InVivoMAb anti-mouse F4/80
Product Description
Specifications
| Isotype | Rat IgG2b, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2b isotype control, anti-keyhole limpet hemocyanin |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | C57BL/6 mouse thioglycollate stimulated peritoneal macrophages |
| Reported Applications |
in vivo Monocyte/Macrophage depletion Functional assays Immunohistochemistry (paraffin) Immunohistochemistry (frozen) Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_10949019 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo Monocyte/Macrophage depletion
Paul, S., et al (2019). "Natural killer T cell activation increases iNOS(+)CD206(-) M1 macrophage and controls the growth of solid tumor" J Immunother Cancer 7(1): 208.
PubMed
BACKGROUND: NKT cells play an important role in anti-tumor immunity. Alpha-galactosylceramide (α-GalCer), a synthetic glycolipid is presented to natural killer T (NKT) cells by most antigen-presenting cells through CD1d molecules leading to activation of NKT cells. However, the precise mechanisms of how α-GalCer-activated NKT regulate the polarization of the macrophages and effector T cells in the solid tumor are not studied adequately. METHODS: We induced solid tumor in C57BL/6 mice by subcutaneous injection of B16F10 cell line (1 X 10(6) cells) and monitored the tumor growth. Animals were given an intraperitoneal injection of α-GalCer (2 μg/injection) in 200 μl PBS on day + 1, + 5, + 10, + 15, and + 20 (with respect to tumor cell injection). Immune cells were characterized using flow cytometry and immunofluorescence staining. NK cells, Gr1(+) cells, and F4/80(+) macrophages in the mice were depleted by intravenous injection of cell-specific antibodies. Statistical analysis was performed using Student’s t-test or one-way ANOVA. RESULTS: Our results showed that intratumoral NKT cells have a lower frequency of CD69, CD25, CD122, and IFN-γR expression; produced less inflammatory cytokines such as IFN-γ, TNF-α, and GM-CSF; higher frequency CD62L(+) NKT cells; and also showed reduced proliferation as compared to the splenic NKT cells. Mice treated with α-GalCer showed a significantly increased frequency of IFN-γ-producing NKT cells, CD8(+) T cells, and effector Th1 cells. Depletion of NK cells in α-GalCer-treated mice showed a lower frequency of IFN-γ-producing CD4(+) and CD8(+) T cells in the tumor and prevented the α-GalCer-induced tumor growth. NKT cell activation with α-GalCer treatment significantly increased the iNOS(+)CD206(-) M1-macrophages and reduced the iNOS(-)CD206(+) M2-macrophages in the spleen and tumor, and depletion of F4/80(+) macrophages prevented the α-GalCer-induced reduction in the tumor growth. CONCLUSIONS: We showed that activation of NKT cell with α-GalCer modulates the frequency of M1-macrophages and effector Th1 cells in the secondary lymphoid tissues and tumor microenvironment and inhibit tumor growth. The finding suggests that activation of NKT cells with α-GalCer may provide an effective anti-cancer outcome.
in vivo Monocyte/Macrophage depletion
Wang, E. C. E., et al (2019). "A Subset of TREM2(+) Dermal Macrophages Secretes Oncostatin M to Maintain Hair Follicle Stem Cell Quiescence and Inhibit Hair Growth" Cell Stem Cell 24(4): 654-669.e656.
PubMed
Hair growth can be induced from resting mouse hair follicles by topical application of JAK inhibitors, suggesting that JAK-STAT signaling is required for maintaining hair follicle stem cells (HFSCs) in a quiescent state. Here, we show that Oncostatin M (OSM), an IL-6 family cytokine, negatively regulates hair growth by signaling through JAK-STAT5 to maintain HFSC quiescence. Genetic deletion of the OSM receptor or STAT5 can induce premature HFSC activation, suggesting that the resting telogen stage is actively maintained by the hair follicle niche. Single-cell RNA sequencing revealed that the OSM source is not intrinsic to the hair follicle itself and is instead a subset of TREM2(+) macrophages that is enriched within the resting follicle and deceases immediately prior to HFSC activation. In vivo inhibition of macrophage function was sufficient to induce HFSC proliferation and hair cycle induction. Together these results clarify how JAK-STAT signaling actively inhibits hair growth.
in vivo Monocyte/Macrophage depletion
Immunohistochemistry (frozen)
Immunohistochemistry (paraffin)
Wang, W., et al (2018). "RIP1 Kinase Drives Macrophage-Mediated Adaptive Immune Tolerance in Pancreatic Cancer" Cancer Cell 34(5): 757-774 e757.
PubMed
Pancreatic ductal adenocarcinoma (PDA) is characterized by immune tolerance and immunotherapeutic resistance. We discovered upregulation of receptor-interacting serine/threonine protein kinase 1 (RIP1) in tumor-associated macrophages (TAMs) in PDA. To study its role in oncogenic progression, we developed a selective small-molecule RIP1 inhibitor with high in vivo exposure. Targeting RIP1 reprogrammed TAMs toward an MHCII(hi)TNFalpha(+)IFNgamma(+) immunogenic phenotype in a STAT1-dependent manner. RIP1 inhibition in TAMs resulted in cytotoxic T cell activation and T helper cell differentiation toward a mixed Th1/Th17 phenotype, leading to tumor immunity in mice and in organotypic models of human PDA. Targeting RIP1 synergized with PD1-and inducible co-stimulator-based immunotherapies. Tumor-promoting effects of RIP1 were independent of its co-association with RIP3. Collectively, our work describes RIP1 as a checkpoint kinase governing tumor immunity.
in vivo Monocyte/Macrophage depletion
Albacker, L. A., et al (2013). "TIM-4, expressed by medullary macrophages, regulates respiratory tolerance by mediating phagocytosis of antigen-specific T cells" Mucosal Immunol 6(3): 580-590.
PubMed
Respiratory exposure to antigen induces T cell tolerance via several overlapping mechanisms that limit the immune response. While the mechanisms involved in the development of Treg cells have received much attention, those that result in T cell deletion are largely unknown. Herein, we show that F4/80(+) lymph node medullary macrophages expressing TIM-4, a phosphatidylserine receptor, remove antigen-specific T cells during respiratory tolerance, thereby reducing secondary T cell responses. Blockade of TIM-4 inhibited the phagocytosis of antigen-specific T cells by TIM-4 expressing lymph node medullary macrophages, resulting in an increase in the number of antigen-specific T cells and the abrogation of respiratory tolerance. Moreover, specific depletion of medullary macrophages inhibited the induction of respiratory tolerance, highlighting the key role of TIM-4 and medullary macrophages in tolerance. Therefore, TIM-4-mediated clearance of antigen specific T cells represents an important previously unrecognized mechanism regulating respiratory tolerance.
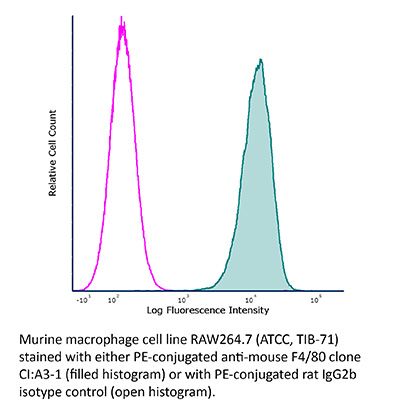
Flow Cytometry
Tittel, A. P., et al (2012). "Functionally relevant neutrophilia in CD11c diphtheria toxin receptor transgenic mice" Nat Methods 9(4): 385-390.
PubMed
Transgenic mice expressing the diphtheria toxin receptor (DTR) in specific cell types are key tools for functional studies in several biological systems. B6.FVB-Tg(Itgax-DTR/EGFP)57Lan/J (CD11c.DTR) and B6.Cg-Tg(Itgax-DTR/OVA/EGFP)1Gjh/Crl (CD11c.DOG) mice express the DTR in CD11c(+) cells, allowing conditional depletion of dendritic cells. We report that dendritic-cell depletion in these models caused polymorphonuclear neutrophil (PMN) release from the bone marrow, which caused chemokine-dependent neutrophilia after 6-24 h and increased bacterial clearance in a mouse pyelonephritis model. We present a transgenic mouse line, B6.Cg-Tg(Itgax-EGFP-CRE-DTR-LUC)2Gjh/Crl (CD11c.LuciDTR), which is unaffected by early neutrophilia. However, CD11c.LuciDTR and CD11c.DTR mice showed late neutrophilia 72 h after dendritic cell depletion, which was independent of PMN release and possibly resulted from increased granulopoiesis. Thus, the time point of dendritic cell depletion and the choice of DTR transgenic mouse line must be considered in experimental settings where neutrophils may be involved.
Flow Cytometry
Winkler, I. G., et al (2012). "Hematopoietic stem cell mobilizing agents G-CSF, cyclophosphamide or AMD3100 have distinct mechanisms of action on bone marrow HSC niches and bone formation" Leukemia 26(7): 1594-1601.
PubMed
The CXCR4 antagonist AMD3100 is progressively replacing cyclophosphamide (CYP) as adjuvant to granulocyte colony-stimulating factor (G-CSF) to mobilize hematopoietic stem cells (HSC) for autologous transplants in patients who failed prior mobilization with G-CSF alone. It has recently emerged that G-CSF mediates HSC mobilization and inhibits bone formation via specific bone marrow (BM) macrophages. We compared the effect of these three mobilizing agents on BM macrophages, bone formation, osteoblasts, HSC niches and HSC reconstitution potential. Both G-CSF and CYP suppressed niche-supportive macrophages and osteoblasts, and inhibited expression of endosteal cytokines resulting in major impairment of HSC reconstitution potential remaining in the mobilized BM. In sharp contrast, although AMD3100 was effective at mobilizing HSC, it did not suppress osteoblasts, endosteal cytokine expression or reconstitution potential of HSC remaining in the mobilized BM. In conclusion, although G-CSF, CYP and AMD3100 efficiently mobilize HSC into the blood, their effects on HSC niches and bone formation are distinct with both G-CSF and CYP targeting HSC niche function and bone formation, whereas AMD3100 directly targets HSC without altering niche function or bone formation.
in vivo Monocyte/Macrophage depletion
Immunohistochemistry (frozen)
Bedoret, D., et al (2009). "Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice" J Clin Invest 119(12): 3723-3738.
PubMed
The respiratory tract is continuously exposed to both innocuous airborne antigens and immunostimulatory molecules of microbial origin, such as LPS. At low concentrations, airborne LPS can induce a lung DC-driven Th2 cell response to harmless inhaled antigens, thereby promoting allergic asthma. However, only a small fraction of people exposed to environmental LPS develop allergic asthma. What prevents most people from mounting a lung DC-driven Th2 response upon exposure to LPS is not understood. Here we have shown that lung interstitial macrophages (IMs), a cell population with no previously described in vivo function, prevent induction of a Th2 response in mice challenged with LPS and an experimental harmless airborne antigen. IMs, but not alveolar macrophages, were found to produce high levels of IL-10 and to inhibit LPS-induced maturation and migration of DCs loaded with the experimental harmless airborne antigen in an IL-10-dependent manner. We further demonstrated that specific in vivo elimination of IMs led to overt asthmatic reactions to innocuous airborne antigens inhaled with low doses of LPS. This study has revealed a crucial role for IMs in maintaining immune homeostasis in the respiratory tract and provides an explanation for the paradox that although airborne LPS has the ability to promote the induction of Th2 responses by lung DCs, it does not provoke airway allergy under normal conditions.
Immunohistochemistry (paraffin)
Joffre, O., et al (2008). "Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes" Nat Med 14(1): 88-92.
PubMed
A major challenge in transplantation medicine is controlling the very strong immune responses to foreign antigens that are responsible for graft rejection. Although immunosuppressive drugs efficiently inhibit acute graft rejection, a substantial proportion of patients suffer chronic rejection that ultimately leads to functional loss of the graft. Induction of immunological tolerance to transplants would avoid rejection and the need for lifelong treatment with immunosuppressive drugs. Tolerance to self-antigens is ensured naturally by several mechanisms; one major mechanism depends on the activity of regulatory T lymphocytes. Here we show that in mice treated with clinically acceptable levels of irradiation, regulatory CD4+CD25+Foxp3+ T cells stimulated in vitro with alloantigens induced long-term tolerance to bone marrow and subsequent skin and cardiac allografts. Regulatory T cells specific for directly presented donor antigens prevented only acute rejection, despite hematopoietic chimerism. By contrast, regulatory T cells specific for both directly and indirectly presented alloantigens prevented both acute and chronic rejection. Our findings demonstrate the potential of appropriately stimulated regulatory T cells for future cell-based therapeutic approaches to induce lifelong immunological tolerance to allogeneic transplants.
Immunohistochemistry (paraffin)
Lloyd, C. M., et al (2008). "Three-colour fluorescence immunohistochemistry reveals the diversity of cells staining for macrophage markers in murine spleen and liver" J Immunol Methods 334(1-2): 70-81.
PubMed
Macrophages have traditionally been identified in murine tissues using a small range of markers, typically F4/80, CD68 and CD11b. However many studies have suggested that substantial heterogeneity exists in macrophage populations, and no single marker, nor even pair of markers, can necessarily identify all the populations. Further, many of the key monoclonal antibodies have been raised in the same species, making it difficult to combine them in histochemical studies. Here we have optimised a triple colour immunofluorescent staining protocol, utilising an anti-FITC technique, to allow antibodies to macrophage markers to be used simultaneously. We highlight the substantial heterogeneity of cells in both normal liver and spleen that stain for F4/80, CD68, CD11b, and CD11c. Using diet-induced steatohepatitis as a model of liver inflammation, we show that CD11b is expressed by newly migrating macrophage precursors, but is an unreliable marker for macrophage precursors when used alone because it is also expressed by migrating neutrophils. In healthy livers CD11c expression is a unique feature of a population of cells immediately surrounding the sinusoids. However, during hepatic inflammation CD11c can also be co-expressed by other cells, including both infiltrating cells and F4/80+ cells within the liver parenchyma. While no one marker alone is sufficient to account for all macrophage populations, we confirm that F4/80 marks the majority of the tissue-resident macrophages in both the liver and the spleen, although F4/80- populations that are positive for CD68, CD11b, or CD11c also exist. Distinguishing between tissue macrophages and dendritic cells with these markers remains problematic.
Immunohistochemistry (paraffin)
Pull, S. L., et al (2005). "Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury" Proc Natl Acad Sci U S A 102(1): 99-104.
PubMed
We have identified cellular and molecular features of the stem cell niche required for marked amplification of mouse colonic epithelial progenitors (ColEPs) that occurs in response to wounding of the epithelium with dextran sodium sulfate. This regenerative response in areas adjacent to breaches in the epithelial barrier depends on the gut microbiota because ColEP proliferation is markedly diminished in germ-free animals. Analysis of conventionally raised C57BL/6 (B6) knockout mice lacking the Toll-like receptor signal transduction pathway component Myd88 and wild-type animals transplanted with Myd88(-/-) bone marrow, revealed that Myd88-mediated signaling through mesenchymal cells is also required for the ColEP response. Studies of B6 Csf1(op/op) (lacking macrophages) mice, Rag1(-/-) mice, and wild-type mice treated with neutrophil-specific Gr1 mAbs, disclosed that macrophages but not lymphocytes or neutrophils are necessary. GeneChip analysis of laser-capture-microdissected mesenchymal cells coupled with immunohistochemical and electron microscopic studies showed that, during the regenerative response, macrophages in the pericryptal stem cell niche express genes associated with their activation and extend processes to directly contact ColEPs near the crypt base. GeneChip analysis also identified a number of potential molecular mediators of regeneration expressed in the pericryptal progenitor niche, including secreted factors that stimulate epithelial proliferation and proteins involved in extracellular matrix and basement membrane function, stability, and growth factor binding. Together, these studies indicate that the colonic epithelial progenitor niche is a dynamic structure in which macrophages function as mobile “cellular transceivers” that coordinate inputs from luminal microbes and injured epithelium and transmit regenerative signals to neighboring ColEPs.
Functional Assays
Warschkau, H. and A. F. Kiderlen (1999). "A monoclonal antibody directed against the murine macrophage surface molecule F4/80 modulates natural immune response to Listeria monocytogenes" J Immunol 163(6): 3409-3416.
PubMed
Whole spleen cell cultures from SCID mice release high levels of IFN-gamma when exposed to heat-killed Listeria monocytogenes (HKL). This microbe-induced and T cell-independent response depends on both macrophages (MPhi) and NK cells: HKL-stimulated MPhi release TNF-alpha and IL-12, which together activate NK cells for IFN-gamma release. We show here that this cytokine-mediated activation cascade can be modulated by a mAb against the MPhi surface glycoprotein F4/80. HKL-induced IL-12, TNF-alpha, and IFN-gamma in SCID whole spleen cell cultures was inhibited by coincubation with anti-F4/80 mAb whereas IL-1 and IL-10 were enhanced. Both effects were apparent at mRNA and protein release levels. Whereas inhibitory activities were F4/80 Ag specific, stimulatory effects were Fc dependent and nonspecific. Furthermore, cytokine inhibition by anti-F4/80 was only apparent when MPhi and NK cells were present simultaneously and in close vicinity, indicating that direct cell-to-cell contact is a prerequisite. These data suggest a novel pathway for microbe-induced MPhi/NK cell interaction involving direct cell-to-cell signaling and give the first evidence for a functional role of the MPhi surface glycoprotein F4/80.
Product Citations
-
-
In Vivo
-
Immu-depl
-
Cancer Research
-
Immunology and Microbiology
Natural killer T cell activation increases iNOS+CD206- M1 macrophage and controls the growth of solid tumor.
In Journal for Immunotherapy of Cancer on 6 August 2019 by Paul, S., Chhatar, S., et al.
PubMed
NKT cells play an important role in anti-tumor immunity. Alpha-galactosylceramide (α-GalCer), a synthetic glycolipid is presented to natural killer T (NKT) cells by most antigen-presenting cells through CD1d molecules leading to activation of NKT cells. However, the precise mechanisms of how α-GalCer-activated NKT regulate the polarization of the macrophages and effector T cells in the solid tumor are not studied adequately. We induced solid tumor in C57BL/6 mice by subcutaneous injection of B16F10 cell line (1 X 106 cells) and monitored the tumor growth. Animals were given an intraperitoneal injection of α-GalCer (2 μg/injection) in 200 μl PBS on day + 1, + 5, + 10, + 15, and + 20 (with respect to tumor cell injection). Immune cells were characterized using flow cytometry and immunofluorescence staining. NK cells, Gr1+ cells, and F4/80+ macrophages in the mice were depleted by intravenous injection of cell-specific antibodies. Statistical analysis was performed using Student's t-test or one-way ANOVA. Our results showed that intratumoral NKT cells have a lower frequency of CD69, CD25, CD122, and IFN-γR expression; produced less inflammatory cytokines such as IFN-γ, TNF-α, and GM-CSF; higher frequency CD62L+ NKT cells; and also showed reduced proliferation as compared to the splenic NKT cells. Mice treated with α-GalCer showed a significantly increased frequency of IFN-γ-producing NKT cells, CD8+ T cells, and effector Th1 cells. Depletion of NK cells in α-GalCer-treated mice showed a lower frequency of IFN-γ-producing CD4+ and CD8+ T cells in the tumor and prevented the α-GalCer-induced tumor growth. NKT cell activation with α-GalCer treatment significantly increased the iNOS+CD206- M1-macrophages and reduced the iNOS-CD206+ M2-macrophages in the spleen and tumor, and depletion of F4/80+ macrophages prevented the α-GalCer-induced reduction in the tumor growth. We showed that activation of NKT cell with α-GalCer modulates the frequency of M1-macrophages and effector Th1 cells in the secondary lymphoid tissues and tumor microenvironment and inhibit tumor growth. The finding suggests that activation of NKT cells with α-GalCer may provide an effective anti-cancer outcome.
-
-
-
Cancer Research
-
Immunology and Microbiology
FLT3L combined with GM-CSF induced dendritic cells drive broad tumor-specific CD8+ T cell responses and remodel the tumor microenvironment to enhance anti-tumor efficacy.
In Front Immunol on 22 September 2025 by Zheng, Q., Zhang, J., et al.
PubMed
Dendritic cells (DCs) play a crucial role in anti-tumor immunity by capturing, processing, and presenting tumor antigens to T cells, making DC-based immunotherapy a promising approach for cancer treatment. However, the most commonly used clinical strategy still relies on inducing DCs in vitro using granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL - 4) (GM/IL4-DCs), which often results in a heterogeneous cell population with suboptimal anti-tumor function. Here, we compared DCs generated by co-stimulating with FMS-like tyrosine kinase 3 ligand (FLT3L) and GM-CSF (FL/GM-DCs) with the conventional GM/IL4-DCs.
-
-
-
Immunology and Microbiology
-
COVID-19
Toll-like receptor 7 (TLR7)-mediated antiviral response protects mice from lethal SARS-CoV-2 infection.
In J Virol on 20 May 2025 by Ghimire, R., Shrestha, R., et al.
PubMed
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced impaired antiviral immunity and excessive inflammatory responses cause lethal pneumonia. However, the in vivo roles of key pattern recognition receptors that elicit protective antiviral and fatal inflammatory responses, specifically in the lungs, are not well described. Coronaviruses possess single-stranded RNA genome that activates TLR7/8 to induce an antiviral interferon (IFN) and robust inflammatory cytokine response. Here, using wild-type and TLR7-deficient (TLR7-/-) mice infected with mouse-adapted SARS-CoV-2 (MA-CoV-2), we examined the role of TLR7 in the lung antiviral and inflammatory response and severe pneumonia. We showed that TLR7 deficiency significantly increased lung virus loads and morbidity/mortality, which correlated with reduced levels of type I IFNs (Ifna/b), type III IFNs (Ifnl), and IFN-stimulated genes (ISGs) in the lungs. A detailed evaluation of MA-CoV-2-infected lungs revealed increased neutrophil accumulation and lung pathology in TLR7-/- mice. We further showed that blocking type I IFN receptor (IFNAR) signaling enhanced SARS-CoV-2 replication in the lungs and caused severe lung pathology, leading to 100% mortality compared to infected control mice. Moreover, immunohistochemical assessment of the lungs revealed increased numbers of SARS-CoV-2 antigen-positive macrophages, pneumocytes, and bronchial epithelial cells in TLR7-/- and IFNAR-deficient mice compared to control mice. In summary, we conclusively demonstrated that despite TLR7-induced robust lung inflammation, TLR7-induced IFN/ISG responses suppress lung virus replication and pathology and provide protection against SARS-CoV-2-induced fatal pneumonia. Additionally, given the similar disease outcomes in control, TLR7-/-, and IFNAR-deficient MA-CoV-2-infected mice and coronavirus disease 2019 (COVID-19) patients, we propose that MA-CoV-2-infected mice constitute an excellent model for studying COVID-19.IMPORTANCESevere coronavirus disease 2019 (COVID-19) is caused by a delicate balance between a strong antiviral and an exuberant inflammatory response. A robust antiviral immunity and regulated inflammation are protective, while a weak antiviral response and excessive inflammation are detrimental. However, the key host immune sensors that elicit protective antiviral and inflammatory responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) challenge are poorly defined. Here, we examined the role of viral RNA-mediated TLR7 activation in the lung antiviral and inflammatory responses in SARS-CoV-2-infected mice. We demonstrate that TLR7 deficiency led to a high rate of morbidity and mortality, which correlated with an impaired antiviral interferon (IFN)-I/III response, enhanced lung virus replication, and severe lung pathology. Furthermore, we show that blocking IFN-I signaling using anti-IFN receptor antibody promoted SARS-CoV-2 replication in the lungs and caused severe disease. These results provide conclusive evidence that TLR7 and IFN-I receptor deficiencies lead to severe disease in mice, replicating clinical features observed in COVID-19 patients.
-
-
-
Immunology and Microbiology
Engineered oncolytic virus coated with anti-PD-1 and alendronate for ameliorating intratumoral T cell hypofunction.
In Exp Hematol Oncol on 15 February 2025 by Zhu, Y., Zhang, X., et al.
PubMed
Glioblastoma is a highly aggressive and devastating primary brain tumor that is resistant to conventional therapies. Oncolytic viruses represent a promising therapeutic approach for glioblastoma by selectively lysing tumor cells and eliciting an anti-tumor immune response. However, the clinical efficacy of oncolytic viruses is often hindered by challenges such as short persistence, host antiviral immune responses, and T cell dysfunction.
-
-
A mosquito salivary protein-driven influx of myeloid cells facilitates flavivirus transmission.
In EMBO J on 1 May 2024 by Wang, Z. Y., Nie, K., et al.
PubMed
Mosquitoes transmit many disease-relevant flaviviruses. Efficient viral transmission to mammalian hosts requires mosquito salivary factors. However, the specific salivary components facilitating viral transmission and their mechanisms of action remain largely unknown. Here, we show that a female mosquito salivary gland-specific protein, here named A. aegypti Neutrophil Recruitment Protein (AaNRP), facilitates the transmission of Zika and dengue viruses. AaNRP promotes a rapid influx of neutrophils, followed by virus-susceptible myeloid cells toward mosquito bite sites, which facilitates establishment of local infection and systemic dissemination. Mechanistically, AaNRP engages TLR1 and TLR4 of skin-resident macrophages and activates MyD88-dependent NF-κB signaling to induce the expression of neutrophil chemoattractants. Inhibition of MyD88-NF-κB signaling with the dietary phytochemical resveratrol reduces AaNRP-mediated enhancement of flavivirus transmission by mosquitoes. These findings exemplify how salivary components can aid viral transmission, and suggest a potential prophylactic target.
-
-
Cardiovascular biology
Myeloid Cell Derived IL1β Contributes to Pulmonary Hypertension in HFpEF.
In Circ Res on 10 November 2023 by Agrawal, V., Kropski, J. A., et al.
PubMed
Pulmonary hypertension (PH) in heart failure with preserved ejection fraction (HFpEF) is a common and highly morbid syndrome, but mechanisms driving PH-HFpEF are poorly understood. We sought to determine whether a well-accepted murine model of HFpEF also displays features of PH, and we sought to identify pathways that might drive early remodeling of the pulmonary vasculature in HFpEF.
-
-
-
Immunohistochemistry-immunofluorescence
Chemical modification of AAV9 capsid with N-ethyl maleimide alters vector tissue tropism.
In Sci Rep on 25 May 2023 by Mulcrone, P. L., Lam, A. K., et al.
PubMed
Although more adeno-associated virus AAV-based drugs enter the clinic, vector tissue tropism remains an unresolved challenge that limits its full potential despite that the tissue tropism of naturally occurring AAV serotypes can be altered by genetic engineering capsid vie DNA shuffling, or molecular evolution. To further expand the tropism and thus potential applications of AAV vectors, we utilized an alternative approach that employs chemical modifications to covalently link small molecules to reactive exposed Lysine residues of AAV capsids. We demonstrated that AAV9 capsid modified with N-ethyl Maleimide (NEM) increased its tropism more towards murine bone marrow (osteoblast lineage) while decreased transduction of liver tissue compared to the unmodified capsid. In the bone marrow, AAV9-NEM transduced Cd31, Cd34, and Cd90 expressing cells at a higher percentage than unmodified AAV9. Moreover, AAV9-NEM localized strongly in vivo to cells lining the calcified trabecular bone and transduced primary murine osteoblasts in culture, while WT AAV9 transduced undifferentiated bone marrow stromal cells as well as osteoblasts. Our approach could provide a promising platform for expanding clinical AAV development to treat bone pathologies such as cancer and osteoporosis. Thus, chemical engineering the AAV capsid holds great potential for development of future generations of AAV vectors.
-
-
-
Cancer Research
Topical therapy for regression and melanoma prevention of congenital giant nevi.
In Cell on 9 June 2022 by Choi, Y. S., Erlich, T. H., et al.
PubMed
Giant congenital melanocytic nevi are NRAS-driven proliferations that may cover up to 80% of the body surface. Their most dangerous consequence is progression to melanoma. This risk often triggers preemptive extensive surgical excisions in childhood, producing severe lifelong challenges. We have presented preclinical models, including multiple genetically engineered mice and xenografted human lesions, which enabled testing locally applied pharmacologic agents to avoid surgery. The murine models permitted the identification of proliferative versus senescent nevus phases and treatments targeting both. These nevi recapitulated the histologic and molecular features of human giant congenital nevi, including the risk of melanoma transformation. Cutaneously delivered MEK, PI3K, and c-KIT inhibitors or proinflammatory squaric acid dibutylester (SADBE) achieved major regressions. SADBE triggered innate immunity that ablated detectable nevocytes, fully prevented melanoma, and regressed human giant nevus xenografts. These findings reveal nevus mechanistic vulnerabilities and suggest opportunities for topical interventions that may alter the therapeutic options for children with congenital giant nevi.
-
-
-
In vivo experiments
-
Cancer Research
Reprogramming NK cells and macrophages via combined antibody and cytokine therapy primes tumors for elimination by checkpoint blockade.
In Cell Rep on 23 November 2021 by Wang, C., Cui, A., et al.
PubMed
Treatments aiming to augment immune checkpoint blockade (ICB) in cancer often focus on T cell immunity, but innate immune cells may have important roles to play. Here, we demonstrate a single-dose combination treatment (termed AIP) using a pan-tumor-targeting antibody surrogate, half-life-extended interleukin-2 (IL-2), and anti-programmed cell death 1 (PD-1), which primes tumors to respond to subsequent ICB and promotes rejection of large established tumors in mice. Natural killer (NK) cells and macrophages activated by AIP treatment underwent transcriptional reprogramming; rapidly killed cancer cells; governed the recruitment of cross-presenting dendritic cells (DCs) and other leukocytes; and induced normalization of the tumor vasculature, facilitating further immune infiltration. Thus, innate cell-activating therapies can initiate critical steps leading to a self-sustaining cycle of T cell priming driven by ICB.
-
-
-
Stem Cells and Developmental Biology
N2-Polarized Neutrophils Guide Bone Mesenchymal Stem Cell Recruitment and Initiate Bone Regeneration: A Missing Piece of the Bone Regeneration Puzzle.
In Adv Sci (Weinh) on 1 October 2021 by Cai, B., Lin, D., et al.
PubMed
The role of neutrophils in bone regeneration remains elusive. In this study, it is shown that intramuscular implantation of interleukin-8 (IL-8) (commonly recognized as a chemotactic cytokine for neutrophils) at different levels lead to outcomes resembling those of fracture hematoma at various stages. Ectopic endochondral ossification is induced by certain levels of IL-8, during which neutrophils are recruited to the implanted site and are N2-polarized, which then secrete stromal cell-derived factor-1α (SDF-1α) for bone mesenchymal stem cell (BMSC) chemotaxis via the SDF-1/CXCR4 (C-X-C motif chemokine receptor 4) axis and its downstream phosphatidylinositol 3'-kinase (PI3K)/Akt pathway and β-catenin-mediated migration. Neutrophils are pivotal for recruiting and orchestrating innate and adaptive immunocytes, as well as BMSCs at the initial stage of bone healing and regeneration. The results in this study delineate the mechanism of neutrophil-initiated bone regeneration and interaction between neutrophils and BMSCs, and innate and adaptive immunities. This work lays the foundation for research in the fields of bone regenerative therapy and biomaterial development, and might inspire further research into novel therapeutic options.
-
-
-
Immunology and Microbiology
Lactobacillus stress protein GroEL prevents colonic inflammation.
In J Gastroenterol on 1 May 2021 by Dias, A. M. M., Douhard, R., et al.
PubMed
We previously showed that supernatants of Lactobacillus biofilms induced an anti-inflammatory response by affecting the secretion of macrophage-derived cytokines, which was abrogated upon immunodepletion of the stress protein GroEL.
-
-
-
Flow cytometry/Cell sorting
Sequencing-Based Protein Analysis of Single Extracellular Vesicles.
In ACS Nano on 23 March 2021 by Ko, J., Wang, Y., et al.
PubMed
Circulating extracellular vesicles (EVs)-biological nanomaterials shed from most mammalian cells-have emerged as promising biomarkers, drug delivery vesicles, and treatment modulators. While different types of vesicles are being explored for these applications, it is becoming clear that human EVs are quite heterogeneous even in homogeneous or monoclonal cell populations. Since it is the surface EV protein composition that will largely dictate their biological behavior, high-throughput single EV profiling methods are needed to better define EV subpopulations. Here, we present an antibody-based immunosequencing method that allows multiplexed measurement of protein molecules from individual nanometer-sized EVs. We use droplet microfluidics to compartmentalize and barcode individual EVs. The barcodes/antibody-DNA are then sequenced to determine protein composition. Using this highly sensitive technology, we detected specific proteins at the single EV level. We expect that this technology can be further adapted for multiplexed protein analysis of any nanoparticle.
-
-
-
Flow cytometry/Cell sorting
Preperitoneal Fat Grafting Inhibits the Formation of Intra-abdominal Adhesions in Mice.
In J Gastrointest Surg on 1 December 2020 by Laukka, M., Hoppela, E., et al.
PubMed
Adhesion formation contributes to postoperative complications in abdominal and gynaecological surgery. Thus far, the prevention and treatment strategies have focused on mechanical barriers in solid and liquid form, but these methods are not in routine use. As autologous fat grafting has become popular in treatment of hypertrophic scars because of its immunomodulatory effects, we postulated that fat grafting could also prevent peritoneal adhesion through similar mechanisms.
-
-
-
Cancer Research
-
Genetics
Targeting Obesity-Induced Macrophages during Preneoplastic Growth Promotes Mammary Epithelial Stem/Progenitor Activity, DNA Damage, and Tumor Formation.
In Cancer Res on 15 October 2020 by Chamberlin, T., Clack, M., et al.
PubMed
Obesity enhances breast cancer risk in postmenopausal women and premenopausal women with genetic or familial risk factors. We have shown previously that within breast tissue, obesity increases macrophage-driven inflammation and promotes expansion of luminal epithelial cell populations that are hypothesized to be the cells of origin for the most common subtypes of breast cancer. However, it is not clear how these changes within the microenvironment of the breast alter cancer risk and tumor growth. Using a high-fat diet to induce obesity, we examined preneoplastic changes associated with epithelial cell-specific loss of Trp53. Obesity significantly enhanced the incidence of tumors of diverse histotypes and increased stromal cells within the tumor microenvironment. Obesity also promoted the growth of preneoplastic lesions containing elevated numbers of luminal epithelial progenitor cells, which were surrounded by macrophages. To understand how macrophage-driven inflammation due to obesity enhances tumor formation, mice were treated with IgG or anti-F4/80 antibodies to deplete macrophages during preneoplastic growth. Unexpectedly, depletion of macrophages in obese mice enhanced mammary epithelial cell stem/progenitor activity, elevated expression of estrogen receptor alpha, and increased DNA damage in cells. Together, these results suggest that in obesity, macrophages reduce epithelial cells with DNA damage, which may limit the progression of preneoplastic breast lesions, and uncovers complex macrophage function within the evolving tumor microenvironment. Understanding how obesity alters the function of macrophages during tumor formation may lead to chemoprevention options for at-risk obese women. SIGNIFICANCE: Understanding how obesity impacts early tumor growth and response to macrophage-targeted therapies may improve therapeutics for obese patients with breast cancer and identify patient populations that would benefit from macrophage-targeted therapies.
-
-
-
Cancer Research
-
Immunology and Microbiology
Tristetraprolin Promotes Hepatic Inflammation and Tumor Initiation but Restrains Cancer Progression to Malignancy.
In Cell Mol Gastroenterol Hepatol on 29 September 2020 by Dolicka, D., Sobolewski, C., et al.
PubMed
Tristetraprolin (TTP) is a key post-transcriptional regulator of inflammatory and oncogenic transcripts. Accordingly, TTP was reported to act as a tumor suppressor in specific cancers. Herein, we investigated how TTP contributes to the development of liver inflammation and fibrosis, which are key drivers of hepatocarcinogenesis, as well as to the onset and progression of hepatocellular carcinoma (HCC).
-
-
-
Immunology and Microbiology
Obesity reduces mammary epithelial cell TGFβ1 activity through macrophage-mediated extracellular matrix remodeling.
In FASEB J on 1 June 2020 by Chamberlin, T., Thompson, V., et al.
PubMed
Obesity is a risk factor for breast cancer in postmenopausal and high-risk premenopausal women. Changes within the obese breast microenvironment may increase breast cancer risk. Transforming growth factor beta-1 (TGFβ1) is a major regulator of mammary epithelial stem/progenitor cells, and its activity is dysregulated under conditions of obesity. Using a high-fat diet model of obesity in mice and breast tissue from women, we observed that TGFβ1 activity is reduced in breast epithelial cells in obesity. Breast ducts and lobules demonstrated increased decorin in the extracellular matrix (ECM) surrounding epithelial cells, and we observed that decorin and latent TGFβ1 complexed together. Under conditions of obesity, macrophages expressed higher levels of decorin and were significantly increased in number surrounding breast epithelial cells. To investigate the relationship between macrophages and decorin expression, we treated obese mice with either IgG control or anti-F4/80 antibodies to deplete macrophages. Mice treated with anti-F4/80 antibodies demonstrated reduced decorin surrounding mammary ducts and enhanced TGFβ1 activity within mammary epithelial cells. Given the role of TGFβ1 as a tumor suppressor, reduced epithelial TGFβ1 activity and enhanced TGFβ1 within the ECM of obese mammary tissue may enhance breast cancer risk.
-
-
-
Cancer Research
Obesity Promotes Cooperation of Cancer Stem-Like Cells and Macrophages to Enhance Mammary Tumor Angiogenesis.
In Cancers (Basel) on 21 February 2020 by Hillers-Ziemer, L. E., McMahon, R. Q., et al.
PubMed
Obesity is correlated with worsened prognosis and treatment resistance in breast cancer. Macrophage-targeted therapies are currently in clinical trials, however, little is known about how obesity may impact treatment efficacy. Within breast adipose tissue, obesity leads to chronic, macrophage-driven inflammation, suggesting that obese breast cancer patients may benefit from these therapies. Using a high fat diet model of obesity, we orthotopically transplanted cancer cell lines into the mammary glands of obese and lean mice. We quantified changes in tumor invasiveness, angiogenesis and metastasis, and examined the efficacy of macrophage depletion to diminish tumor progression in obese and lean mice. Mammary tumors from obese mice grew significantly faster, were enriched for cancer stem-like cells (CSCs) and were more locally invasive and metastatic. Tumor cells isolated from obese mice demonstrated enhanced expression of stem cell-related pathways including Sox2 and Notch2. Despite more rapid growth, mammary tumors from obese mice had reduced necrosis, higher blood vessel density, and greater macrophage recruitment. Depletion of macrophages in obese tumor-bearing mice resulted in increased tumor necrosis, reduced endothelial cells, and enhanced recruitment of CD8+ T cells compared to IgG-treated controls. Macrophages may be an important clinical target to improve treatment options for obese breast cancer patients.
-
-
-
In vivo experiments
-
In vivo experiments
-
Stem Cells and Developmental Biology
A Subset of TREM2+ Dermal Macrophages Secretes Oncostatin M to Maintain Hair Follicle Stem Cell Quiescence and Inhibit Hair Growth.
In Cell Stem Cell on 4 April 2019 by Wang, E. C. E., Dai, Z., et al.
PubMed
Hair growth can be induced from resting mouse hair follicles by topical application of JAK inhibitors, suggesting that JAK-STAT signaling is required for maintaining hair follicle stem cells (HFSCs) in a quiescent state. Here, we show that Oncostatin M (OSM), an IL-6 family cytokine, negatively regulates hair growth by signaling through JAK-STAT5 to maintain HFSC quiescence. Genetic deletion of the OSM receptor or STAT5 can induce premature HFSC activation, suggesting that the resting telogen stage is actively maintained by the hair follicle niche. Single-cell RNA sequencing revealed that the OSM source is not intrinsic to the hair follicle itself and is instead a subset of TREM2+ macrophages that is enriched within the resting follicle and deceases immediately prior to HFSC activation. In vivo inhibition of macrophage function was sufficient to induce HFSC proliferation and hair cycle induction. Together these results clarify how JAK-STAT signaling actively inhibits hair growth.
-
-
-
Cancer Research
-
Immunology and Microbiology
RIP1 Kinase Drives Macrophage-Mediated Adaptive Immune Tolerance in Pancreatic Cancer.
In Cancer Cell on 12 November 2018 by Wang, W., Marinis, J. M., et al.
PubMed
Pancreatic ductal adenocarcinoma (PDA) is characterized by immune tolerance and immunotherapeutic resistance. We discovered upregulation of receptor-interacting serine/threonine protein kinase 1 (RIP1) in tumor-associated macrophages (TAMs) in PDA. To study its role in oncogenic progression, we developed a selective small-molecule RIP1 inhibitor with high in vivo exposure. Targeting RIP1 reprogrammed TAMs toward an MHCIIhiTNFα+IFNγ+ immunogenic phenotype in a STAT1-dependent manner. RIP1 inhibition in TAMs resulted in cytotoxic T cell activation and T helper cell differentiation toward a mixed Th1/Th17 phenotype, leading to tumor immunity in mice and in organotypic models of human PDA. Targeting RIP1 synergized with PD1-and inducible co-stimulator-based immunotherapies. Tumor-promoting effects of RIP1 were independent of its co-association with RIP3. Collectively, our work describes RIP1 as a checkpoint kinase governing tumor immunity.
-