InVivoMAb anti-mouse CD8 (Lyt 2.1)
Product Description
Specifications
| Isotype | Mouse IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG2a isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | CE mouse spleen cells and thymocytes |
| Reported Applications |
in vivo CD8+ T cell depletion Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_10949065 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo CD8+ T cell depletion
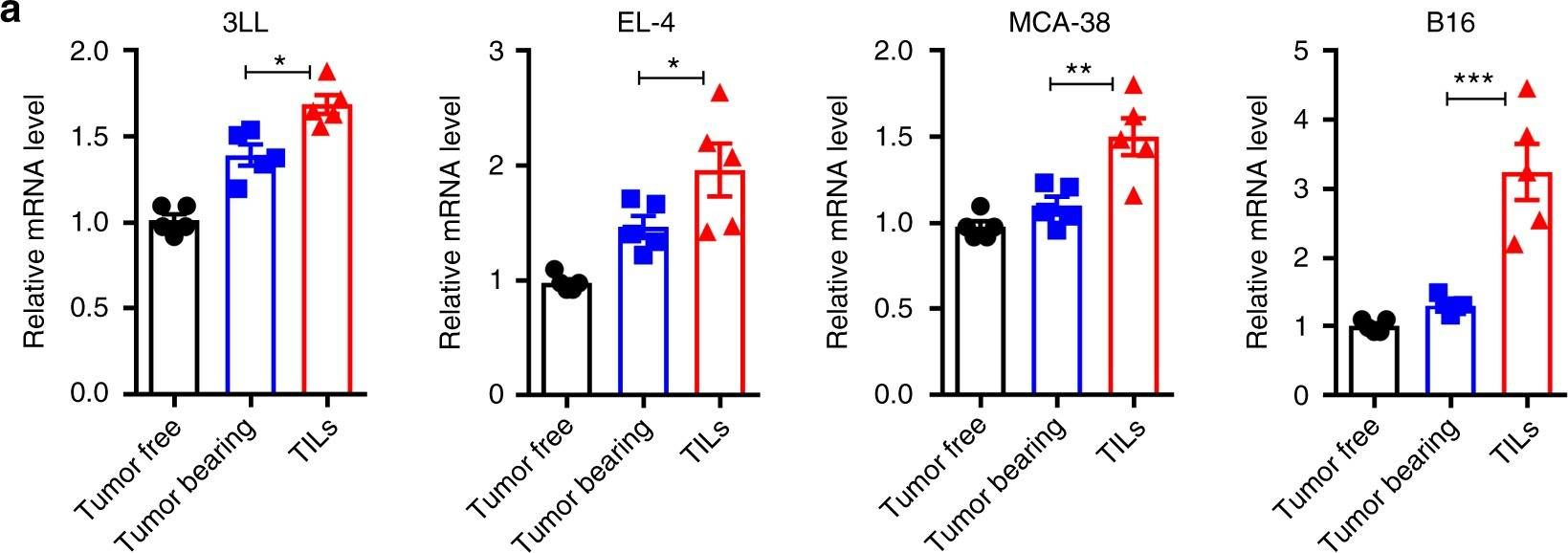
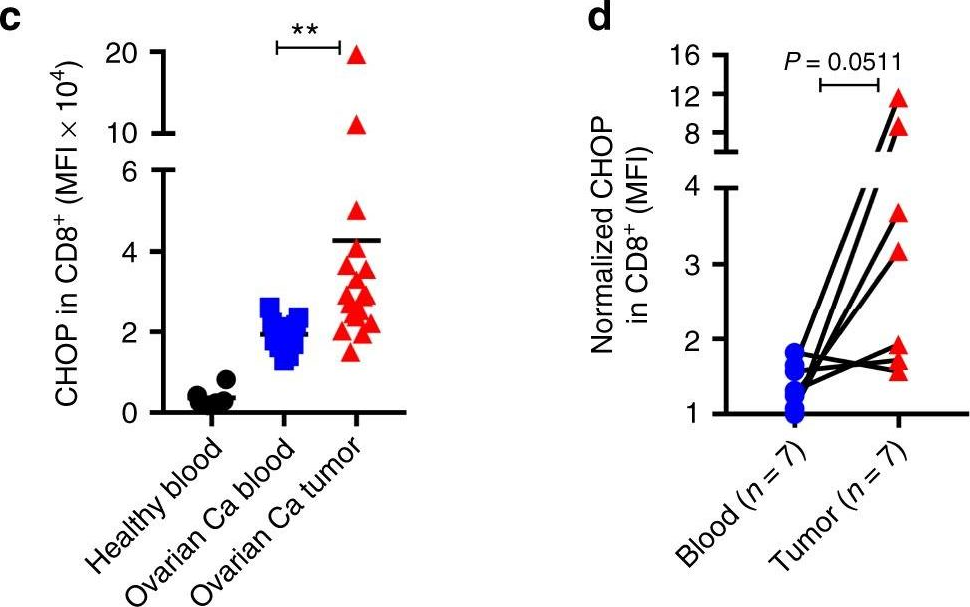
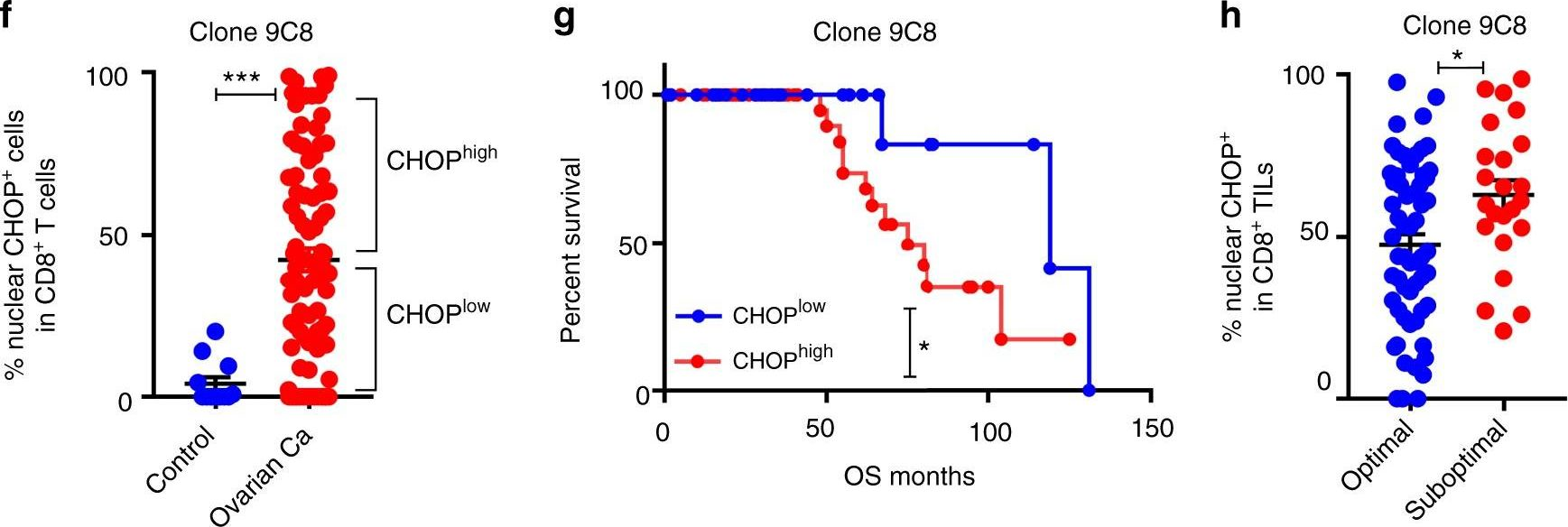
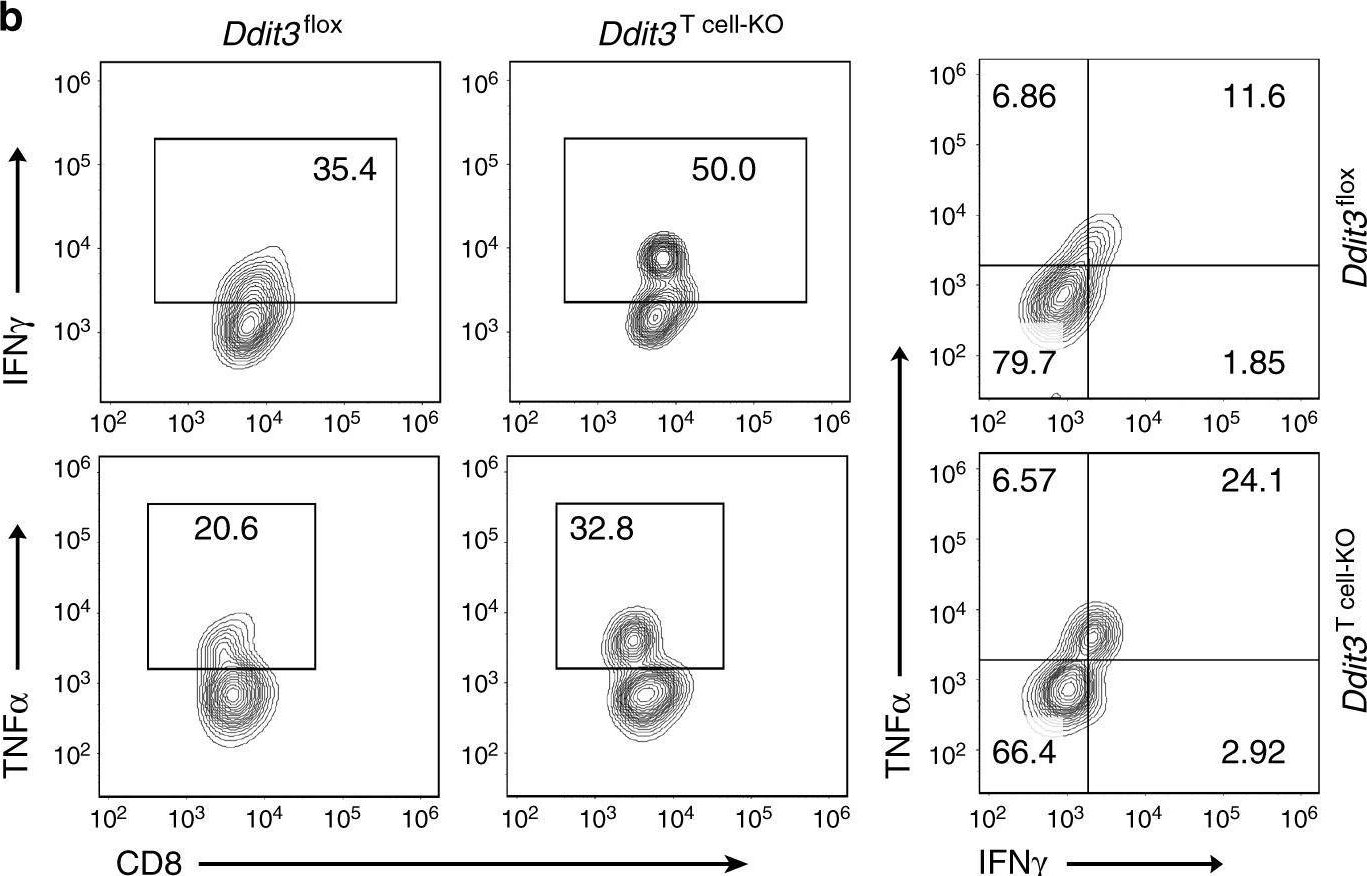
Cao, Y., et al (2019). "ER stress-induced mediator C/EBP homologous protein thwarts effector T cell activity in tumors through T-bet repression" Nat Commun 10(1): 1280.
PubMed
Understanding the intrinsic mediators that render CD8(+) T cells dysfunctional in the tumor microenvironment is a requirement to develop more effective cancer immunotherapies. Here, we report that C/EBP homologous protein (Chop), a downstream sensor of severe endoplasmic reticulum (ER) stress, is a major negative regulator of the effector function of tumor-reactive CD8(+) T cells. Chop expression is increased in tumor-infiltrating CD8(+) T cells, which correlates with poor clinical outcome in ovarian cancer patients. Deletion of Chop in T cells improves spontaneous antitumor CD8(+) T cell immunity and boosts the efficacy of T cell-based immunotherapy. Mechanistically, Chop in CD8(+) T cells is elevated primarily through the ER stress-associated kinase Perk and a subsequent induction of Atf4; and directly represses the expression of T-bet, a master regulator of effector T cell function. These findings demonstrate the primary role of Chop in tumor-induced CD8(+) T cell dysfunction and the therapeutic potential of blocking Chop or ER stress to unleash T cell-mediated antitumor immunity.
in vivo CD8+ T cell depletion
Racine, J. J., et al (2014). "Induction of mixed chimerism depletes pre-existing and de novo-developed autoreactive B cells in autoimmune NOD mice" Diabetes 63(6): 2051-2062.
PubMed
Destruction of pancreatic islet beta-cells in type 1 diabetes (T1D) is mainly mediated by autoimmune T and B lymphocytes. We reported that induction of major histocompatibility complex (MHC)-mismatched mixed chimerism reversed autoimmunity and reestablished thymic negative selection of autoreactive T cells in NOD mice, but it is still unclear how mixed chimerism tolerizes autoreactive B cells. The current studies were designed to reveal the mechanisms on how mixed chimerism tolerizes autoreactive B cells in T1D. Accordingly, mixed chimerism was induced in NOD mice through radiation-free nonmyeloablative anti-CD3/CD8 conditioning and infusion of donor CD4(+) T cell-depleted spleen and whole bone marrow (BM) cells or through myeloablative total body irradiation conditioning and reconstitution with T cell-depleted BM cells from donor and host. Kinetic analysis of percentage and yield of preplasma and plasma B cells, newly developed B-cell subsets, and their apoptosis was performed 30-60 days after transplantation. Induction of MHC-mismatched mixed chimerism results in depleting host-type pre-existing preplasma and plasma B cells as well as augmenting apoptosis of immature transitional T1 B cells, including insulin-specific B cells in a donor B cell-dependent manner. Therefore, induction of MHC-mismatched mixed chimerism depletes pre-existing and de novo-developed autoreactive B cells
in vivo CD8+ T cell depletion
Wang, M., et al (2014). "MHC-mismatched chimerism is required for induction of transplantation tolerance in autoimmune nonobese diabetic recipients" J Immunol 193(4): 2005-2015.
PubMed
In nonautoimmune recipients, induction of mixed and complete chimerism with hematopoietic progenitor cells from MHC (HLA)-matched or -mismatched donors are effective approaches for induction of organ transplantation immune tolerance in both animal models and patients. But it is still unclear whether this is the case in autoimmune recipients. With the autoimmune diabetic NOD mouse model, we report that, although mixed and complete MHC-mismatched chimerism provide immune tolerance to donor-type islet and skin transplants, neither mixed nor complete MHC-matched chimerism does. The MHC-mismatched chimerism not only tolerizes the de novo developed, but also the residual pre-existing host-type T cells in a mismatched MHC class II-dependent manner. In the MHC-mismatched chimeras, the residual host-type peripheral T cells appear to be anergic with upregulation of PD-1 and downregulation of IL-7Ralpha. Conversely, in the MHC-matched chimeras, the residual host-type peripheral T cells manifest both alloreactivity and autoreactivity; they not only mediate insulitis and sialitis in the recipient, but also reject allogeneic donor-type islet and skin grafts. Interestingly, transgenic autoreactive BDC2.5 T cells from Rag1(+/+), but not from Rag1(-/-), NOD mice show alloreactivity and mediate both insulitis and rejection of allografts. Taken together, MHC-mismatched, but not MHC-matched, chimerism can effectively provide transplantation immune tolerance in autoimmune recipients.
in vivo CD8+ T cell depletion
Yang, Y., et al (2012). "Antitumor T-cell responses contribute to the effects of dasatinib on c-KIT mutant murine mastocytoma and are potentiated by anti-OX40" Blood 120(23): 4533-4543.
PubMed
Targeted and immune-based therapies are thought to eradicate cancer cells by different mechanisms, and these approaches could possibly complement each other when used in combination. In this study, we report that the in vivo antitumor effects of the c-KIT inhibitor, dasatinib, on the c-KIT mutant P815 mastocytoma tumor were substantially dependent on T cell-mediated immunity. We found that dasatinib treatment significantly decreased levels of Tregs while specifically enhancing tumor antigen-specific T-cell responses. We sought to further enhance this therapy with the addition of anti-OX40 antibody, which is known to provide a potent costimulatory signal to T cells. The combination of dasatinib and anti-OX40 antibody resulted in substantially better therapeutic efficacy compared with either drug alone, and this was associated with enhanced accumulation of tumor antigen-specific T cells in the tumor microenvironment. Furthermore, the combination regimen inhibited the function of Tregs and also resulted in significantly up-regulated expression of the IFN-gamma-induced chemokines CXCL9, 10, and 11 in the tumor microenvironment, which provides a feasible mechanism for the enhanced intratumoral CTL infiltration. These studies delineate a strategy by which targeted therapy and immunotherapy may be combined to achieve superior antitumor responses in cancer patients.
in vivo CD8+ T cell depletion
Makhlouf, L., et al (2003). "Allorecognition and effector pathways of islet allograft rejection in normal versus nonobese diabetic mice" J Am Soc Nephrol 14(8): 2168-2175.
PubMed
Islet transplantation is becoming an accepted therapy to cure type I diabetes mellitus. The exact mechanisms of islet allograft rejection remain unclear, however. In vivo CD4(+) and CD8(+) T cell-depleting strategies and genetically altered mice that did not express MHC class I or class II antigens were used to study the allorecognition and effector pathways of islet allograft rejection in different strains of mice, including autoimmunity-prone nonobese diabetic (NOD) mice. In BALB/c mice, islet rejection depended on both CD4(+) and CD8(+) T cells. In C57BL/6 mice, CD8(+) T cells could eventually mediate islet rejection by themselves, but they produced rejection more efficiently with help from CD4(+) T cells stimulated through either the direct or indirect pathway. In C57BL/6 mice, CD4(+) T cells alone caused islet rejection when only the direct pathway was available but not when only the indirect pathway was available. In contrast, in NOD mice, CD4(+) T cells alone, with only the indirect pathway, could mediate islet and cardiac allograft rejection. These findings indicate that different mouse strains can make use of different pathways for T cell-mediated rejection of islet allografts. In addition, they demonstrate that NOD mice, which develop autoimmunity and are known to be resistant to tolerance induction, have an unusually powerful CD4(+) cell indirect mechanism that can cause rejection of both islet and cardiac allografts. These data shed light on the mechanisms of islet allograft rejection in different responder strains, including those with autoimmunity.
in vivo CD8+ T cell depletion
Kang, E. S. and J. Iacomini (2002). "Induction of central deletional T cell tolerance by gene therapy" J Immunol 169(4): 1930-1935.
PubMed
Transgenic mice expressing an alloreactive TCR specific for the MHC class I Ag K(b) were used to examine the mechanism by which genetic engineering of bone marrow induces T cell tolerance. Reconstitution of lethally irradiated mice with bone marrow infected with retroviruses carrying the MHC class I gene H-2K(b) resulted in lifelong expression of K(b) on bone marrow-derived cells. While CD8 T cells expressing the transgenic TCR developed in control mice reconstituted with mock-transduced bone marrow, CD8 T cells expressing the transgenic TCR failed to develop in mice reconstituted with H-2K(b) transduced bone marrow. Analysis of transgene-expressing CD8 T cells in the thymus and periphery of reconstituted mice revealed that CD8 T cells expressing the transgenic TCR underwent negative selection in the thymus of mice reconstituted with K(b) transduced bone marrow. Negative selection induced by gene therapy resulted in tolerance to K(b). Thus, genetic engineering of bone marrow can be used to alter T cell education in the thymus by inducing negative selection.
Product Citations
-
-
Immunology and Microbiology
Engineered oncolytic virus coated with anti-PD-1 and alendronate for ameliorating intratumoral T cell hypofunction.
In Exp Hematol Oncol on 15 February 2025 by Zhu, Y., Zhang, X., et al.
PubMed
Glioblastoma is a highly aggressive and devastating primary brain tumor that is resistant to conventional therapies. Oncolytic viruses represent a promising therapeutic approach for glioblastoma by selectively lysing tumor cells and eliciting an anti-tumor immune response. However, the clinical efficacy of oncolytic viruses is often hindered by challenges such as short persistence, host antiviral immune responses, and T cell dysfunction.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
METTL3-VISTA axis-based combination immunotherapy for APC truncation colorectal cancer.
In J Immunother Cancer on 9 December 2024 by Wu, L., Bai, R., et al.
PubMed
Although immune checkpoint blockade (ICB) therapy represents a bright spot in antitumor immunotherapy, its clinical benefits in colorectal cancer (CRC) are limited. Therefore, a new target for mediating CRC immunosuppression is urgently needed. Adenomatous polyposis coli (APC) mutations have been reported as early-stage characteristic events in CRC, but the role of truncated APC in the CRC immune microenvironment remains unclear and its clinical significance has yet to be explored.
-
-
-
Cancer Research
High-grade serous ovarian cancer development and anti-PD-1 resistance is driven by IRE1α activity in neutrophils.
In Oncoimmunology on 4 October 2024 by Emmanuelli, A., Salvagno, C., et al.
PubMed
High-grade serious ovarian cancer (HGSOC) is an aggressive malignancy that remains refractory to current immunotherapies. While advanced stage disease has been extensively studied, the cellular and molecular mechanisms that promote early immune escape in HGSOC remain largely unexplored. Here, we report that primary HGSO tumors program neutrophils to inhibit T cell anti-tumor function by activating the endoplasmic reticulum (ER) stress sensor IRE1α. We found that intratumoral neutrophils exhibited overactivation of ER stress response markers compared with their counterparts at non-tumor sites. Selective deletion of IRE1α in neutrophils delayed primary ovarian tumor growth and extended the survival of mice with HGSOC by enabling early T cell-mediated tumor control. Notably, loss of IRE1α in neutrophils sensitized tumor-bearing mice to PD-1 blockade, inducing HGSOC regression and long-term survival in ~ 50% of the treated hosts. Hence, neutrophil-intrinsic IRE1α facilitates early adaptive immune escape in HGSOC and targeting this ER stress sensor might be used to unleash endogenous and immunotherapy-elicited immunity that controls metastatic disease.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
High-grade serous ovarian cancer development and anti-PD-1 resistance is driven by IRE1α activity in neutrophils
In bioRxiv on 7 August 2024 by Emmanuelli, A., Salvagno, C., et al.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Targeting KDM4 family epigenetically triggers antitumour immunity via enhancing tumour-intrinsic innate sensing and immunogenicity.
In Clin Transl Med on 1 February 2024 by Sun, M., Han, X., et al.
PubMed
Despite the remarkable clinical efficacy of cancer immunotherapy, considerable patients fail to benefit from it due to primary or acquired resistance. Tumours frequently hijack diverse epigenetic mechanisms to evade immune detection, thereby highlighting the potential for pharmacologically targeting epigenetic regulators to restore the impaired immunosurveillance and re-sensitise tumours to immunotherapy. Herein, we demonstrated that KDM4-targeting chemotherapeutic drug JIB-04, epigenetically triggered the tumour-intrinsic innate immune responses and immunogenic cell death (ICD), resulting in impressive antitumour effects. Specifically, JIB-04 induced H3K9 hypermethylation through specific inhibition of the KDM4 family (KDM4A-D), leading to impaired DNA repair signalling and subsequent DNA damage. As a result, JIB-04 not only activated the tumour-intrinsic cyclic GMP-AMP synthase (cGAS)-STING pathway via DNA-damage-induced cytosolic DNA accumulation, but also promoted ICD, releasing numerous damage-associated molecular patterns. Furthermore, JIB-04 induced adaptive resistance through the upregulation of programmed death-ligand 1 (PD-L1), which could be overcome with additional PD-L1 blockade. In human tumours, KDM4B expression was negatively correlated with clinical outcomes, type I interferon signatures, and responses to immunotherapy. In conclusion, our results demonstrate that targeting KDM4 family can activate tumour-intrinsic innate sensing and immunogenicity, and synergise with immunotherapy to improve antitumour outcomes.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
-
Genetics
Targeting nucleic acid sensors in tumor cells to reprogram biogenesis and RNA cargo of extracellular vesicles for T cell-mediated cancer immunotherapy.
In Cell Rep Med on 19 September 2023 by Heidegger, S., Stritzke, F., et al.
PubMed
Tumor-derived extracellular vesicles (EVs) have been associated with immune evasion and tumor progression. We show that the RNA-sensing receptor RIG-I within tumor cells governs biogenesis and immunomodulatory function of EVs. Cancer-intrinsic RIG-I activation releases EVs, which mediate dendritic cell maturation and T cell antitumor immunity, synergizing with immune checkpoint blockade. Intact RIG-I, autocrine interferon signaling, and the GTPase Rab27a in tumor cells are required for biogenesis of immunostimulatory EVs. Active intrinsic RIG-I signaling governs composition of the tumor EV RNA cargo including small non-coding stimulatory RNAs. High transcriptional activity of EV pathway genes and RIG-I in melanoma samples associate with prolonged patient survival and beneficial response to immunotherapy. EVs generated from human melanoma after RIG-I stimulation induce potent antigen-specific T cell responses. We thus define a molecular pathway that can be targeted in tumors to favorably alter EV immunomodulatory function. We propose "reprogramming" of tumor EVs as a personalized strategy for T cell-mediated cancer immunotherapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Neuroscience
-
Stem Cells and Developmental Biology
Neoantigen-specific stem cell memory-like CD4+ T cells mediate CD8+ T cell-dependent immunotherapy of MHC class II-negative solid tumors.
In Nat Immunol on 1 August 2023 by Brightman, S. E., Becker, A., et al.
PubMed
CD4+ T cells play key roles in a range of immune responses, either as direct effectors or through accessory cells, including CD8+ T lymphocytes. In cancer, neoantigen (NeoAg)-specific CD8+ T cells capable of direct tumor recognition have been extensively studied, whereas the role of NeoAg-specific CD4+ T cells is less well understood. We have characterized the murine CD4+ T cell response against a validated NeoAg (CLTCH129>Q) expressed by the MHC-II-deficient squamous cell carcinoma tumor model (SCC VII) at the level of single T cell receptor (TCR) clonotypes and in the setting of adoptive immunotherapy. We find that the natural CLTCH129>Q-specific repertoire is diverse and contains TCRs with distinct avidities as measured by tetramer-binding assays and CD4 dependence. Despite these differences, CD4+ T cells expressing high or moderate avidity TCRs undergo comparable in vivo proliferation to cross-presented antigen from growing tumors and drive similar levels of therapeutic immunity that is dependent on CD8+ T cells and CD40L signaling. Adoptive cellular therapy (ACT) with NeoAg-specific CD4+ T cells is most effective when TCR-engineered cells are differentiated ex vivo with IL-7 and IL-15 rather than IL-2 and this was associated with both increased expansion as well as the acquisition and stable maintenance of a T stem cell memory (TSCM)-like phenotype in tumor-draining lymph nodes (tdLNs). ACT with TSCM-like CD4+ T cells results in lower PD-1 expression by CD8+ T cells in the tumor microenvironment and an increased frequency of PD-1+CD8+ T cells in tdLNs. These findings illuminate the role of NeoAg-specific CD4+ T cells in mediating antitumor immunity via providing help to CD8+ T cells and highlight their therapeutic potential in ACT.
-
-
-
Immunology and Microbiology
-
In vivo experiments
-
Mus musculus (Mouse)
Development of a Heat-Killed fbp1 Mutant Strain as a Therapeutic Agent To Treat Invasive Cryptococcus Infection.
In Microbiol Spectr on 31 January 2023 by Wang, Y., Wang, K., et al.
PubMed
In previous studies, we determined that the F-box protein Fbp1, a subunit of the SCF(Fbp1) E3 ligase in Cryptococcus neoformans, is essential for fungal pathogenesis. Heat-killed fbp1Δ cells (HK-fbp1) can confer vaccine-induced immunity against lethal challenge with clinically important invasive fungal pathogens, e.g., C. neoformans, C. gattii, and Aspergillus fumigatus. In this study, we found that either CD4+ T cells or CD8+ T cells were sufficient to confer protection against lethal challenge by C. neoformans in HK-fbp1-induced immunity. Given the potent effect of HK-fbp1 as a preventative vaccine, we further tested the potential efficacy of administering HK-fbp1 cells as a therapeutic agent for treating animals after infection. Remarkably, administration of HK-fbp1 provided robust host protection against preexisting C. neoformans infection. The mice infected with wild-type H99 cells and then treated with HK-fbp1 showed significant reduction of fungal burden in the infected lung and no dissemination of fungal cells to the brain and spleen. We find that early treatment is critical for the effective use of HK-fbp1 as a therapeutic agent. Immune analysis revealed that early treatment with HK-fbp1 cells elicited Th1-biased protective immune responses that help block fungal dissemination and promote better host protection. Our data thus suggest that HK-fbp1 is both an effective prophylactic vaccine candidate against C. neoformans infection in both immunocompetent and immunocompromised populations and a potential novel therapeutic strategy to treat early-stage cryptococcosis. IMPORTANCE Invasive fungal infections, e.g., cryptococcosis, are often life threatening and difficult to treat with very limited therapeutic options. There is no vaccine available in clinical use to prevent or treat fungal infections. Our previous studies demonstrated that heat-killed fbp1Δ cells (HK-fbp1) in Cryptococcus neoformans can be harnessed to confer protection against a challenge by the virulent parental strain, even in immunocompromised animals, such as ones lacking CD4+ T cells. In this study, we further determined that T cells are required for vaccine-induced protection against homologous challenge and that either CD4+ or CD8+ cells are sufficient. This finding is particularly important for the potential utility of this vaccine candidate in the context of HIV/AIDS-induced immune deficiency, the main risk factor for cryptococcosis in humans. Furthermore, in addition to the utility of HK-fbp1 as a prophylactic vaccine, we found that HK-fbp1 administration can inhibit disease dissemination when animals are treated at an early stage during Cryptococcus infection. Our findings could significantly expand the utility of HK-fbp1 not only as a prophylactic vaccine but also as a novel therapy against cryptococcosis. In all, our studies showed that the HK-fbp1 strain can be used both preventively and therapeutically to elicit robust host protection against cryptococcosis.
-
-
-
Mus musculus (Mouse)
Development of a heat-killedfbp1mutant strain as a therapeutic agent to treat invasiveCryptococcusinfection
In bioRxiv on 9 December 2022 by Wang, Y., Wang, K., et al.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Targeting of Cdc42 GTPase in regulatory T cells unleashes antitumor T-cell immunity.
In J Immunother Cancer on 1 November 2022 by Kalim, K. W., Yang, J. Q., et al.
PubMed
Cancer immunotherapy has taken center stage in cancer treatment. However, the current immunotherapies only benefit a small proportion of patients with cancer, necessitating better understanding of the mechanisms of tumor immune evasion and improved cancer immunotherapy strategies. Regulatory T (Treg) cells play an important role in maintaining immune tolerance through inhibiting effector T-cell function. In the tumor microenvironment, Treg cells are used by tumor cells to counteract effector T cell-mediated tumor suppression. Targeting Treg cells may thus unleash the antitumor activity of effector T cells. While systemic depletion of Treg cells can cause excessive effector T-cell responses and subsequent autoimmune diseases, controlled targeting of Treg cells may benefit patients with cancer.
-
-
-
Flow cytometry/Cell sorting
-
Mus musculus (Mouse)
Binary outcomes of enhancer activity underlie stable random monoallelic expression.
In Elife on 26 May 2022 by Kissiov, D. U., Ethell, A., et al.
PubMed
Mitotically stable random monoallelic gene expression (RME) is documented for a small percentage of autosomal genes. We developed an in vivo genetic model to study the role of enhancers in RME using high-resolution single-cell analysis of natural killer (NK) cell receptor gene expression and enhancer deletions in the mouse germline. Enhancers of the RME NK receptor genes were accessible and enriched in H3K27ac on silent and active alleles alike in cells sorted according to allelic expression status, suggesting enhancer activation and gene expression status can be decoupled. In genes with multiple enhancers, enhancer deletion reduced gene expression frequency, in one instance converting the universally expressed gene encoding NKG2D into an RME gene, recapitulating all aspects of natural RME including mitotic stability of both the active and silent states. The results support the binary model of enhancer action, and suggest that RME is a consequence of general properties of gene regulation by enhancers rather than an RME-specific epigenetic program. Therefore, many and perhaps all genes may be subject to some degree of RME. Surprisingly, this was borne out by analysis of several genes that define different major hematopoietic lineages, that were previously thought to be universally expressed within those lineages: the genes encoding NKG2D, CD45, CD8α, and Thy-1. We propose that intrinsically probabilistic gene allele regulation is a general property of enhancer-controlled gene expression, with previously documented RME representing an extreme on a broad continuum.
-
-
-
Cancer Research
-
Immunology and Microbiology
Anti‑VEGF antibody triggers the effect of anti‑PD‑L1 antibody in PD‑L1low and immune desert‑like mouse tumors.
In Oncol Rep on 1 February 2022 by Ishikura, N., Sugimoto, M., et al.
PubMed
The efficacy of programmed cell death‑ligand 1 (PD‑L1)/programmed cell death protein 1 (PD‑1) blockade therapy has been demonstrated but is limited in patients with PD‑L1low or immune desert tumors. This limitation can be overcome by combination therapies that include anti‑vascular endothelial growth factor (VEGF) therapy. Such combinations have been investigated in clinical trials for a number of cancer types; however, evidence on the mechanisms underlying their effects in these types of patients is still not sufficient. Therefore, the present study investigated the efficacy and effects on CD8+ T cell and C‑X‑C motif chemokine receptor 3 (CXCR3) ligand expression in tumors by combining anti‑PD‑L1 and anti‑VEGF antibodies using an OV2944‑HM‑1 mouse model with PD‑L1low and immune desert‑like phenotypes. Although the model exhibited anti‑PD‑L1 insensitivity, anti‑PD‑L1 antibody treatment combined with anti‑VEGF antibody inhibited tumor growth compared with anti‑VEGF monotherapy, which itself inhibited tumor growth compared with the control treatment on Day 25. In combination‑treated mice, a higher percentage of CD8+ T cells and higher levels of CXCR3 ligands were observed in tumor tissues compared with those in the anti‑VEGF antibody treatment group, which was not significantly different from control treatment on Day 8. The increase in the intratumoral percentage of CD8+ T cells following the combination treatment was reversed by CXCR3 blocking to the same level as the control. In an anti‑PD‑L1 insensitive model with PD‑L1low and immune desert‑like phenotypes, although anti‑PD‑L1 antibody alone was not effective, anti‑PD‑L1 antibody in combination with anti‑VEGF antibody exhibited antitumor combination efficacy with an increase of CD8+ T cell infiltration, which was suggested to be dependent on the increase of intratumoral CXCR3 ligands. This mechanism could explain the efficacy of anti‑PD‑L1 antibody and anti‑VEGF antibody combination therapy in the clinical setting.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Targeting of Cdc42 GTPase in regulatory T cells unleashes anti-tumor T cell immunity
In bioRxiv on 24 September 2021 by Kalim, K. W., Yang, J., et al.
-
-
Binary outcomes of enhancer activity underlie stable random monoallelic expression
In bioRxiv on 28 August 2021 by Kissiov, D. U., Ethell, A., et al.
-
-
Flow cytometry/Cell sorting
-
Cancer Research
-
Immunology and Microbiology
-
Mus musculus (Mouse)
PLGA-particle vaccine carrying TLR3/RIG-I ligand Riboxxim synergizes with immune checkpoint blockade for effective anti-cancer immunotherapy.
In Nat Commun on 18 May 2021 by Koerner, J., Horvath, D., et al.
PubMed
With emerging supremacy, cancer immunotherapy has evolved as a promising therapeutic modality compared to conventional antitumor therapies. Cancer immunotherapy composed of biodegradable poly(lactic-co-glycolic acid) (PLGA) particles containing antigens and toll-like receptor ligands induces vigorous antitumor immune responses in vivo. Here, we demonstrate the supreme adjuvant effect of the recently developed and pharmaceutically defined double-stranded (ds)RNA adjuvant Riboxxim especially when incorporated into PLGA particles. Encapsulation of Riboxxim together with antigens potently activates murine and human dendritic cells, and elevated tumor-specific CD8+ T cell responses are superior to those obtained using classical dsRNA analogues. This PLGA particle vaccine affords primary tumor growth retardation, prevention of metastases, and prolonged survival in preclinical tumor models. Its advantageous therapeutic potency was further enhanced by immune checkpoint blockade that resulted in reinvigoration of cytotoxic T lymphocyte responses and tumor ablation. Thus, combining immune checkpoint blockade with immunotherapy based on Riboxxim-bearing PLGA particles strongly increases its efficacy.
-
-
-
In vivo experiments
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
-
In vivo experiments
ER stress-induced mediator C/EBP homologous protein thwarts effector T cell activity in tumors through T-bet repression.
In Nat Commun on 20 March 2019 by Cao, Y., Trillo-Tinoco, J., et al.
PubMed
Understanding the intrinsic mediators that render CD8+ T cells dysfunctional in the tumor microenvironment is a requirement to develop more effective cancer immunotherapies. Here, we report that C/EBP homologous protein (Chop), a downstream sensor of severe endoplasmic reticulum (ER) stress, is a major negative regulator of the effector function of tumor-reactive CD8+ T cells. Chop expression is increased in tumor-infiltrating CD8+ T cells, which correlates with poor clinical outcome in ovarian cancer patients. Deletion of Chop in T cells improves spontaneous antitumor CD8+ T cell immunity and boosts the efficacy of T cell-based immunotherapy. Mechanistically, Chop in CD8+ T cells is elevated primarily through the ER stress-associated kinase Perk and a subsequent induction of Atf4; and directly represses the expression of T-bet, a master regulator of effector T cell function. These findings demonstrate the primary role of Chop in tumor-induced CD8+ T cell dysfunction and the therapeutic potential of blocking Chop or ER stress to unleash T cell-mediated antitumor immunity.
-