InVivoMAb anti-mouse Galectin-9
Product Description
Specifications
| Isotype | Rat IgG2b, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2b isotype control, anti-keyhole limpet hemocyanin |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Recombinant mouse galectin-9 |
| Reported Applications | in vivo Galectin-9 blockade in vitro Galectin-9 blockade |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687702 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo Galectin-9 blockade
in vitro Galectin-9 blockade
de Mingo Pulido, A., et al (2018). "TIM-3 Regulates CD103(+) Dendritic Cell Function and Response to Chemotherapy in Breast Cancer" Cancer Cell 33(1): 60-74 e66.
PubMed
Intratumoral CD103(+) dendritic cells (DCs) are necessary for anti-tumor immunity. Here we evaluated the expression of immune regulators by CD103(+) DCs in a murine model of breast cancer and identified expression of TIM-3 as a target for therapy. Anti-TIM-3 antibody improved response to paclitaxel chemotherapy in models of triple-negative and luminal B disease, with no evidence of toxicity. Combined efficacy was CD8(+) T cell dependent and associated with increased granzyme B expression; however, TIM-3 expression was predominantly localized to myeloid cells in both human and murine tumors. Gene expression analysis identified upregulation of Cxcl9 within intratumoral DCs during combination therapy, and therapeutic efficacy was ablated by CXCR3 blockade, Batf3 deficiency, or Irf8 deficiency.
in vivo Galectin-9 blockade
Daley, D., et al (2016). "gammadelta T Cells Support Pancreatic Oncogenesis by Restraining alphabeta T Cell Activation" Cell 166(6): 1485-1499 e1415.
PubMed
Inflammation is paramount in pancreatic oncogenesis. We identified a uniquely activated gammadeltaT cell population, which constituted approximately 40% of tumor-infiltrating T cells in human pancreatic ductal adenocarcinoma (PDA). Recruitment and activation of gammadeltaT cells was contingent on diverse chemokine signals. Deletion, depletion, or blockade of gammadeltaT cell recruitment was protective against PDA and resulted in increased infiltration, activation, and Th1 polarization of alphabetaT cells. Although alphabetaT cells were dispensable to outcome in PDA, they became indispensable mediators of tumor protection upon gammadeltaT cell ablation. PDA-infiltrating gammadeltaT cells expressed high levels of exhaustion ligands and thereby negated adaptive anti-tumor immunity. Blockade of PD-L1 in gammadeltaT cells enhanced CD4(+) and CD8(+) T cell infiltration and immunogenicity and induced tumor protection suggesting that gammadeltaT cells are critical sources of immune-suppressive checkpoint ligands in PDA. We describe gammadeltaT cells as central regulators of effector T cell activation in cancer via novel cross-talk.
in vivo Galectin-9 blockade
Dolina, J. S., et al (2014). "Liver-primed CD8+ T cells suppress antiviral adaptive immunity through galectin-9-independent T-cell immunoglobulin and mucin 3 engagement of high-mobility group box 1 in mice" Hepatology 59(4): 1351-1365.
PubMed
The liver is a tolerogenic environment exploited by persistent infections, such as hepatitis B (HBV) and C (HCV) viruses. In a murine model of intravenous hepatotropic adenovirus infection, liver-primed antiviral CD8(+) T cells fail to produce proinflammatory cytokines and do not display cytolytic activity characteristic of effector CD8(+) T cells generated by infection at an extrahepatic, that is, subcutaneous, site. Importantly, liver-generated CD8(+) T cells also appear to have a T-regulatory (Treg) cell function exemplified by their ability to limit proliferation of antigen-specific T-effector (Teff ) cells in vitro and in vivo via T-cell immunoglobulin and mucin 3 (Tim-3) expressed by the CD8(+) Treg cells. Regulatory activity did not require recognition of the canonical Tim-3 ligand, galectin-9, but was dependent on CD8(+) Treg cell-surface Tim-3 binding to the alarmin, high-mobility group box 1 (HMGB-1). CONCLUSION: Virus-specific Tim-3(+) CD8(+) T cells operating through HMGB-1 recognition in the setting of acute and chronic viral infections of the liver may act to dampen hepatic T-cell responses in the liver microenvironment and, as a consequence, limit immune-mediated tissue injury or promote the establishment of persistent infections.
Product Citations
-
-
Immunology and Microbiology
Targeting hepatocytic TβRI ameliorates liver metastatic outcomes by revitalizing stem-like CD8+ Tex subsets.
In Nat Commun on 27 November 2025 by Wang, H., Zhou, Y., et al.
PubMed
Stem-like CD8⁺ exhausted T cells (Tex) sustain antitumor immunity, whereas TGFβ signaling acts as a major immunosuppressive pathway. In patients with colorectal liver metastases, we observe that elevated TβRI expression in peri-metastatic hepatocytes correlates with poor prognosis. We therefore investigate whether disrupting hepatocytic TGFβ signaling can reinvigorate stem-like CD8⁺ Tex cells to restrict liver metastasis. In support of this hypothesis, mice with hepatocyte-specific TβRI depletion exhibit reduced liver metastatic burden across multiple tumor models. Mechanistically, hepatocytic TβRI blockade suppresses Galectin-9 secretion, which reshapes the transcriptional program of intra-tumoral CD8⁺ T cells. This reprogramming promotes a phenotypic transition from terminal exhaustion toward stem-like and effector states, yielding T cell subsets with enhanced metastasis-control capacity. Importantly, this axis functions independently of macrophages and CD4⁺ T cells. Furthermore, therapeutic delivery of Galunisertib using choline-modified lipid nanoparticles synergizes with αPD-1, fostering the conversion of exhausted CD8⁺ T cells into responsive Ly108⁺CX3CR1⁺ subsets and suppressing liver metastases. Collectively, our results identify hepatocyte TGFβ signaling as a targetable checkpoint against liver metastases.
-
-
-
Binding experiments
-
Genetics
-
Immunology and Microbiology
-
Binding experiments
The inhibitory receptor TIM-3 limits activation of the cGAS-STING pathway in intra-tumoral dendritic cells by suppressing extracellular DNA uptake.
In Immunity on 8 June 2021 by de Mingo Pulido, A., Hänggi, K., et al.
PubMed
Blockade of the inhibitory receptor TIM-3 shows efficacy in cancer immunotherapy clinical trials. TIM-3 inhibits production of the chemokine CXCL9 by XCR1+ classical dendritic cells (cDC1), thereby limiting antitumor immunity in mammary carcinomas. We found that increased CXCL9 expression by splenic cDC1s upon TIM-3 blockade required type I interferons and extracellular DNA. Chemokine expression as well as combinatorial efficacy of TIM-3 blockade and paclitaxel chemotherapy were impaired by deletion of Cgas and Sting. TIM-3 blockade increased uptake of extracellular DNA by cDC1 through an endocytic process that resulted in cytoplasmic localization. DNA uptake and efficacy of TIM-3 blockade required DNA binding by HMGB1, while galectin-9-induced cell surface clustering of TIM-3 was necessary for its suppressive function. Human peripheral blood cDC1s also took up extracellular DNA upon TIM-3 blockade. Thus, TIM-3 regulates endocytosis of extracellular DNA and activation of the cytoplasmic DNA sensing cGAS-STING pathway in cDC1s, with implications for understanding the mechanisms underlying TIM-3 immunotherapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Western Blotting
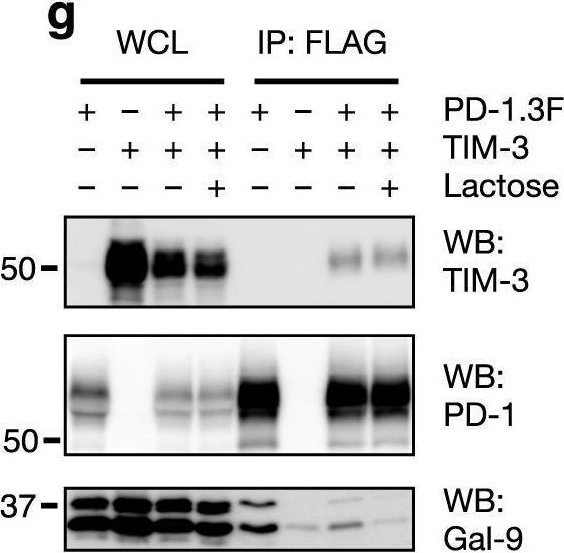
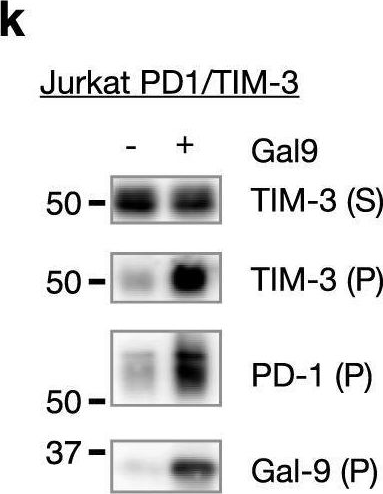
Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy.
In Nat Commun on 5 February 2021 by Yang, R. Y., Sun, L., et al.
PubMed
The two T cell inhibitory receptors PD-1 and TIM-3 are co-expressed during exhausted T cell differentiation, and recent evidence suggests that their crosstalk regulates T cell exhaustion and immunotherapy efficacy; however, the molecular mechanism is unclear. Here we show that PD-1 contributes to the persistence of PD-1+TIM-3+ T cells by binding to the TIM-3 ligand galectin-9 (Gal-9) and attenuates Gal-9/TIM-3-induced cell death. Anti-Gal-9 therapy selectively expands intratumoral TIM-3+ cytotoxic CD8 T cells and immunosuppressive regulatory T cells (Treg cells). The combination of anti-Gal-9 and an agonistic antibody to the co-stimulatory receptor GITR (glucocorticoid-induced tumor necrosis factor receptor-related protein) that depletes Treg cells induces synergistic antitumor activity. Gal-9 expression and secretion are promoted by interferon β and γ, and high Gal-9 expression correlates with poor prognosis in multiple human cancers. Our work uncovers a function for PD-1 in exhausted T cell survival and suggests Gal-9 as a promising target for immunotherapy.
-
-
-
In vivo experiments
-
Cancer Research
-
Immunology and Microbiology
TIM-3 Regulates CD103+ Dendritic Cell Function and Response to Chemotherapy in Breast Cancer.
In Cancer Cell on 8 January 2018 by de Mingo Pulido, A., Gardner, A., et al.
PubMed
Intratumoral CD103+ dendritic cells (DCs) are necessary for anti-tumor immunity. Here we evaluated the expression of immune regulators by CD103+ DCs in a murine model of breast cancer and identified expression of TIM-3 as a target for therapy. Anti-TIM-3 antibody improved response to paclitaxel chemotherapy in models of triple-negative and luminal B disease, with no evidence of toxicity. Combined efficacy was CD8+ T cell dependent and associated with increased granzyme B expression; however, TIM-3 expression was predominantly localized to myeloid cells in both human and murine tumors. Gene expression analysis identified upregulation of Cxcl9 within intratumoral DCs during combination therapy, and therapeutic efficacy was ablated by CXCR3 blockade, Batf3 deficiency, or Irf8 deficiency.
-
-
-
In vivo experiments
-
Immunology and Microbiology
γδ T Cells Support Pancreatic Oncogenesis by Restraining αβ T Cell Activation.
In Cell on 8 September 2016 by Daley, D., Zambirinis, C. P., et al.
PubMed
Inflammation is paramount in pancreatic oncogenesis. We identified a uniquely activated γδT cell population, which constituted ∼40% of tumor-infiltrating T cells in human pancreatic ductal adenocarcinoma (PDA). Recruitment and activation of γδT cells was contingent on diverse chemokine signals. Deletion, depletion, or blockade of γδT cell recruitment was protective against PDA and resulted in increased infiltration, activation, and Th1 polarization of αβT cells. Although αβT cells were dispensable to outcome in PDA, they became indispensable mediators of tumor protection upon γδT cell ablation. PDA-infiltrating γδT cells expressed high levels of exhaustion ligands and thereby negated adaptive anti-tumor immunity. Blockade of PD-L1 in γδT cells enhanced CD4(+) and CD8(+) T cell infiltration and immunogenicity and induced tumor protection suggesting that γδT cells are critical sources of immune-suppressive checkpoint ligands in PDA. We describe γδT cells as central regulators of effector T cell activation in cancer via novel cross-talk.
-