InVivoMAb anti-mouse CD276 (B7-H3)
Product Description
Specifications
| Isotype | Rat IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG1 isotype control, anti-horseradish peroxidase |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse B7-H3 IgG2a fusion protein |
| Reported Applications |
in vivo B7-H3 blockade Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_10950149 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Flow Cytometry
Kamachi, F., et al (2015). "ICOS promotes group 2 innate lymphoid cell activation in lungs" Biochem Biophys Res Commun 463(4): 739-745.
PubMed
Group 2 innate lymphoid cells (ILC2s) are newly identified, potent producers of type 2 cytokines, such as IL-5 and IL-13, and contribute to the development of allergic lung inflammation induced by cysteine proteases. Although it has been shown that inducible costimulator (ICOS), a costimulatory molecule, is expressed on ILC2s, the role of ICOS in ILC2 responses is largely unknown. In the present study, we investigated whether the interaction of ICOS with its ligand B7-related protein-1 (B7RP-1) can promote ILC2 activation. Cytokine production in ILC2s purified from mouse lungs was significantly increased by coculture with B7RP-1-transfected cells, and increased cytokine production was inhibited by monoclonal antibody-mediated blocking of the ICOS/B7RP-1 interaction. ILC2 expansion and eosinophil influx induced by papain, a cysteine protease antigen, in mouse lungs were significantly abrogated by blocking the ICOS/B7RP-1 interaction. Dendritic cells (DCs) in the lungs expressed B7RP-1 and the number of DCs markedly increased with papain administration. B7RP-1 expression on lung DCs was reduced after papain administration. This downregulation of B7RP-1 expression may be an indication of ICOS/B7RP-1 binding. These results indicate that ILC2s might interact with B7RP-1-expressing DCs in allergic inflammatory lung, and ICOS signaling can positively regulate the protease allergen-induced ILC2 activation followed by eosinophil infiltration into the lungs.
in vivo B7-H3 blockade
Yamato, I., et al (2009). "Clinical importance of B7-H3 expression in human pancreatic cancer" Br J Cancer 101(10): 1709-1716.
PubMed
BACKGROUND: B7-H3 is a new member of the B7 ligand family and regulates T-cell responses in various conditions. However, the role of B7-H3 in tumour immunity is largely unknown. The purpose of this study was to evaluate the clinical significance of B7-H3 expression in human pancreatic cancer and the therapeutic potential for cancer immunotherapy. METHODS: We investigated B7-H3 expression in 59 patients with pancreatic cancer by immunohistochemistry and real-time PCR. Furthermore, we examined the anti-tumour effect of B7-H3-blocking monoclonal antibody in vivo in a murine pancreatic cancer model. RESULTS: Tumour-related B7-H3 expression was abundant in most human pancreatic cancer tissues and was significantly higher compared with that in non-cancer tissue or normal pancreas. Moreover, its expression was significantly more intense in cases with lymph node metastasis and advanced pathological stage. B7-H3 blockade promoted CD8(+) T-cell infiltration into the tumour and induced a substantial anti-tumour effect on murine pancreatic cancer. In addition, the combination of gemcitabine with B7-H3 blockade showed a synergistic anti-tumour effect without overt toxicity. CONCLUSION: Our data show for the first time that B7-H3 may have a critical role in pancreatic cancer and provide the rationale for developing a novel cancer immunotherapy against this fatal disease.
Flow Cytometry
Nagashima, O., et al (2008). "B7-H3 contributes to the development of pathogenic Th2 cells in a murine model of asthma" J Immunol 181(6): 4062-4071.
PubMed
B7-H3 is a new member of the B7 family. The receptor for B7-H3 has not been identified, but it seems to be expressed on activated T cells. Initial studies have shown that B7-H3 provides a stimulatory signal to T cells. However, recent studies suggest a negative regulatory role for B7-H3 in T cell responses. Thus, the immunological function of B7-H3 is controversial and unclear. In this study, we investigated the effects of neutralizing anti-B7-H3 mAb in a mouse model of allergic asthma to determine whether B7-H3 contributes to the development of pathogenic Th2 cells and pulmonary inflammation. Administration of anti-B7-H3 mAb significantly reduced airway hyperreactivity with a concomitant decrease in eosinophils in the lung as compared with control IgG-treated mice. Treatment with anti-B7-H3 mAb also resulted in decreased production of Th2 cytokines (IL-4, IL-5, and IL-13) in the draining lymph node cells. Although blockade of B7-H3 during the induction phase abrogated the development of asthmatic responses, B7-H3 blockade during the effector phase did not inhibit asthmatic responses. These results indicated an important role for B7-H3 in the development of pathogenic Th2 cells during the induction phase in a murine model of asthma.
Product Citations
-
-
In vivo experiments
-
Cancer Research
-
Immunology and Microbiology
Targeting B7-H3 inhibition-induced activation of fatty acid synthesis boosts anti-B7-H3 immunotherapy in triple-negative breast cancer.
In J Immunother Cancer on 12 April 2025 by Jiang, Y., Qian, Z., et al.
PubMed
Triple-negative breast cancer (TNBC) is the most malignant breast cancer, highlighting the need for effective immunotherapeutic targets. The immune checkpoint molecule B7-H3 has recently gained attention as a promising therapeutic target due to its pivotal role in promoting tumorigenesis and cancer progression. However, the therapeutic impact of B7-H3 inhibitors (B7-H3i) remains unclear.
-
-
-
Cancer Research
Altered Atlas of Exercise-Responsive MicroRNAs Revealing miR-29a-3p Attacks Armored and Cold Tumors and Boosts Anti-B7-H3 Therapy.
In Research (Wash D C) on 23 January 2025 by Mei, J., Luo, Z., et al.
PubMed
Increasing evidence has shown that physical exercise remarkably inhibits oncogenesis and progression of numerous cancers and exercise-responsive microRNAs (miRNAs) exert a marked role in exercise-mediated tumor suppression. In this research, expression and prognostic values of exercise-responsive miRNAs were examined in breast cancer (BRCA) and further pan-cancer types. In addition, multiple independent public and in-house cohorts, in vitro assays involving multiple, macrophages, fibroblasts, and tumor cells, and in vivo models were utilized to uncover the tumor-suppressive roles of miR-29a-3p in cancers. Here, we reported that miR-29a-3p was the exercise-responsive miRNA, which was lowly expressed in tumor tissues and associated with unfavorable prognosis in BRCA. Mechanistically, miR-29a-3p targeted macrophages, fibroblasts, and tumor cells to down-regulate B7 homolog 3 (B7-H3) expression. Single-cell RNA sequencing (scRNA-seq) and cytometry by time-of-flight (CyTOF) demonstrated that miR-29a-3p attacked the armored and cold tumors, thereby shaping an immuno-hot tumor microenvironment (TME). Translationally, liposomes were developed and loaded with miR-29a-3p (lipo@miR-29a-3p), and lipo@miR-29a-3p exhibited promising antitumor effects in a mouse model with great biocompatibility. In conclusion, we uncovered that miR-29a-3p is a critical exercise-responsive miRNA, which attacked armored and cold tumors by inhibiting B7-H3 expression. Thus, miR-29a-3p restoration could be an alternative strategy for antitumor therapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
CD276-dependent efferocytosis by tumor-associated macrophages promotes immune evasion in bladder cancer.
In Nat Commun on 1 April 2024 by Cheng, M., Chen, S., et al.
PubMed
Interplay between innate and adaptive immune cells is important for the antitumor immune response. However, the tumor microenvironment may turn immune suppressive, and tumor associated macrophages are playing a role in this transition. Here, we show that CD276, expressed on tumor-associated macrophages (TAM), play a role in diminishing the immune response against tumors. Using a model of tumors induced by N-butyl-N-(4-hydroxybutyl) nitrosamine in BLCA male mice we show that genetic ablation of CD276 in TAMs blocks efferocytosis and enhances the expression of the major histocompatibility complex class II (MHCII) of TAMs. This in turn increases CD4 + and cytotoxic CD8 + T cell infiltration of the tumor. Combined single cell RNA sequencing and functional experiments reveal that CD276 activates the lysosomal signaling pathway and the transcription factor JUN to regulate the expression of AXL and MerTK, resulting in enhanced efferocytosis in TAMs. Proving the principle, we show that simultaneous blockade of CD276 and PD-1 restrain tumor growth better than any of the components as a single intervention. Taken together, our study supports a role for CD276 in efferocytosis by TAMs, which is potentially targetable for combination immune therapy.
-
-
-
Cancer Research
High B7-H3 expression with low PD-L1 expression identifies armored-cold tumors in triple-negative breast cancer.
In NPJ Breast Cancer on 27 January 2024 by Mei, J., Cai, Y., et al.
PubMed
Triple-negative breast cancer (TNBC) is generally regarded as the most aggressive subtype among breast cancers, but exhibits higher chemotherapeutic and immunotherapeutic responses due to its unique immunogenicity. Thus, appropriate discrimination of subtypes is critical for guiding therapeutic options in clinical practice. In this research, using multiple in-house and public cohorts, we investigated the expression features and immuno-correlations of B7-H3 in breast cancer and checked the anti-tumor effect of the B7-H3 monoclonal antibody in a mouse model. We also developed a novel classifier combining B7-H3 and PD-L1 expression in TNBC. B7-H3 was revealed to be related to immuno-cold features and accumulated collagen in TNBC. In addition, targeting B7-H3 using the monoclonal antibody significantly suppressed mouse TNBC growth, reversed the armored-cold phenotype, and also boosted anti-PD-1 immunotherapy. In addition, patients with B7-H3 high and PD-L1 low expression showed the lowest anti-tumor immune infiltration, the highest collagen level, and the lowest therapeutic responses to multiple therapies, which mostly belong to armored-cold tumors. Overall, this research provides a novel subtyping strategy based on the combination of B7-H3/PD-L1 expression, which leads to a novel approach for the management of TNBC.
-
-
-
Cancer Research
The anti-B7-H3 blocking antibody MJ18 does not recognize B7-H3 in murine tumor models
In bioRxiv on 17 November 2023 by Nammor, T., Frizzell, J., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
Immune checkpoint B7-H3 is a therapeutic vulnerability in prostate cancer harboring PTEN and TP53 deficiencies.
In Sci Transl Med on 10 May 2023 by Shi, W., Wang, Y., et al.
PubMed
Checkpoint immunotherapy has yielded meaningful responses across many cancers but has shown modest efficacy in advanced prostate cancer. B7 homolog 3 protein (B7-H3/CD276) is an immune checkpoint molecule and has emerged as a promising therapeutic target. However, much remains to be understood regarding B7-H3's role in cancer progression, predictive biomarkers for B7-H3-targeted therapy, and combinatorial strategies. Our multi-omics analyses identified B7-H3 as one of the most abundant immune checkpoints in prostate tumors containing PTEN and TP53 genetic inactivation. Here, we sought in vivo genetic evidence for, and mechanistic understanding of, the role of B7-H3 in PTEN/TP53-deficient prostate cancer. We found that loss of PTEN and TP53 induced B7-H3 expression by activating transcriptional factor Sp1. Prostate-specific deletion of Cd276 resulted in delayed tumor progression and reversed the suppression of tumor-infiltrating T cells and NK cells in Pten/Trp53 genetically engineered mouse models. Furthermore, we tested the efficacy of the B7-H3 inhibitor in preclinical models of castration-resistant prostate cancer (CRPC). We demonstrated that enriched regulatory T cells and elevated programmed cell death ligand 1 (PD-L1) in myeloid cells hinder the therapeutic efficacy of B7-H3 inhibition in prostate tumors. Last, we showed that B7-H3 inhibition combined with blockade of PD-L1 or cytotoxic T lymphocyte-associated protein 4 (CTLA-4) achieved durable antitumor effects and had curative potential in a PTEN/TP53-deficient CRPC model. Given that B7-H3-targeted therapies have been evaluated in early clinical trials, our studies provide insights into the potential of biomarker-driven combinatorial immunotherapy targeting B7-H3 in prostate cancer, among other malignancies.
-
-
-
Cancer Research
-
Immunology and Microbiology
mTORC1 upregulates B7-H3/CD276 to inhibit antitumor T cells and drive tumor immune evasion.
In Nat Commun on 3 March 2023 by Liu, H. J., Du, H., et al.
PubMed
Identifying the mechanisms underlying the regulation of immune checkpoint molecules and the therapeutic impact of targeting them in cancer is critical. Here we show that high expression of the immune checkpoint B7-H3 (CD276) and high mTORC1 activity correlate with immunosuppressive phenotypes and worse clinical outcomes in 11,060 TCGA human tumors. We find that mTORC1 upregulates B7-H3 expression via direct phosphorylation of the transcription factor YY2 by p70 S6 kinase. Inhibition of B7-H3 suppresses mTORC1-hyperactive tumor growth via an immune-mediated mechanism involving increased T-cell activity and IFN-γ responses coupled with increased tumor cell expression of MHC-II. CITE-seq reveals strikingly increased cytotoxic CD38+CD39+CD4+ T cells in B7-H3-deficient tumors. In pan-human cancers, a high cytotoxic CD38+CD39+CD4+ T-cell gene signature correlates with better clinical prognosis. These results show that mTORC1-hyperactivity, present in many human tumors including tuberous sclerosis complex (TSC) and lymphangioleiomyomatosis (LAM), drives B7-H3 expression leading to suppression of cytotoxic CD4+ T cells.
-
-
-
In vivo experiments
-
Cardiovascular biology
-
Immunology and Microbiology
B7H3-dependent myeloid-derived suppressor cell recruitment and activation in pulmonary fibrosis.
In Front Immunol on 2 September 2022 by Liu, T., Gonzalez De Los Santos, F., et al.
PubMed
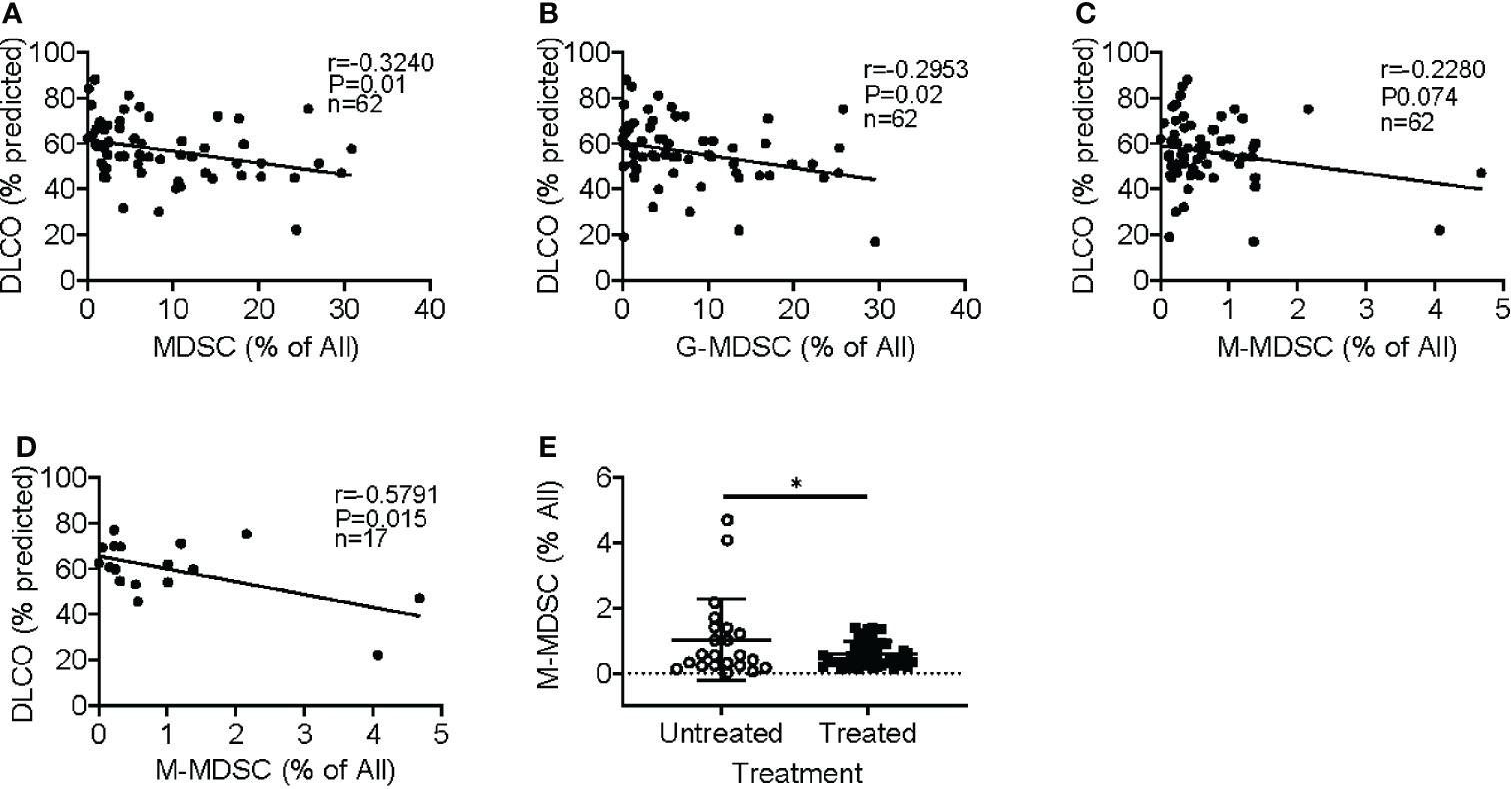
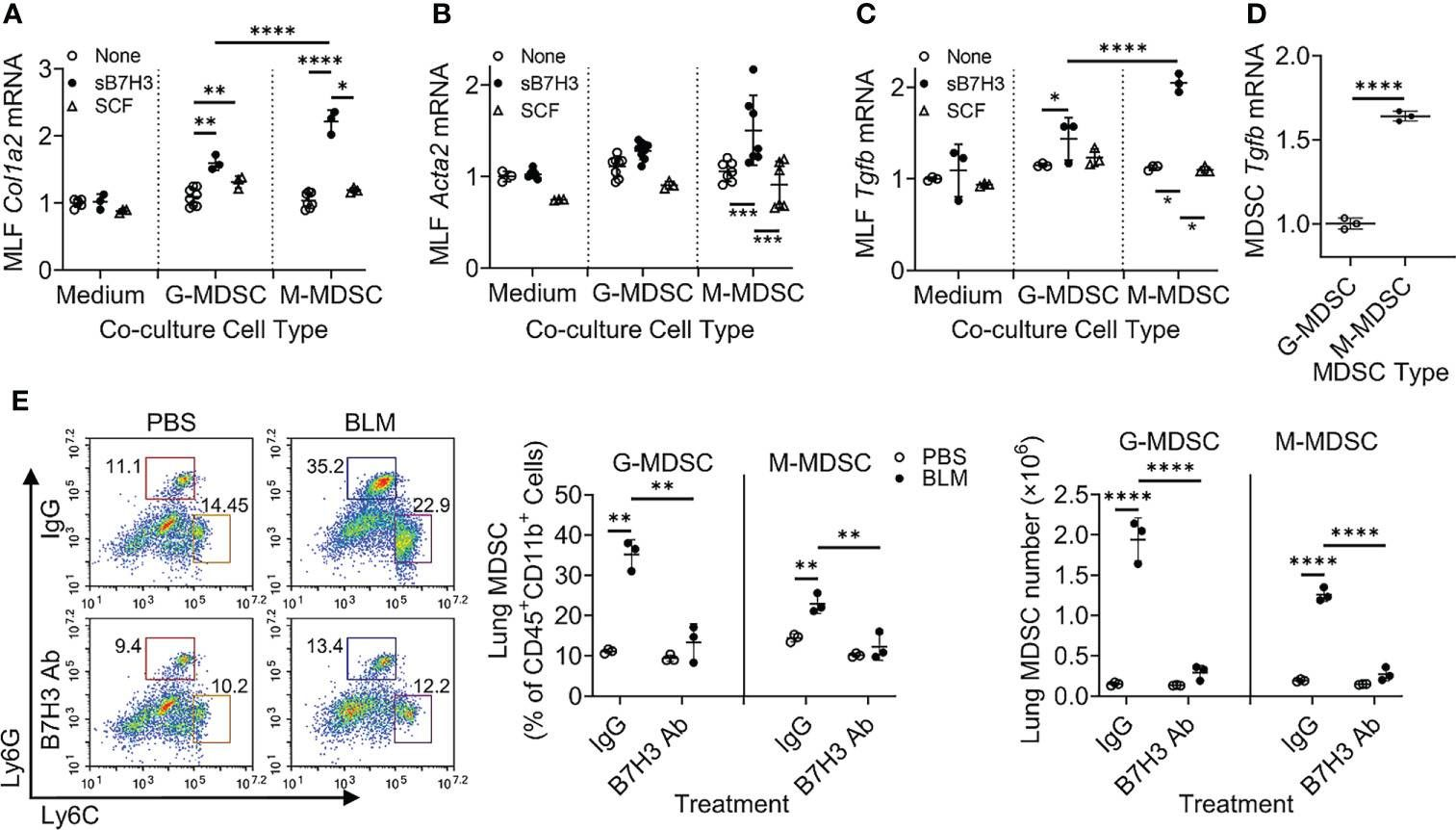
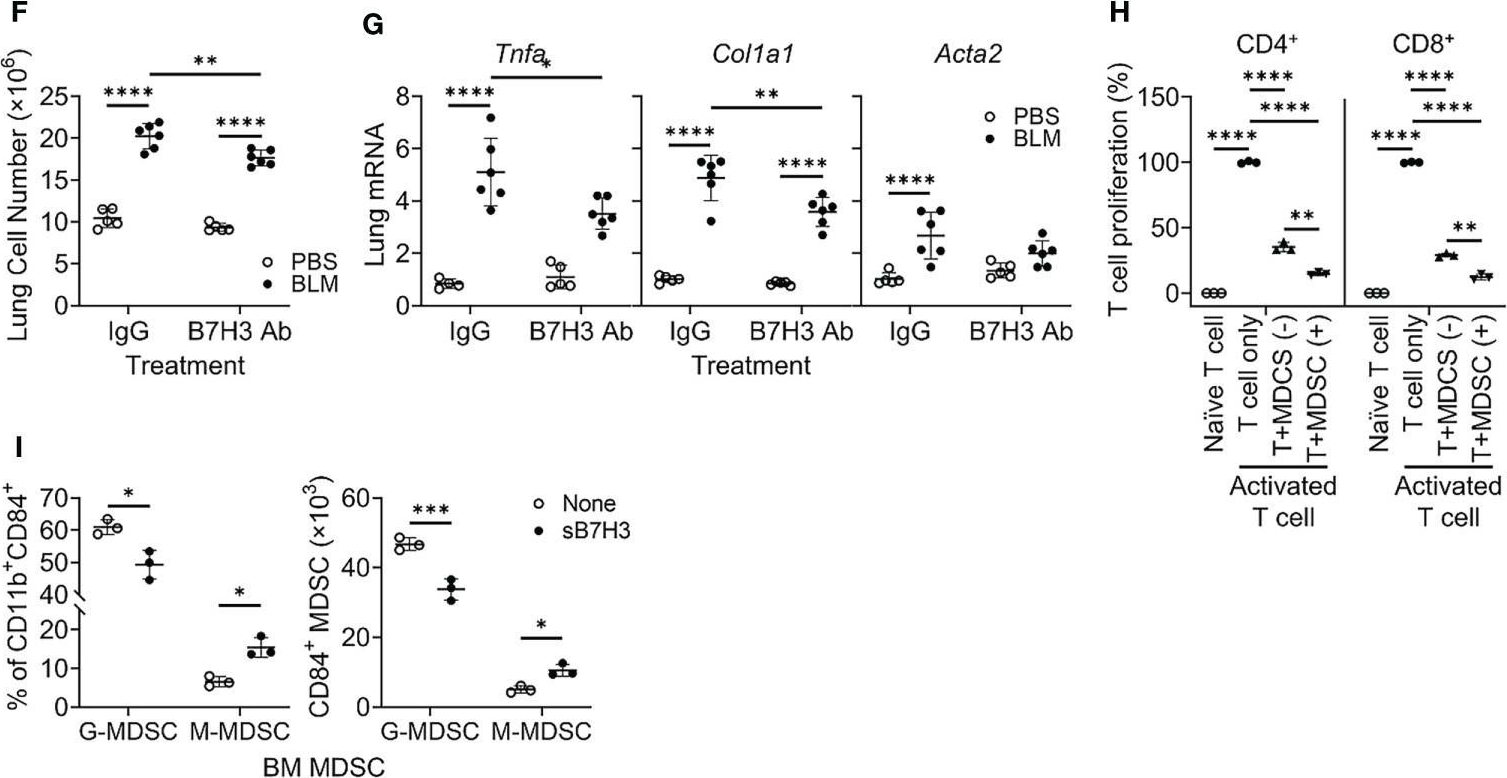
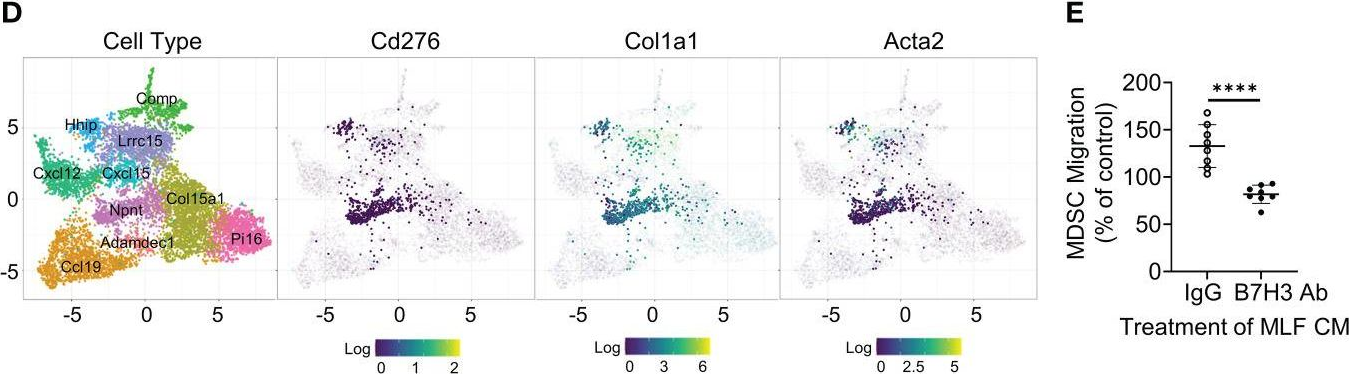
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic lung disease without effective curative therapy. Recent evidence shows increased circulating myeloid-derived suppressor cells (MDSCs) in cancer, inflammation, and fibrosis, with some of these cells expressing B7H3. We sought to investigate the role of MDSCs in IPF and its potential mediation via B7H3. Here we prospectively collected peripheral blood samples from IPF patients to analyze for circulating MDSCs and B7H3 expression to assess their clinical significance and potential impact on co-cultured lung fibroblasts and T-cell activation. In parallel, we assess MDSC recruitment and potential B7H3 dependence in a mouse model of pulmonary fibrosis. Expansion of MDSCs in IPF patients correlated with disease severity. Co-culture of soluble B7H3 (sB7H3)-treated mouse monocytic MDSCs (M-MDSCs), but not granulocytic MDSCs (G-MDSCs), activated lung fibroblasts and myofibroblast differentiation. Additionally, sB7H3 significantly enhanced MDSC suppression of T-cell proliferation. Activated M-MDSCs displayed elevated TGFβ and Arg1 expression relative to that in G-MDSCs. Treatment with anti-B7H3 antibodies inhibited bone marrow-derived MDSC recruitment into the bleomycin-injured lung, accompanied by reduced expression of inflammation and fibrosis markers. Selective telomerase reverse transcriptase (TERT) deficiency in myeloid cells also diminished MDSC recruitment associated with the reduced plasma level of sB7H3, lung recruitment of c-Kit+ hematopoietic progenitors, myofibroblast differentiation, and fibrosis. Lung single-cell RNA sequencing (scRNA-seq) revealed fibroblasts as a predominant potential source of sB7H3, and indeed the conditioned medium from activated mouse lung fibroblasts had a chemotactic effect on bone marrow (BM)-MDSC, which was abolished by B7H3 blocking antibody. Thus, in addition to their immunosuppressive activity, TERT and B7H3-dependent MDSC expansion/recruitment from BM could play a paracrine role to activate myofibroblast differentiation during pulmonary fibrosis with potential significance for disease progression mediated by sB7H3.
-
-
-
Flow cytometry/Cell sorting
-
Cancer Research
Elimination of acquired resistance to PD-1 blockade via the concurrent depletion of tumour cells and immunosuppressive cells.
In Nat Biomed Eng on 1 November 2021 by Xue, G., Wang, Z., et al.
PubMed
Antigen release resulting from the death of tumour cells induced by chemotherapies and targeted therapies can augment the antitumour responses induced by immune checkpoint blockade (ICB). However, tumours responding to ICB therapies often become resistant to them. Here we show that the specific targeting of tumour cells promotes the growth of tumour-cell variants that are resistant to ICB, and that the acquired resistance can be overcome via the concurrent depletion of tumour cells and of major types of immunosuppressive cell via a monoclonal antibody binding the enzyme CD73, which we identified as highly expressed on tumour cells and on regulatory T cells, myeloid-derived suppressor cells and tumour-associated macrophages, but not on cytolytic T lymphocytes, natural killer cells and dendritic cells. In mice with murine tumours, the systemic administration of anti-PD1 antibodies and anti-CD73 antibodies conjugated to a near-infrared dye prevented near-infrared-irradiated tumours from acquiring resistance to ICB and resulted in the eradication of advanced tumours. The elimination of immunosuppressive cells may overcome acquired resistance to ICB across a range of tumour types and combination therapies.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
-
In vivo experiments
CD276 expression enables squamous cell carcinoma stem cells to evade immune surveillance.
In Cell Stem Cell on 2 September 2021 by Wang, C., Li, Y., et al.
PubMed
Immunosurveillance is a critical mechanism guarding against tumor development and progression. Checkpoint inhibitors have shown significant success in cancer treatment, but expression of key factors such as PD-L1 in putative cancer stem cell (CSC) populations in squamous cell carcinoma has been inconclusive, suggesting that CSCs may have developed other mechanisms to escape immune surveillance. Here we show that CSCs upregulate the immune checkpoint molecule CD276 (B7-H3) to evade host immune responses. CD276 is highly expressed by CSCs in mouse and human head and neck squamous cell carcinoma (HNSCC) and can be used to prospectively isolate tumorigenic CSCs. Anti-CD276 antibodies eliminate CSCs in a CD8+ T cell-dependent manner, inhibiting tumor growth and lymph node metastases in a mouse HNSCC model. Single-cell RNA sequencing (RNA-seq) showed that CD276 blockade remodels SCC heterogeneity and reduces epithelial-mesenchymal transition. These results show that CSCs utilize CD276 for immune escape and suggest that targeting CD276 may reduce CSCs in HNSCC.
-