terry, R. L., et al.
PubMed
Differentiating infiltrating myeloid cells from resident microglia in neuroinflammatory disease is challenging, because bone marrow-derived inflammatory monocytes infiltrating the inflamed brain adopt a 'microglia-like' phenotype. This precludes the accurate identification of either cell type without genetic manipulation, which is important to understand their temporal contribution to disease and inform effective intervention in its pathogenesis. During West Nile virus (WNV) encephalitis, widespread neuronal infection drives substantial CNS infiltration of inflammatory monocytes, causing severe immunopathology and/or death, but the role of microglia in this remains unclear.
-