Catalog #BE0223
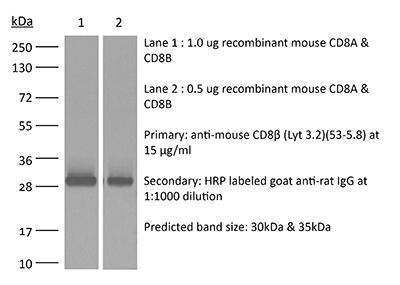
InVivoMAb anti-mouse CD8β (Lyt 3.2)
Clone
53-5.8
Reactivities
Mouse
Product Citations
139
Isotype
Rat IgG1, κ