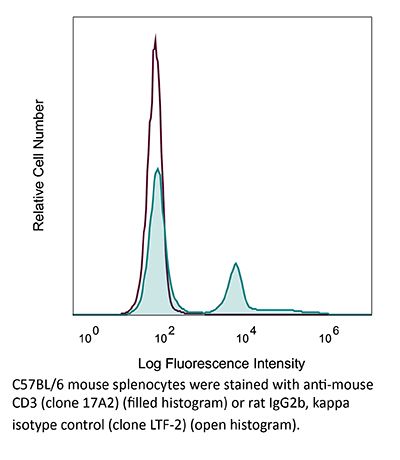
InVivoMAb anti-mouse CD3
Product Description
Specifications
| Isotype | Rat IgG2b, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2b isotype control, anti-keyhole limpet hemocyanin |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | γδ TCR-positive T-T hybridoma D1 |
| Reported Applications | in vitro T cell stimulation/activation |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107630 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vitro T cell stimulation/activation
Shen, P. X., et al (2021). "Urolithin A ameliorates experimental autoimmune encephalomyelitis by targeting aryl hydrocarbon receptor" EBioMedicine 64: 103227.
PubMed
BACKGROUND: Urolithin A (URA) is an intestinal microbiota metabolic product from ellagitannin-containing foods with multiple biological activities. However, its role in autoimmune diseases is largely unknown. Here, for first time, we demonstrate the therapeutic effect of URA in an experimental autoimmune encephalomyelitis (EAE) animal model. METHODS: Therapeutic effect was evaluated via an active and passive EAE animal model in vivo. The function of URA on bone marrow-derived dendritic cells (BM-DCs), T cells, and microglia were tested in vitro. FINDINGS: Oral URA (25 mg/kg/d) suppressed disease progression at prevention, induction, and effector phases of preclinical EAE. Histological evaluation showed that significantly fewer inflammatory cells, decreased demyelination, lower numbers of M1-type microglia and activated DCs, as well as reduced infiltrating Th1/Th17 cells were present in the central nervous system (CNS) of the URA-treated group. URA treatment at 25 μM inhibited the activation of BM-DCs in vitro, restrained Th17 cell differentiation in T cell polarization conditions, and in a DC-CD4(+) T cell co-culture system. Moreover, we confirmed URA inhibited pathogenicity of Th17 cells in adoptive EAE. Mechanism of URA action was directly targeting Aryl Hydrocarbon Receptor (AhR) and modulating the signaling pathways. INTERPRETATION: Collectively, our study offers new evidence that URA, as a human microbial metabolite, is valuable to use as a prospective therapeutic candidate for autoimmune diseases.
in vitro T cell stimulation/activation
Edwards-Hicks, J., et al (2020). "Metabolic Dynamics of In Vitro CD8+ T Cell Activation" Metabolites 11(1).
PubMed
CD8+ T cells detect and kill infected or cancerous cells. When activated from their naïve state, T cells undergo a complex transition, including major metabolic reprogramming. Detailed resolution of metabolic dynamics is needed to advance the field of immunometabolism. Here, we outline methodologies that when utilized in parallel achieve broad coverage of the metabolome. Specifically, we used a combination of 2 flow injection analysis (FIA) and 3 liquid chromatography (LC) methods in combination with positive and negative mode high-resolution mass spectrometry (MS) to study the transition from naïve to effector T cells with fine-grained time resolution. Depending on the method, between 54% and 98% of measured metabolic features change in a time-dependent manner, with the major changes in both polar metabolites and lipids occurring in the first 48 h. The statistical analysis highlighted the remodeling of the polyamine biosynthesis pathway, with marked differences in the dynamics of precursors, intermediates, and cofactors. Moreover, phosphatidylcholines, the major class of membrane lipids, underwent a drastic shift in acyl chain composition with polyunsaturated species decreasing from 60% to 25% of the total pool and specifically depleting species containing a 20:4 fatty acid. We hope that this data set with a total of over 11,000 features recorded with multiple MS methodologies for 9 time points will be a useful resource for future work.
in vitro T cell stimulation/activation
Choi, Y. S., et al (2015). "LEF-1 and TCF-1 orchestrate TFH differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6" Nat Immunol 16(9): 980-990.
PubMed
Follicular helper T cells (TFH cells) are specialized effector CD4(+) T cells that help B cells develop germinal centers (GCs) and memory. However, the transcription factors that regulate the differentiation of TFH cells remain incompletely understood. Here we report that selective loss of Lef1 or Tcf7 (which encode the transcription factor LEF-1 or TCF-1, respectively) resulted in TFH cell defects, while deletion of both Lef1 and Tcf7 severely impaired the differentiation of TFH cells and the formation of GCs. Forced expression of LEF-1 enhanced TFH differentiation. LEF-1 and TCF-1 coordinated such differentiation by two general mechanisms. First, they established the responsiveness of naive CD4(+) T cells to TFH cell signals. Second, they promoted early TFH differentiation via the multipronged approach of sustaining expression of the cytokine receptors IL-6Ralpha and gp130, enhancing expression of the costimulatory receptor ICOS and promoting expression of the transcriptional repressor Bcl6.
in vitro T cell stimulation/activation
Nance, J. P., et al (2015). "Bcl6 middle domain repressor function is required for T follicular helper cell differentiation and utilizes the corepressor MTA3" Proc Natl Acad Sci U S A. pii : 201507312.
PubMed
T follicular helper (Tfh) cells are essential providers of help to B cells. The transcription factor B-cell CLL/lymphoma 6 (Bcl6) is a lineage-defining regulator of Tfh cells and germinal center B cells. In B cells, Bcl6 has the potential to recruit distinct transcriptional corepressors through its BTB domain or its poorly characterized middle domain (also known as RDII), but in Tfh cells the roles of the Bcl6 middle domain have yet to be clarified. Mimicked acetylation of the Bcl6 middle domain (K379Q) in CD4 T cells results in significant reductions in Tfh differentiation in vivo. Blimp1 (Prdm1) is a potent inhibitor of Tfh cell differentiation. Although Bcl6 K379Q still bound to the Prdm1 cis-regulatory elements in Tfh cells, Prdm1 expression was derepressed. This was a result of the failure of Bcl6 K379Q to recruit metastasis-associated protein 3 (MTA3). The loss of Bcl6 function in Bcl6 K379Q-expressing CD4 T cells could be partially rescued by abrogating Prdm1 expression. In addition to Prdm1, we found that Bcl6 recruits MTA3 to multiple genes involved in Tfh cell biology, including genes important for cell migration, cell survival, and alternative differentiation pathways. Thus, Bcl6 middle domain mediated repression is a major mechanism of action by which Bcl6 controls CD4 T-cell fate and function.
in vitro T cell stimulation/activation
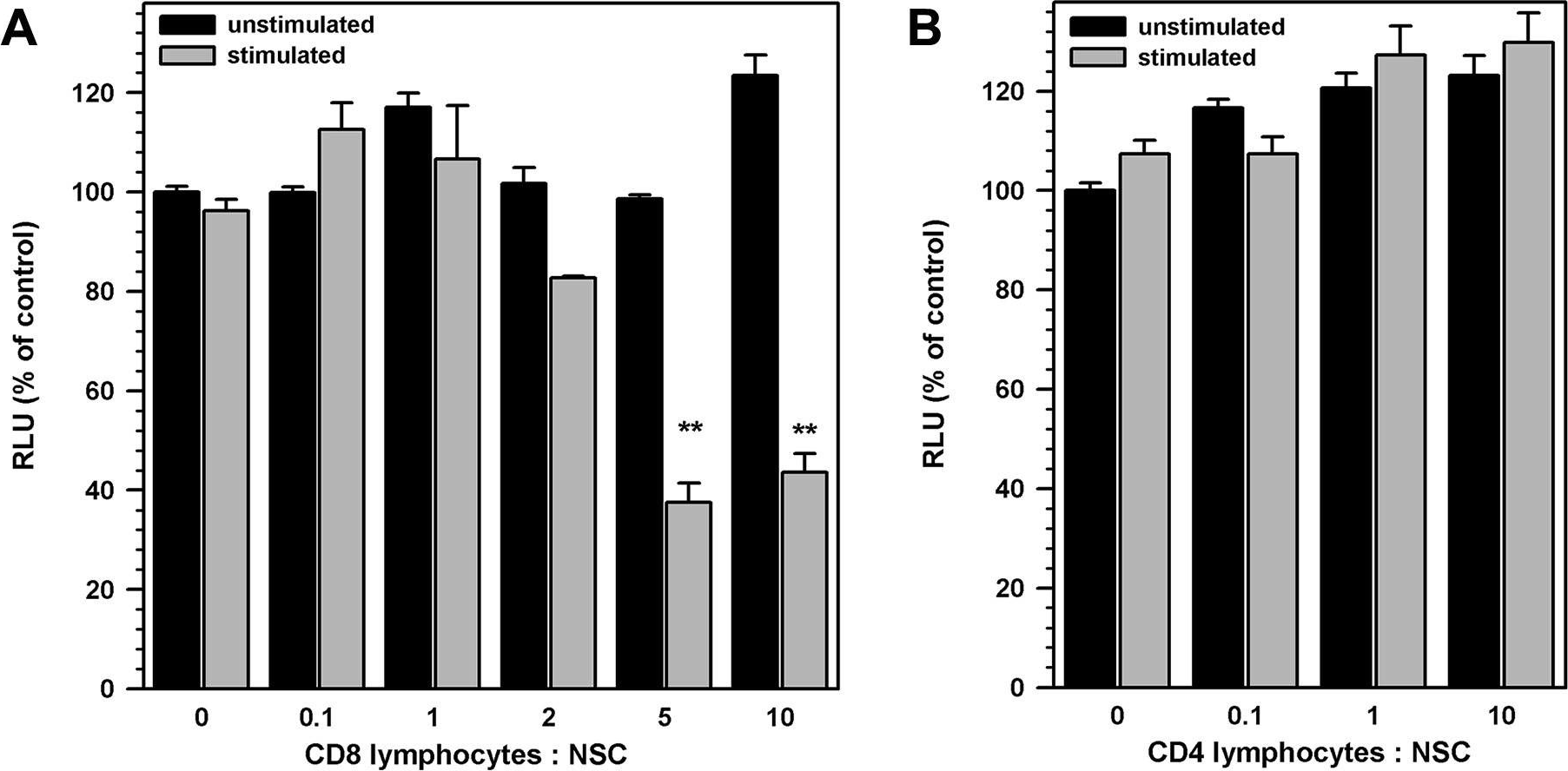
Hu, S., et al (2014). "Activated CD8+ T lymphocytes inhibit neural stem/progenitor cell proliferation: role of interferon-gamma" PLoS One 9(8): e105219.
PubMed
The ability of neural stem/progenitor cells (NSCs) to self-renew, migrate to damaged sites, and differentiate into neurons has renewed interest in using them in therapies for neurodegenerative disorders. Neurological diseases, including viral infections of the brain, are often accompanied by chronic inflammation, whose impact on NSC function remains unexplored. We have previously shown that chronic neuroinflammation, a hallmark of experimental herpes simplex encephalitis (HSE) in mice, is dominated by brain-infiltrating activated CD8 T-cells. In the present study, activated CD8 lymphocytes were found to suppress NSC proliferation profoundly. Luciferase positive (luc+) NSCs co-cultured with activated, MHC-matched, CD8+ lymphocytes (luc-) showed two- to five-fold lower luminescence than co-cultures with un-stimulated lymphocytes. On the other hand, similarly activated CD4+ lymphocytes did not suppress NSC growth. This differential lymphocyte effect on proliferation was confirmed by decreased BrdU uptake by NSC cultured with activated CD8 T-cells. Interestingly, neutralizing antibodies to interferon-gamma (IFN-gamma) reversed the impact of CD8 lymphocytes on NSCs. Antibodies specific to the IFN-gamma receptor-1 subunit complex abrogated the inhibitory effects of both CD8 lymphocytes and IFN-gamma, indicating that the inhibitory effect of these cells was mediated by IFN-gamma in a receptor-specific manner. In addition, activated CD8 lymphocytes decreased levels of nestin and Sox2 expression in NSCs while increasing GFAP expression, suggesting possible induction of an altered differentiation state. Furthermore, NSCs obtained from IFN-gamma receptor-1 knock-out embryos were refractory to the inhibitory effects of activated CD8+ T lymphocytes on cell proliferation and Sox2 expression. Taken together, the studies presented here demonstrate a role for activated CD8 T-cells in regulating NSC function mediated through the production of IFN-gamma. This cytokine may influence neuro-restorative processes and ultimately contribute to the long-term sequelae commonly seen following herpes encephalitis.
in vitro T cell stimulation/activation
Choi, Y. S., et al (2013). "Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory" J Immunol 190(8): 4014-4026.
PubMed
Follicular helper CD4 T (Tfh) cells are a distinct type of differentiated CD4 T cells uniquely specialized for B cell help. In this study, we examined Tfh cell fate commitment, including distinguishing features of Tfh versus Th1 proliferation and survival. Using cell transfer approaches at early time points after an acute viral infection, we demonstrate that early Tfh cells and Th1 cells are already strongly cell fate committed by day 3. Nevertheless, Tfh cell proliferation was tightly regulated in a TCR-dependent manner. The Tfh cells still depend on extrinsic cell fate cues from B cells in their physiological in vivo environment. Unexpectedly, we found that Tfh cells share a number of phenotypic parallels with memory precursor CD8 T cells, including selective upregulation of IL-7Ralpha and a collection of coregulated genes. As a consequence, the early Tfh cells can progress to robustly form memory cells. These data support the hypothesis that CD4 and CD8 T cells share core aspects of a memory cell precursor gene expression program involving Bcl6, and a strong relationship exists between Tfh cells and memory CD4 T cell development.
Product Citations
-
-
Immunology and Microbiology
Harnessing the dual immunomodulatory function of myeloid-derived suppressor cells to reshape the inflammatory microenvironment for osteoarthritis therapy.
In Mater Today Bio on 1 December 2025 by Guo, Z., Chen, T., et al.
PubMed
Osteoarthritis (OA) pathogenesis is profoundly influenced by dysregulated immune dynamics, where persistent interleukin-17 (IL-17)/T helper 17 (Th17) cell mediated inflammation coordinates with failed regenerative processes to perpetuate joint destruction. Here, we unveil the role of myeloid-derived suppressor cells (MDSCs) as dual-phase regulators that paradoxically orchestrate both inflammatory escalation and tissue repair in OA progression. Intra-articular administration of MDSCs in OA mice amplified IL-17 dependent inflammatory cascades and chemokine-driven leukocyte recruitment, revealing a context-dependent pro-inflammatory phenotype. Unexpectedly, MDSC depletion failed to attenuate joint damage, implying their indispensable yet multifaceted role in OA pathogenesis. Mechanistically, MDSCs exhibited functional plasticity by upregulating arginase-1 to polarize M2 macrophages, fostering a regenerative niche alongside their inflammatory activity. To resolve this duality, we developed a bio-responsive hydrogel-microsphere system integrating transforming growth factor β1 (TGF-β1) and interleukin-1 β1 antibody (anti-IL-1β) loaded mesoporous silica nanoparticles (MSNs). This spatiotemporally controlled platform selectively suppressed MDSC-mediated Th17 cell expansion while harnessing their intrinsic capacity to drive M2 macrophage polarization and chondrogenesis. The resultant shift from a pro-inflammatory to pro-regenerative microenvironment significantly attenuated cartilage erosion and restored joint integrity in OA models. Our findings redefine MDSCs as bifunctional immune orchestrators in OA and establish precision biomaterial guided immune decoding as a paradigm-shifting therapeutic strategy. By engineering MDSCs plasticity through antagonistic cytokine delivery, this work provides a blueprint for microenvironment remodeling in degenerative joint diseases.
-
-
-
Cancer Research
-
Immunology and Microbiology
β-adrenergic signaling blockade attenuates metastasis through activation of cytotoxic CD4 T cells.
In Nat Commun on 17 November 2025 by Fjæstad, K. Y., Johansen, A. Z., et al.
PubMed
β-adrenergic signaling has been suggested to promote tumor growth, and β-blockers are being evaluated for repurposing for cancer treatment. Here, we identify a β-adrenergic signaling axis involved in metastasis formation. We show that the β-blocker propranolol has strong anti-metastatic activity in multiple murine models, with this effect being completely dependent on CD4 + T cells and independent of NK or CD8 + T cells. We also observe that CD4 + T cells are required for the anti-tumor effect of propranolol in a syngeneic subcutaneous model of colon cancer. Mechanistically, propranolol induces a Th1-polarized and cytotoxic CD4 + T cell response, which requires MHC class II expression by cancer cells for full efficacy. We also report propanolol-driven systemic changes in the monocyte compartment, and upon depletion of monocytes, propranolol loses its anti-tumor effects. Finally, we show that propranolol treatment synergizes with anti-CTLA-4 therapy to further enhance CD4 + T cell infiltration and control metastasis. Thus, we show that β-adrenergic signaling limits CD4 T cell-mediated anti-tumor immunity, highlighting the potential of repurposing β-blockers for cancer treatment.
-
-
-
Immunology and Microbiology
-
Cell Biology
-
Cancer Research
-
Biochemistry and Molecular biology
Targeting pyruvate metabolism generates distinct CD8+ T cell responses to gammaherpesvirus and B lymphoma.
In JCI Insight on 22 August 2025 by Kang, T., Usherwood, Y. K., et al.
PubMed
T cells rely on different metabolic pathways to differentiate into effector or memory cells, and metabolic intervention is a promising strategy to optimize T cell function for immunotherapy. Pyruvate dehydrogenase (PDH) is a nexus between glycolytic and mitochondrial metabolism, regulating pyruvate conversion to either lactate or acetyl-CoA. Here, we retrovirally transduced pyruvate dehydrogenase kinase 1 (PDK1) or pyruvate dehydrogenase phosphatase 1 (PDP1), which control PDH activity, into CD8+ T cells to test effects on T cell function. Although PDK1 and PDP1 were expected to influence PDH in opposing directions, by several criteria they induced similar changes relative to control T cells. Seahorse metabolic flux assays showed both groups exhibited increased glycolysis and oxidative phosphorylation. Both groups had improved primary and memory recall responses following infection with murine gammaherpesvirus-68. However, metabolomics using labeled fuels indicated differential usage of key fuels by metabolic pathways. Importantly, CD8+ T cell populations after B cell lymphoma challenge were smaller in both groups, resulting in poorer protection, which was rescued by glutamine and acetate supplementation. Overall, this study indicates that PDK1 and PDP1 both enhance metabolic capacity, but the context of the antigenic challenge significantly influences the consequences for T cell function.
-
-
-
Immunology and Microbiology
Alpha-1 Antitrypsin Overexpressing Mesenchymal Stem/Stromal Cells Reverses Type 1 Diabetes via Promoting Treg Function and CD8+T cell exhaustion
In bioRxiv on 21 April 2025 by Wei, H., Gou, W., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
A novel HVEM-Fc recombinant protein for lung cancer immunotherapy.
In J Exp Clin Cancer Res on 20 February 2025 by Yao, Y., Li, B., et al.
PubMed
The ubiquitously expressed transmembrane protein, Herpesvirus Entry Mediator (HVEM), functions as a molecular switch, capable of both activating and inhibiting the immune response depending on its interacting ligands. HVEM-Fc is a novel recombinant fusion protein with the potential to eradicate tumor cells.
-
-
-
Immunology and Microbiology
Engineered oncolytic virus coated with anti-PD-1 and alendronate for ameliorating intratumoral T cell hypofunction.
In Exp Hematol Oncol on 15 February 2025 by Zhu, Y., Zhang, X., et al.
PubMed
Glioblastoma is a highly aggressive and devastating primary brain tumor that is resistant to conventional therapies. Oncolytic viruses represent a promising therapeutic approach for glioblastoma by selectively lysing tumor cells and eliciting an anti-tumor immune response. However, the clinical efficacy of oncolytic viruses is often hindered by challenges such as short persistence, host antiviral immune responses, and T cell dysfunction.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Chimeric antigen receptor macrophages (CAR-M) sensitize HER2+ solid tumors to PD1 blockade in pre-clinical models.
In Nat Commun on 15 January 2025 by Pierini, S., Gabbasov, R., et al.
PubMed
We previously developed human CAR macrophages (CAR-M) and demonstrated redirection of macrophage anti-tumor function leading to tumor control in immunodeficient xenograft models. Here, we develop clinically relevant fully immunocompetent syngeneic models to evaluate the potential for CAR-M to remodel the tumor microenvironment (TME), induce T cell anti-tumor immunity, and sensitize solid tumors to PD1/PDL1 checkpoint inhibition. In vivo, anti-HER2 CAR-M significantly reduce tumor burden, prolong survival, remodel the TME, increase intratumoral T cell and natural killer (NK) cell infiltration, and induce antigen spreading. CAR-M therapy protects against antigen-negative relapses in a T cell dependent fashion, confirming long-term anti-tumor immunity. In HER2+ solid tumors with limited sensitivity to anti-PD1 (aPD1) monotherapy, the combination of CAR-M and aPD1 significantly improves tumor growth control, survival, and remodeling of the TME in pre-clinical models. These results demonstrate synergy between CAR-M and T cell checkpoint blockade and provide a strategy to potentially enhance response to aPD1 therapy for patients with non-responsive tumors.
-
-
-
-
Mus musculus (Mouse)
-
Cell Biology
InTraSeq: A Multimodal Assay that Uncovers New Single-Cell Biology and Regulatory Mechanisms
In Research Square on 9 December 2024 by Beausoleil, S., Ariss, M., et al.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Calcium nanoparticles target and activate T cells to enhance anti-tumor function.
In Nat Commun on 21 November 2024 by Yang, W., Feng, Z., et al.
PubMed
Calcium signaling plays a crucial role in the activation of T lymphocytes. However, modulating calcium levels to control T cell activation in vivo remains a challenge. In this study, we investigate T cell activation using 12-myristate 13-acetate (PMA)-encapsulated CaCO3 nanoparticles. We find that anti-PD-1 antibody-conjugated CaCO3 nanoparticles can be internalized by T cells via receptor-mediated endocytosis and then gradually release calcium. This results in an increase in cytosolic calcium, which triggers the activation of NFAT and NF-κB pathways, especially when the surface of the CaCO3 nanoparticles is loaded with PMA. Animal studies demonstrate that the PMA-loaded calcium nanoparticles enhance the activation and proliferation of cytotoxic T cells, leading to improved tumor suppression without additional toxicity. When tested in metastatic tumor models, T cells loaded with the calcium nanoparticles prior to adoptive cell transfer control tumor growth better, resulting in prolonged animal survival. Our approach offers an alternative T cell activation strategy to potentiate immunotherapy by targeting a fundamental signaling pathway.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
PARP inhibitors enhance antitumor immune responses by triggering pyroptosis via TNF-caspase 8-GSDMD/E axis in ovarian cancer.
In J Immunother Cancer on 4 October 2024 by Xia, Y., Huang, P., et al.
PubMed
In addition to their established action of synthetic lethality in tumor cells, poly(ADP-ribose) polymerase inhibitors (PARPis) also orchestrate tumor immune microenvironment (TIME) that contributes to suppressing tumor growth. However, it remains not fully understood whether and how PARPis trigger tumor-targeting immune responses.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
The type 2 cytokine Fc-IL-4 revitalizes exhausted CD8+ T cells against cancer.
In Nature on 1 October 2024 by Feng, B., Bai, Z., et al.
PubMed
Current cancer immunotherapy predominately focuses on eliciting type 1 immune responses fighting cancer; however, long-term complete remission remains uncommon1,2. A pivotal question arises as to whether type 2 immunity can be orchestrated alongside type 1-centric immunotherapy to achieve enduring response against cancer3,4. Here we show that an interleukin-4 fusion protein (Fc-IL-4), a typical type 2 cytokine, directly acts on CD8+ T cells and enriches functional terminally exhausted CD8+ T (CD8+ TTE) cells in the tumour. Consequently, Fc-IL-4 enhances antitumour efficacy of type 1 immunity-centric adoptive T cell transfer or immune checkpoint blockade therapies and induces durable remission across several syngeneic and xenograft tumour models. Mechanistically, we discovered that Fc-IL-4 signals through both signal transducer and activator of transcription 6 (STAT6) and mammalian target of rapamycin (mTOR) pathways, augmenting the glycolytic metabolism and the nicotinamide adenine dinucleotide (NAD) concentration of CD8+ TTE cells in a lactate dehydrogenase A-dependent manner. The metabolic modulation mediated by Fc-IL-4 is indispensable for reinvigorating intratumoural CD8+ TTE cells. These findings underscore Fc-IL-4 as a potent type 2 cytokine-based immunotherapy that synergizes effectively with type 1 immunity to elicit long-lasting responses against cancer. Our study not only sheds light on the synergy between these two types of immune responses, but also unveils an innovative strategy for advancing next-generation cancer immunotherapy by integrating type 2 immune factors.
-
-
-
Immunology and Microbiology
-
Cancer Research
Fasting reshapes tissue-specific niches to improve NK cell-mediated anti-tumor immunity.
In Immunity on 13 August 2024 by Delconte, R. B., Owyong, M., et al.
PubMed
Fasting is associated with improved outcomes in cancer. Here, we investigated the impact of fasting on natural killer (NK) cell anti-tumor immunity. Cyclic fasting improved immunity against solid and metastatic tumors in an NK cell-dependent manner. During fasting, NK cells underwent redistribution from peripheral tissues to the bone marrow (BM). In humans, fasting also reduced circulating NK cell numbers. NK cells in the spleen of fasted mice were metabolically rewired by elevated concentrations of fatty acids and glucocorticoids, augmenting fatty acid metabolism via increased expression of the enzyme CPT1A, and Cpt1a deletion impaired NK cell survival and function in this setting. In parallel, redistribution of NK cells to the BM during fasting required the trafficking mediators S1PR5 and CXCR4. These cells were primed by an increased pool of interleukin (IL)-12-expressing BM myeloid cells, which improved IFN-γ production. Our findings identify a link between dietary restriction and optimized innate immune responses, with the potential to enhance immunotherapy strategies.
-
-
-
Immunology and Microbiology
Venetoclax Induces BCL-2-Dependent Treg to TH17 Plasticity to Enhance the Antitumor Efficacy of Anti-PD-1 Checkpoint Blockade.
In Cancer Immunol Res on 1 August 2024 by Liao, R., Hsu, J. Y., et al.
PubMed
The specific BCL-2 small molecule inhibitor venetoclax induces apoptosis in a wide range of malignancies, which has led to rapid clinical expansion in its use alone and in combination with chemotherapy and immune-based therapies against a myriad of cancer types. While lymphocytes, and T cells in particular, rely heavily on BCL-2 for survival and function, the effects of small molecule blockade of the BCL-2 family on surviving immune cells is not fully understood. We aimed to better understand the effect of systemic treatment with venetoclax on regulatory T cells (Treg), which are relatively resistant to cell death induced by specific drugging of BCL-2 compared to other T cells. We found that BCL-2 blockade altered Treg transcriptional profiles and mediated Treg plasticity toward a TH17-like Treg phenotype, resulting in increased IL17A production in lymphoid organs and within the tumor microenvironment. Aligned with previously described augmented antitumor effects observed when combining venetoclax with anti-PD-1 checkpoint inhibition, we also demonstrated that Treg-specific genetic BCL-2 knockout combined with anti-PD-1 induced tumor regression and conferred overlapping genetic changes with venetoclax-treated Tregs. As long-term combination therapies using venetoclax gain more traction in the clinic, an improved understanding of the immune-modulatory effects caused by venetoclax may allow expansion of its use against malignancies and immune-related diseases.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
The ATP-exporting channel Pannexin 1 promotes CD8+ T cell effector and memory responses.
In iScience on 19 July 2024 by Vardam-Kaur, T., Banuelos, A., et al.
PubMed
Sensing of extracellular ATP (eATP) controls CD8+ T cell function. Their accumulation can occur through export by specialized molecules, such as the release channel Pannexin 1 (Panx1). Whether Panx1 controls CD8+ T cell immune responses in vivo, however, has not been previously addressed. Here, we report that T-cell-specific Panx1 is needed for CD8+ T cell responses to viral infections and cancer. We found that CD8-specific Panx1 promotes both effector and memory CD8+ T cell responses. Panx1 favors initial effector CD8+ T cell activation through extracellular ATP (eATP) export and subsequent P2RX4 activation, which helps promote full effector differentiation through extracellular lactate accumulation and its subsequent recycling. In contrast, Panx1 promotes memory CD8+ T cell survival primarily through ATP export and subsequent P2RX7 engagement, leading to improved mitochondrial metabolism. In summary, Panx1-mediated eATP export regulates effector and memory CD8+ T cells through distinct purinergic receptors and different metabolic and signaling pathways.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
IL3-Driven T Cell-Basophil Crosstalk Enhances Antitumor Immunity.
In Cancer Immunol Res on 2 July 2024 by Wei, J., Mayberry, C. L., et al.
PubMed
Cytotoxic T lymphocytes (CTL) are pivotal in combating cancer, yet their efficacy is often hindered by the immunosuppressive tumor microenvironment, resulting in CTL exhaustion. This study investigates the role of interleukin-3 (IL3) in orchestrating antitumor immunity through CTL modulation. We found that intratumoral CTLs exhibited a progressive decline in IL3 production, which was correlated with impaired cytotoxic function. Augmenting IL3 supplementation, through intraperitoneal administration of recombinant IL3, IL3-expressing tumor cells, or IL3-engineered CD8+ T cells, conferred protection against tumor progression, concomitant with increased CTL activity. CTLs were critical for this therapeutic efficacy as IL3 demonstrated no impact on tumor growth in Rag1 knockout mice or following CD8+ T-cell depletion. Rather than acting directly, CTL-derived IL3 exerted its influence on basophils, concomitantly amplifying antitumor immunity within CTLs. Introducing IL3-activated basophils retarded tumor progression, whereas basophil depletion diminished the effectiveness of IL3 supplementation. Furthermore, IL3 prompted basophils to produce IL4, which subsequently elevated CTL IFNγ production and viability. Further, the importance of basophil-derived IL4 was evident from the absence of benefits of IL3 supplementation in IL4 knockout tumor-bearing mice. Overall, this research has unveiled a role for IL3-mediated CTL-basophil cross-talk in regulating antitumor immunity and suggests harnessing IL3 sustenance as a promising approach for optimizing and enhancing cancer immunotherapy. See related Spotlight, p. 798.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Regulatory T cells effectively downregulate the autoimmune anti-MPO response and ameliorate anti-MPO induced glomerulonephritis in mice.
In J Autoimmun on 1 July 2024 by Hu, P., Xiao, H., et al.
PubMed
Regulation of autoreactive cells is key for both prevention and amelioration of autoimmune disease. A better understanding of the key cell population(s) responsible for downregulation of autoreactive cells would provide necessary foundational insight for cellular-based therapies in autoimmune disease. Utilizing a mouse model of anti-myeloperoxidase (MPO) glomerulonephritis, we sought to understand which immune cells contribute to downregulation of the anti-MPO autoimmune response. MPO-/- mice were immunized with whole MPO to induce an anti-MPO response. Anti-MPO splenocytes were then transferred into recipient mice (Rag2-/- mice or WT mice). Anti-MPO titers were followed over time. After anti-MPO splenocyte transfer, WT mice are able to downregulate the anti-MPO response while anti-MPO titers persist in Rag2-/- recipients. Reconstitution with WT splenocytes into Rag2-/- recipients prior to anti-MPO splenocyte transfer enabled mice to downregulate the anti-MPO immune response. Therefore, wildtype splenocytes contain a cellular population that is capable of downregulating the autoimmune response. Through splenocyte transfer, antibody depletion experiments, and purified cell population transfers, we confirmed that the regulatory T cell (Treg) population is responsible for the downregulation of the anti-MPO autoimmune response. Further investigation revealed that functional Tregs from WT mice are capable of downregulating anti-MPO antibody production and ameliorate anti-MPO induced glomerulonephritis. These data underscore the importance of functional Tregs for control of autoimmune responses and prevention of end-organ damage due to autoimmunity.
-
-
-
Cancer Research
-
Neuroscience
Sensory nerve release of CGRP increases tumor growth in HNSCC by suppressing TILs.
In Med on 8 March 2024 by Darragh, L. B., Nguyen, A., et al.
PubMed
Perineural invasion (PNI) and nerve density within the tumor microenvironment (TME) have long been associated with worse outcomes in head and neck squamous cell carcinoma (HNSCC). This prompted an investigation into how nerves within the tumor microenvironment affect the adaptive immune system and tumor growth.
-
-
-
Immunology and Microbiology
Homeostatic PD-1 signaling restrains EOMES-dependent oligoclonal expansion of liver-resident CD8 T cells.
In Cell Rep on 29 August 2023 by Le Moine, M., Azouz, A., et al.
PubMed
The co-inhibitory programmed death (PD)-1 signaling pathway plays a major role in the context of tumor-specific T cell responses. Conversely, it also contributes to the maintenance of peripheral tolerance, as patients receiving anti-PD-1 treatment are prone to developing immune-related adverse events. Yet, the physiological role of the PD-1/PDL-1 axis in T cell homeostasis is still poorly understood. Herein, we show that under steady-state conditions, the absence of PD-1 signaling led to a preferential expansion of CD8+ T cells in the liver. These cells exhibit an oligoclonal T cell receptor (TCR) repertoire and a terminally differentiated exhaustion profile. The transcription factor EOMES is required for the clonal expansion and acquisition of this differentiation program. Finally, single-cell transcriptomics coupled with TCR repertoire analysis support the notion that these cells arise locally from liver-resident memory CD8+ T cells. Overall, we show a role for PD-1 signaling in liver memory T cell homeostasis.
-
-
-
Mus musculus (Mouse)
CCR4 and CCR7 differentially regulate thymocyte localization with distinct outcomes for central tolerance.
In Elife on 2 June 2023 by Li, Y., Guaman Tipan, P., et al.
PubMed
Central tolerance ensures autoreactive T cells are eliminated or diverted to the regulatory T cell lineage, thus preventing autoimmunity. To undergo central tolerance, thymocytes must enter the medulla to test their T-cell receptors (TCRs) for autoreactivity against the diverse self-antigens displayed by antigen-presenting cells (APCs). While CCR7 is known to promote thymocyte medullary entry and negative selection, our previous studies implicate CCR4 in these processes, raising the question of whether CCR4 and CCR7 play distinct or redundant roles in central tolerance. Here, synchronized positive selection assays, two-photon time-lapse microscopy, and quantification of TCR-signaled apoptotic thymocytes, demonstrate that CCR4 and CCR7 promote medullary accumulation and central tolerance of distinct post-positive selection thymocyte subsets in mice. CCR4 is upregulated within hours of positive selection signaling and promotes medullary entry and clonal deletion of immature post-positive selection thymocytes. In contrast, CCR7 is expressed several days later and is required for medullary localization and negative selection of mature thymocytes. In addition, CCR4 and CCR7 differentially enforce self-tolerance, with CCR4 enforcing tolerance to self-antigens presented by activated APCs, which express CCR4 ligands. Our findings show that CCR7 expression is not synonymous with medullary localization and support a revised model of central tolerance in which CCR4 and CCR7 promote early and late stages of negative selection, respectively, via interactions with distinct APC subsets.
-
-
-
Immunology and Microbiology
-
Mus musculus (Mouse)
Phosphoinositide acyl chain saturation drives CD8+ effector T cell signaling and function.
In Nat Immunol on 1 March 2023 by Edwards-Hicks, J., Apostolova, P., et al.
PubMed
How lipidome changes support CD8+ effector T (Teff) cell differentiation is not well understood. Here we show that, although naive T cells are rich in polyunsaturated phosphoinositides (PIPn with 3-4 double bonds), Teff cells have unique PIPn marked by saturated fatty acyl chains (0-2 double bonds). PIPn are precursors for second messengers. Polyunsaturated phosphatidylinositol bisphosphate (PIP2) exclusively supported signaling immediately upon T cell antigen receptor activation. In late Teff cells, activity of phospholipase C-γ1, the enzyme that cleaves PIP2 into downstream mediators, waned, and saturated PIPn became essential for sustained signaling. Saturated PIP was more rapidly converted to PIP2 with subsequent recruitment of phospholipase C-γ1, and loss of saturated PIPn impaired Teff cell fitness and function, even in cells with abundant polyunsaturated PIPn. Glucose was the substrate for de novo PIPn synthesis, and was rapidly utilized for saturated PIP2 generation. Thus, separate PIPn pools with distinct acyl chain compositions and metabolic dependencies drive important signaling events to initiate and then sustain effector function during CD8+ T cell differentiation.
-