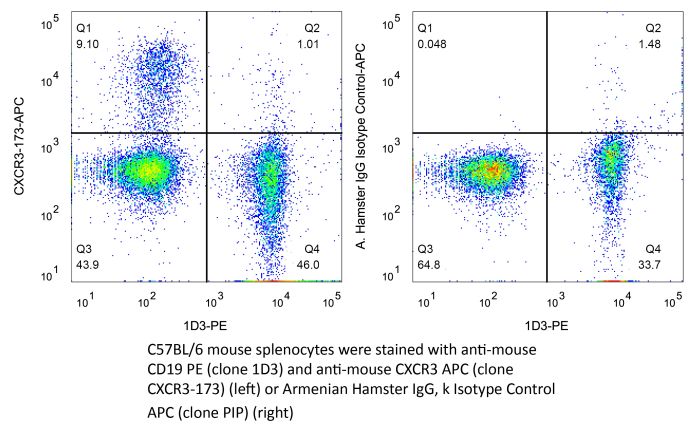
InVivoMAb anti-mouse CXCR3 (CD183)
Product Description
Specifications
| Isotype | Armenian hamster IgG |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb polyclonal Armenian hamster IgG |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Peptide consisting of amino acids 1-37 of mouse CXCR3 |
| Reported Applications |
in vivo CXCR3 neutralization Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687730 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo CXCR3 neutralization
Jacquelot, N., et al (2016). "Chemokine receptor patterns in lymphocytes mirror metastatic spreading in melanoma" J Clin Invest 126(3): 921-937.
PubMed
Melanoma prognosis is dictated by tumor-infiltrating lymphocytes, the migratory and functional behavior of which is guided by chemokine or cytokine gradients. Here, we retrospectively analyzed the expression patterns of 9 homing receptors (CCR/CXCR) in naive and memory CD4+ and CD8+ T lymphocytes in 57 patients with metastatic melanoma (MMel) with various sites of metastases to evaluate whether T cell CCR/CXCR expression correlates with intratumoral accumulation, metastatic progression, and/or overall survival (OS). Homing receptor expression on lymphocytes strongly correlated with MMel dissemination. Loss of CCR6 or CXCR3, but not cutaneous lymphocyte antigen (CLA), on circulating T cell subsets was associated with skin or lymph node metastases, loss of CXCR4, CXCR5, and CCR9 corresponded with lung involvement, and a rise in CCR10 or CD103 was associated with widespread dissemination. High frequencies of CD8+CCR9+ naive T cells correlated with prolonged OS, while neutralizing the CCR9/CCL25 axis in mice stimulated tumor progression. The expansion of CLA-expressing effector memory CD8+ T cells in response to a single administration of CTLA4 blockade predicted disease control at 3 months in 47 patients with MMel. Thus, specific CCR/CXCR expression patterns on circulating T lymphocytes may guide potential diagnostic and therapeutic approaches.
in vivo CXCR3 neutralization
Yang, H., et al (2015). "STAT3 Inhibition Enhances the Therapeutic Efficacy of Immunogenic Chemotherapy by Stimulating Type 1 Interferon Production by Cancer Cells" Cancer Res 75(18): 3812-3822.
PubMed
STAT3 is an oncogenic transcription factor with potent immunosuppressive functions. We found that pharmacologic inhibition of STAT3 or its selective knockout in cancer cells improved the tumor growth-inhibitory efficacy of anthracycline-based chemotherapies. This combined effect of STAT3 inhibition/depletion and anthracyclines was only found in tumors growing on immunocompetent (not in immunodeficient) mice. As compared with Stat3-sufficient control tumors, Stat3(-/-) cancer cells exhibited an increased infiltration by dendritic cells and cytotoxic T lymphocytes after chemotherapy. Anthracyclines are known to induce several stress pathways that enhance the immunogenicity of dying and dead cancer cells, thereby stimulating a dendritic cell-dependent and T lymphocyte-mediated anticancer immune response. Among these therapy-relevant stress pathways, Stat3(-/-) cancer cells manifested one significant improvement, namely an increase in the expression of multiple type-1 interferon-responsive genes, including that of the chemokines Cxcl9 and Cxcl10. This enhanced type-1 interferon response could be suppressed by reintroducing wild-type Stat3 (but not a transactivation-deficient mutant Stat3(Y705F)) into the tumor cells. This maneuver also abolished the improved chemotherapeutic response of Stat3(-/-) cancers. Finally, the neutralization of the common type-1 interferon receptor or that of the chemokine receptor CXCR3 (which binds CXCL9 and CXCL10) abolished the difference in the chemotherapeutic response between Stat3(-/-) and control tumors. Altogether, these results suggest that STAT3 inhibitors may improve the outcome of chemotherapy by enhancing the type-1 interferon response of cancer cells.
in vivo CXCR3 neutralization
Flow Cytometry
Chaturvedi, V., et al (2015). "CXCR3 blockade protects against Listeria monocytogenes infection-induced fetal wastage" J Clin Invest 125(4): 1713-1725.
PubMed
Mammalian pregnancy requires protection against immunological rejection of the developing fetus bearing discordant paternal antigens. Immune evasion in this developmental context entails silenced expression of chemoattractant proteins (chemokines), thereby preventing harmful immune cells from penetrating the maternal-fetal interface. Here, we demonstrate that fetal wastage triggered by prenatal Listeria monocytogenes infection is driven by placental recruitment of CXCL9-producing inflammatory neutrophils and macrophages that promote infiltration of fetal-specific T cells into the decidua. Maternal CD8+ T cells with fetal specificity upregulated expression of the chemokine receptor CXCR3 and, together with neutrophils and macrophages, were essential for L. monocytogenes-induced fetal resorption. Conversely, decidual accumulation of maternal T cells with fetal specificity and fetal wastage were extinguished by CXCR3 blockade or in CXCR3-deficient mice. Remarkably, protection against fetal wastage and in utero L. monocytogenes invasion was maintained even when CXCR3 neutralization was initiated after infection, and this protective effect extended to fetal resorption triggered by partial ablation of immune-suppressive maternal Tregs, which expand during pregnancy to sustain fetal tolerance. Together, our results indicate that functionally overriding chemokine silencing at the maternal-fetal interface promotes the pathogenesis of prenatal infection and suggest that therapeutically reinforcing this pathway represents a universal approach for mitigating immune-mediated pregnancy complications.
in vivo CXCR3 neutralization
Glennie, N. D., et al (2015). "Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection" J Exp Med 212(9): 1405-1414.
PubMed
Leishmaniasis causes a significant disease burden worldwide. Although Leishmania-infected patients become refractory to reinfection after disease resolution, effective immune protection has not yet been achieved by human vaccines. Although circulating Leishmania-specific T cells are known to play a critical role in immunity, the role of memory T cells present in peripheral tissues has not been explored. Here, we identify a population of skin-resident Leishmania-specific memory CD4(+) T cells. These cells produce IFN-gamma and remain resident in the skin when transplanted by skin graft onto naive mice. They function to recruit circulating T cells to the skin in a CXCR3-dependent manner, resulting in better control of the parasites. Our findings are the first to demonstrate that CD4(+) TRM cells form in response to a parasitic infection, and indicate that optimal protective immunity to Leishmania, and thus the success of a vaccine, may depend on generating both circulating and skin-resident memory T cells.
Product Citations
-
-
Immunology and Microbiology
Mucosal unadjuvanted booster vaccines elicit local IgA responses by conversion of pre-existing immunity in mice.
In Nat Immunol on 1 June 2025 by Kwon, D. I., Mao, T., et al.
PubMed
Mucosal delivery of vaccine boosters induces robust local protective immune responses even without any adjuvants. Yet, the mechanisms by which antigen alone induces mucosal immunity in the respiratory tract remain unclear. Here we show that an intranasal booster with an unadjuvanted recombinant SARS-CoV-2 spike protein, after intramuscular immunization with 1 μg of mRNA-LNP vaccine encoding the full-length SARS-CoV-2 spike protein (Pfizer/BioNTech BNT162b2), elicits protective mucosal immunity by retooling the lymph node-resident immune cells. On intranasal boosting, peripheral lymph node-primed B cells rapidly migrated to the lung through CXCR3-CXCL9 and CXCR3-CXCL10 signaling and differentiated into antigen-specific IgA-secreting plasma cells. Memory CD4+ T cells in the lung served as a natural adjuvant for developing mucosal IgA by inducing the expression of chemokines CXCL9 and CXCL10 for memory B cell recruitment. Furthermore, CD40 and TGFβ signaling had important roles in mucosal IgA development. Repeated mucosal boosting with an unadjuvanted protein amplified anamnestic IgA responses in both the upper and the lower respiratory tracts. These findings help explain why nasal boosters do not require an adjuvant to induce robust mucosal immunity at the respiratory mucosa and can be used to design safe and effective vaccines against respiratory pathogens.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
SIN3B Loss Heats up Cold Tumor Microenvironment to Boost Immunotherapy in Pancreatic Cancer.
In Adv Sci (Weinh) on 1 November 2024 by Zhang, Z., Tang, Y., et al.
PubMed
Despite progress significant advances in immunotherapy for some solid tumors, pancreatic ductal adenocarcinoma (PDAC) remains unresponsive poorly responsive to such interventions, largely due to its highly immunosuppressive tumor microenvironment (TME) with limited CD8+ T cell infiltration. This study explores the role of the epigenetic factor Sin3B in the PDAC TME. Using murine PDAC models, we found that tumor cell-intrinsic Sin3B loss reshapes the TME, increasing CD8+ T cell infiltration and cytotoxicity, thus impeding tumor progression and enhancing sensitivity to anti-PD1 treatment. Sin3B-deficient tumor cells exhibited amplified CXCL9/10 secretion in response to Interferon-gamma (IFNγ), creating a positive feedback loop via the CXCL9/10-CXCR3 axis, thereby intensifying the anti-tumor immune response against PDAC. Mechanistically, extensive epigenetic regulation is uncovered by Sin3B loss, particularly enhanced H3K27Ac distribution on genes related to immune responses in PDAC cells. Consistent with the murine model findings, analysis of human PDAC samples revealed a significant inverse correlation between SIN3B levels and both CD8+ T cell infiltration and CXCL9/10 expression. Notebly, PDAC patients with lower SIN3B expression showed a more favorable response to anti-PD1 therapy. The findings suggest that targeting SIN3B can enhance cytotoxic T cell infiltration into the tumor site and improve immunotherapy efficacy in PDAC, offering potential avenues for therapeutic biomarker or target in this challenging disease.
-
-
-
Immunology and Microbiology
Validation of the C-X-C chemokine receptor 3 (CXCR3) as a target for PET imaging of T cell activation.
In EJNMMI Res on 28 August 2024 by Martin, S., Wendlinger, L., et al.
PubMed
CXCR3 is expressed on activated T cells and plays a crucial role in T-cell recruitment to the tumor microenvironment (TME) during cell-based and immune checkpoint inhibitor (ICI) immunotherapy. This study utilized a 64Cu-labeled NOTA-α-CXCR3 antibody to assess CXCR3 expression in the TME and validate it as a potential T cell activation biomarker in vivo.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
CXCL9/10-engineered dendritic cells promote T cell activation and enhance immune checkpoint blockade for lung cancer.
In Cell Rep Med on 16 April 2024 by Lim, R. J., Salehi-Rad, R., et al.
PubMed
Immune checkpoint blockade (ICB) with PD-1/PD-L1 inhibition has revolutionized the treatment of non-small cell lung cancer (NSCLC). Durable responses, however, are observed only in a subpopulation of patients. Defective antigen presentation and an immunosuppressive tumor microenvironment (TME) can lead to deficient T cell recruitment and ICB resistance. We evaluate intratumoral (IT) vaccination with CXCL9- and CXCL10-engineered dendritic cells (CXCL9/10-DC) as a strategy to overcome resistance. IT CXCL9/10-DC leads to enhanced T cell infiltration and activation in the TME and tumor inhibition in murine NSCLC models. The antitumor efficacy of IT CXCL9/10-DC is dependent on CD4+ and CD8+ T cells, as well as CXCR3-dependent T cell trafficking from the lymph node. IT CXCL9/10-DC, in combination with ICB, overcomes resistance and establishes systemic tumor-specific immunity in murine models. These studies provide a mechanistic understanding of CXCL9/10-DC-mediated host immune activation and support clinical translation of IT CXCL9/10-DC to augment ICB efficacy in NSCLC.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
A Novel Therapeutic Approach using CXCR3 Blockade to Treat Immune Checkpoint Inhibitor-mediated Myocarditis
In bioRxiv on 2 February 2024 by Huang, Y. V., Lee, D., et al.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
INHBA/Activin A promotes tumor growth and induces resistance to anti-PD-L1 therapy by suppressing IFN-γ signaling
In bioRxiv on 8 December 2023 by Li, F., Gu, L., et al.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
T cell-specific P2RX7 favors lung parenchymal CD4+ T cell accumulation in response to severe lung infections.
In Cell Rep on 28 November 2023 by Santiago-Carvalho, I., Almeida-Santos, G., et al.
PubMed
CD4+ T cells are key components of the immune response during lung infections and can mediate protection against tuberculosis (TB) or influenza. However, CD4+ T cells can also promote lung pathology during these infections, making it unclear how these cells control such discrepant effects. Using mouse models of hypervirulent TB and influenza, we observe that exaggerated accumulation of parenchymal CD4+ T cells promotes lung damage. Low numbers of lung CD4+ T cells, in contrast, are sufficient to protect against hypervirulent TB. In both situations, lung CD4+ T cell accumulation is mediated by CD4+ T cell-specific expression of the extracellular ATP (eATP) receptor P2RX7. P2RX7 upregulation in lung CD4+ T cells promotes expression of the chemokine receptor CXCR3, favoring parenchymal CD4+ T cell accumulation. Our findings suggest that direct sensing of lung eATP by CD4+ T cells is critical to induce tissue CD4+ T cell accumulation and pathology during lung infections.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Low-dose radiotherapy combined with dual PD-L1 and VEGFA blockade elicits antitumor response in hepatocellular carcinoma mediated by activated intratumoral CD8+ exhausted-like T cells.
In Nat Commun on 24 November 2023 by Li, S., Li, K., et al.
PubMed
Atezolizumab (anti-PD-L1) combined with bevacizumab (anti-VEGFA) is the first-line immunotherapy for advanced hepatocellular carcinoma (HCC), but the number of patients who benefit from this regimen remains limited. Here, we combine dual PD-L1 and VEGFA blockade (DPVB) with low-dose radiotherapy (LDRT), which rapidly inflames tumors, rendering them vulnerable to immunotherapy. The combinatorial therapy exhibits superior antitumor efficacy mediated by CD8+ T cells in various preclinical HCC models. Treatment efficacy relies upon mobilizing exhausted-like CD8+ T cells (CD8+ Tex) with effector function and cytolytic capacity. Mechanistically, LDRT sensitizes tumors to DPVB by recruiting stem-like CD8+ Tpex, the progenitor exhausted CD8+ T cells, from draining lymph nodes (dLNs) into the tumor via the CXCL10/CXCR3 axis. Together, these results further support the rationale for combining LDRT with atezolizumab and bevacizumab, and its clinical translation.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
HDAC3 Inhibition Promotes Antitumor Immunity by Enhancing CXCL10-Mediated Chemotaxis and Recruiting of Immune Cells.
In Cancer Immunol Res on 3 May 2023 by Li, L., Hao, S., et al.
PubMed
It is generally believed that histone deacetylase (HDAC) inhibitors, which represent a new class of anticancer agents, exert their antitumor activity by directly causing cell-cycle arrest and apoptosis of tumor cells. However, in this study, we demonstrated that class I HDAC inhibitors, such as Entinostat and Panobinostat, effectively suppressed tumor growth in immunocompetent but not immunodeficient mice. Further studies with Hdac1, 2, or 3 knockout tumor cells indicated that tumor-specific inactivation of HDAC3 suppressed tumor growth by activating antitumor immunity. Specifically, we found that HDAC3 could directly bind to promotor regions and inhibit the expression of CXCL9, 10, and 11 chemokines. Hdac3-deficient tumor cells expressed high levels of these chemokines, which suppressed tumor growth in immunocompetent mice by recruiting CXCR3+ T cells into the tumor microenvironment (TME). Furthermore, the inverse correlation between HDAC3 and CXCL10 expression in hepatocellular carcinoma tumor tissues also suggested HDAC3 might be involved in antitumor immune regulation and patient survival. Thus, our studies have illustrated that HDAC3 inhibition suppresses tumor growth by enhancing immune cell infiltration into the TME. This antitumor mechanism may be helpful in guiding HDAC3 inhibitor-based treatment.
-
-
-
Immunology and Microbiology
-
Mus musculus (Mouse)
-
Flow cytometry/Cell sorting
Expansion of circulating stem-like CD8+ T cells by adding CD122-directed IL-2 complexes to radiation and anti-PD1 therapies in mice.
In Nat Commun on 12 April 2023 by Onyshchenko, K., Luo, R., et al.
PubMed
Combination of radiation therapy (RT) with immune checkpoint blockade can enhance systemic anti-tumor T cell responses. Here, using two mouse tumor models, we demonstrate that adding long-acting CD122-directed IL-2 complexes (IL-2c) to RT/anti-PD1 further increases tumor-specific CD8+ T cell numbers. The highest increase (>50-fold) is found in the blood circulation. Compartmental analysis of exhausted T cell subsets shows that primarily undifferentiated, stem-like, tumor-specific CD8+ T cells expand in the blood; these cells express the chemokine receptor CXCR3, which is required for migration into tumors. In tumor tissue, effector-like but not terminally differentiated exhausted CD8+ T cells increase. Consistent with the surge in tumor-specific CD8+ T cells in blood that are migration and proliferation competent, we observe a CD8-dependent and CXCR3-dependent enhancement of the abscopal effect against distant/non-irradiated tumors and find that CD8+ T cells isolated from blood after RT/anti-PD1/IL-2c triple treatment can be a rich source of tumor-specific T cells for adoptive transfers.
-
-
-
Flow cytometry/Cell sorting
-
Mus musculus (Mouse)
-
Immunology and Microbiology
OX40 agonism enhances PD-L1 checkpoint blockade by shifting the cytotoxic T cell differentiation spectrum.
In Cell Rep Med on 21 March 2023 by van der Sluis, T. C., Beyrend, G., et al.
PubMed
Immune checkpoint therapy (ICT) has the power to eradicate cancer, but the mechanisms that determine effective therapy-induced immune responses are not fully understood. Here, using high-dimensional single-cell profiling, we interrogate whether the landscape of T cell states in the peripheral blood predict responses to combinatorial targeting of the OX40 costimulatory and PD-1 inhibitory pathways. Single-cell RNA sequencing and mass cytometry expose systemic and dynamic activation states of therapy-responsive CD4+ and CD8+ T cells in tumor-bearing mice with expression of distinct natural killer (NK) cell receptors, granzymes, and chemokines/chemokine receptors. Moreover, similar NK cell receptor-expressing CD8+ T cells are also detected in the blood of immunotherapy-responsive cancer patients. Targeting the NK cell and chemokine receptors in tumor-bearing mice shows the functional importance of these receptors for therapy-induced anti-tumor immunity. These findings provide a better understanding of ICT and highlight the use and targeting of dynamic biomarkers on T cells to improve cancer immunotherapy.
-
-
-
Immunology and Microbiology
-
Neuroscience
-
Mus musculus (Mouse)
Infection induces tissue-resident memory NK cells that safeguard tissue health.
In Immunity on 14 March 2023 by Schuster, I. S., Sng, X. Y. X., et al.
PubMed
Tissue health is dictated by the capacity to respond to perturbations and then return to homeostasis. Mechanisms that initiate, maintain, and regulate immune responses in tissues are therefore essential. Adaptive immunity plays a key role in these responses, with memory and tissue residency being cardinal features. A corresponding role for innate cells is unknown. Here, we have identified a population of innate lymphocytes that we term tissue-resident memory-like natural killer (NKRM) cells. In response to murine cytomegalovirus infection, we show that circulating NK cells were recruited in a CX3CR1-dependent manner to the salivary glands where they formed NKRM cells, a long-lived, tissue-resident population that prevented autoimmunity via TRAIL-dependent elimination of CD4+ T cells. Thus, NK cells develop adaptive-like features, including long-term residency in non-lymphoid tissues, to modulate inflammation, restore immune equilibrium, and preserve tissue health. Modulating the functions of NKRM cells may provide additional strategies to treat inflammatory and autoimmune diseases.
-
-
-
Immunology and Microbiology
-
Mus musculus (Mouse)
IL9 Polarizes Macrophages to M1 and Induces the Infiltration of Antitumor Immune Cells via MIP-1 and CXCR3 Chemokines.
In Cancer Res Commun on 1 January 2023 by Do-Thi, V. A., Park, S. M., et al.
PubMed
Tumor-associated macrophages (TAM) are involved in tumor progression, metastasis, and immunosuppression. Because TAMs are highly plastic and could alter their phenotypes to proinflammatory M1 in response to environmental stimuli, reeducating TAMs has emerged as a promising approach to overcoming the challenges of solid cancer treatment. This study investigated the effect of IL9 on macrophage M1 polarization and verified its antitumor potential to retrain TAMs and promote chemokine secretion. We demonstrated that IL9 stimulated macrophage proliferation and polarized them toward the proinflammatory M1 phenotype in an IFNγ-dependent manner. Tumor-localized IL9 also polarized TAMs toward M1 in vivo and made them release CCL3/4 and CXCL9/10 to recruit antitumor immune cells, including T and natural killer cells, into the tumor microenvironment. Furthermore, peritoneal treatment with recombinant IL9 delayed the growth of macrophage-enriched B16F10 melanoma and 4T1 breast cancer in syngeneic mice, although IL9 treatment did not reduce tumor growth in the absence of macrophage enrichment. These results demonstrate the efficacy of IL9 in macrophage polarization to trigger antitumor immunity.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Mucosal plasma cells are required to protect the upper airway and brain from infection.
In Immunity on 8 November 2022 by Wellford, S. A., Moseman, A. P., et al.
PubMed
While blood antibodies mediate protective immunity in most organs, whether they protect nasal surfaces in the upper airway is unclear. Using multiple viral infection models in mice, we found that blood-borne antibodies could not defend the olfactory epithelium. Despite high serum antibody titers, pathogens infected nasal turbinates, and neurotropic microbes invaded the brain. Using passive antibody transfers and parabiosis, we identified a restrictive blood-endothelial barrier that excluded circulating antibodies from the olfactory mucosa. Plasma cell depletions demonstrated that plasma cells must reside within olfactory tissue to achieve sterilizing immunity. Antibody blockade and genetically deficient models revealed that this local immunity required CD4+ T cells and CXCR3. Many vaccine adjuvants failed to generate olfactory plasma cells, but mucosal immunizations established humoral protection of the olfactory surface. Our identification of a blood-olfactory barrier and the requirement for tissue-derived antibody has implications for vaccinology, respiratory and CNS pathogen transmission, and B cell fate decisions.
-
-
-
Cancer Research
Inhibition of HCK in myeloid cells restricts pancreatic tumor growth and metastasis.
In Cell Rep on 11 October 2022 by Poh, A. R., O'Brien, M., et al.
PubMed
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive disease with a low 5-year survival rate and is associated with poor response to therapy. Elevated expression of the myeloid-specific hematopoietic cell kinase (HCK) is observed in PDAC and correlates with reduced patient survival. To determine whether aberrant HCK signaling in myeloid cells is involved in PDAC growth and metastasis, we established orthotopic and intrasplenic PDAC tumors in wild-type and HCK knockout mice. Genetic ablation of HCK impaired PDAC growth and metastasis by inducing an immune-stimulatory endotype in myeloid cells, which in turn reduced the desmoplastic microenvironment and enhanced cytotoxic effector cell infiltration. Consequently, genetic ablation or therapeutic inhibition of HCK minimized metastatic spread, enhanced the efficacy of chemotherapy, and overcame resistance to anti-PD1, anti-CTLA4, or stimulatory anti-CD40 immunotherapy. Our results provide strong rationale for HCK to be developed as a therapeutic target to improve the response of PDAC to chemo- and immunotherapy.
-
-
-
Enzyme-linked immunosorbent assay
-
Mus musculus (Mouse)
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Cancer cell autophagy, reprogrammed macrophages, and remodeled vasculature in glioblastoma triggers tumor immunity.
In Cancer Cell on 10 October 2022 by Chryplewicz, A., Scotton, J., et al.
PubMed
Glioblastoma (GBM) is poorly responsive to therapy and invariably lethal. One conceivable strategy to circumvent this intractability is to co-target distinctive mechanistic components of the disease, aiming to concomitantly disrupt multiple capabilities required for tumor progression and therapeutic resistance. We assessed this concept by combining vascular endothelial growth factor (VEGF) pathway inhibitors that remodel the tumor vasculature with the tricyclic antidepressant imipramine, which enhances autophagy in GBM cancer cells and unexpectedly reprograms immunosuppressive tumor-associated macrophages via inhibition of histamine receptor signaling to become immunostimulatory. While neither drug is efficacious as monotherapy, the combination of imipramine with VEGF pathway inhibitors orchestrates the infiltration and activation of CD8 and CD4 T cells, producing significant therapeutic benefit in several GBM mouse models. Inclusion up front of immune-checkpoint blockade with anti-programmed death-ligand 1 (PD-L1) in eventually relapsing tumors markedly extends survival benefit. The results illustrate the potential of mechanism-guided therapeutic co-targeting of disparate biological vulnerabilities in the tumor microenvironment.
-
-
-
Flow cytometry/Cell sorting
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Breast cancer cell-derived microRNA-155 suppresses tumor progression via enhancing immune cell recruitment and antitumor function.
In J Clin Invest on 3 October 2022 by Wang, J., Wang, Q., et al.
PubMed
Evidence suggests that increased microRNA-155 (miR-155) expression in immune cells enhances antitumor immune responses. However, given the reported association of miR-155 with tumorigenesis in various cancers, a debate is provoked on whether miR-155 is oncogenic or tumor suppressive. We aimed to interrogate the impact of tumor miR-155 expression, particularly that of cancer cell-derived miR-155, on antitumor immunity in breast cancer. We performed bioinformatic analysis of human breast cancer databases, murine experiments, and human specimen examination. We revealed that higher tumor miR-155 levels correlate with a favorable antitumor immune profile and better patient outcomes. Murine experiments demonstrated that miR-155 overexpression in breast cancer cells enhanced T cell influx, delayed tumor growth, and sensitized the tumors to immune checkpoint blockade (ICB) therapy. Mechanistically, miR-155 overexpression in breast cancer cells upregulated their CXCL9/10/11 production, which was mediated by SOCS1 inhibition and increased phosphorylated STAT1 (p-STAT1)/p-STAT3 ratios. We further found that serum miR-155 levels in breast cancer patients correlated with tumor miR-155 levels and tumor immune status. Our findings suggest that high serum and tumor miR-155 levels may be a favorable prognostic marker for breast cancer patients and that therapeutic elevation of miR-155 in breast tumors may improve the efficacy of ICB therapy via remodeling the antitumor immune landscape.
-
-
-
Flow cytometry/Cell sorting
-
Mus musculus (Mouse)
-
Cardiovascular biology
-
Genetics
-
Immunology and Microbiology
-
Flow cytometry/Cell sorting
Single-Cell RNA sequencing reveals immune cell dynamics and local intercellular communication in acute murine cardiac allograft rejection.
In Theranostics on 29 September 2022 by Chen, Z., Xu, H., et al.
PubMed
Rationale: Transplant rejection is a major impediment to long-term allograft survival, in which the actions of immune cells are of fundamental importance. However, the immune cell dynamics and local intercellular communication of acute cardiac allograft rejection are not completely clear. Methods: Here we performed single-cell RNA sequencing on CD45+ immune cells isolated from cardiac grafts and spleens in a model of murine heterotopic heart transplantation. Moreover, we applied unsupervised clustering, functional enrichment analysis, cell trajectory construction and intercellular communication analysis to explore the immune cell dynamics and local intercellular communication of acute cardiac allograft rejection at single-cell level. The effect of CXCR3 antagonist and neutralizing antibody against its ligand on allograft rejection and T cell function was evaluated in murine heart transplantation model. Results: We presented the immune cell landscape of acute murine cardiac allograft rejection at single-cell resolution, and uncovered the functional characteristics and differentiation trajectory of several alloreactive cell subpopulations, including Mki67hi CTLs, Ccl5hi CTLs, activated Tregs and alloreactive B cells. We demonstrated local intercellular communication and revealed the upregulation of CXCR3 and its ligands in cardiac allografts. Finally, CXCR3 blockade significantly suppressed acute cardiac allograft rejection and inhibited the alloreactive T cell function. Conclusions: These results provide a new insight into the immune cell dynamics and local intercellular communication of acute cardiac allograft rejection, and suggest CXCR3 pathway may serve as a potential therapeutic target for transplant rejection.
-
-
-
Cancer Research
-
Immunology and Microbiology
IFI16-dependent STING signaling is a crucial regulator of anti-HER2 immune response in HER2+ breast cancer.
In Proc Natl Acad Sci U S A on 2 August 2022 by Ong, L. T., Lee, W. C., et al.
PubMed
Relapse to anti-HER2 monoclonal antibody (mAb) therapies, such as trastuzumab in HER2+ breast cancer (BC), is associated with residual disease progression due to resistance to therapy. Here, we identify interferon-γ inducible protein 16 (IFI16)-dependent STING signaling as a significant determinant of trastuzumab responses in HER2+ BC. We show that down-regulation of immune-regulated genes (IRG) is specifically associated with poor survival of HER2+, but not other BC subtypes. Among IRG, IFI16 is identified as a direct target of EZH2, the underexpression of which leads to deficient STING activation and downstream CXCL10/11 expression in response to trastuzumab treatment. Dual inhibition of EZH2 and histone deacetylase (HDAC) significantly activates IFI16-dependent immune responses to trastuzumab. Notably, a combination of a novel histone methylation inhibitor with an HDAC inhibitor induces complete tumor eradication and long-term T cell memory in a HER2+ BC mouse model. Our findings demonstrate an epigenetic regulatory mechanism suppressing the expression of the IFI16-CXCL10/11 signaling pathway that provides a survival advantage to HER2+ BC to confer resistance to trastuzumab treatment.
-
-
-
Cancer Research
-
Immunology and Microbiology
Therapeutic inhibition of the SRC-kinase HCK facilitates T cell tumor infiltration and improves response to immunotherapy.
In Sci Adv on 24 June 2022 by Poh, A. R., Love, C. G., et al.
PubMed
Although immunotherapy has revolutionized cancer treatment, many immunogenic tumors remain refractory to treatment. This can be largely attributed to an immunologically "cold" tumor microenvironment characterized by an accumulation of immunosuppressive myeloid cells and exclusion of activated T cells. Here, we demonstrate that genetic ablation or therapeutic inhibition of the myeloid-specific hematopoietic cell kinase (HCK) enables activity of antagonistic anti-programmed cell death protein 1 (anti-PD1), anti-CTLA4, or agonistic anti-CD40 immunotherapies in otherwise refractory tumors and augments response in treatment-susceptible tumors. Mechanistically, HCK ablation reprograms tumor-associated macrophages and dendritic cells toward an inflammatory endotype and enhances CD8+ T cell recruitment and activation when combined with immunotherapy in mice. Meanwhile, therapeutic inhibition of HCK in humanized mice engrafted with patient-derived xenografts counteracts tumor immunosuppression, improves T cell recruitment, and impairs tumor growth. Collectively, our results suggest that therapeutic targeting of HCK activity enhances response to immunotherapy by simultaneously stimulating immune cell activation and inhibiting the immunosuppressive tumor microenvironment.
-