InVivoMAb anti-mouse CD132 (common γ chain)
Product Description
Specifications
| Isotype | Rat IgG2b |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2b isotype control, anti-keyhole limpet hemocyanin |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Rat myeloma YB2/0 transfected with mouse cytoplasmic-tailless CD132 |
| Reported Applications |
in vivo γc blockade Functional assays Immunoprecipitation Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687794 |
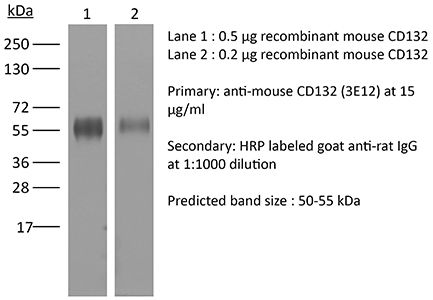
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo γc blockade
Li, X. C., et al (2001). "IL-15 and IL-2: a matter of life and death for T cells in vivo" Nat Med 7(1): 114-118.
PubMed
Interleukin (IL)-2 and IL-15 are redundant in stimulating T-cell proliferation in vitro. Their precise role in vivo in governing T-cell expansion and T-cell homeostasis is less clear. Each may have distinct functions and regulate distinct aspects of T-cell activation. The functional receptors for IL-2 and IL-15 consist of a private alpha-chain, which defines the binding specificity for IL-2 or IL-15, and shared IL-2 receptor beta- and gamma-chains. The gamma-chain is also a critical signaling component of IL-4, IL-7 and IL-9 receptors. Thus, the gamma-chain is called the common gamma or gamma-c. As these receptor subunits can be expressed individually or in various combinations resulting in the formation of receptors with different affinities, distinct signaling capabilities or both, we hypothesized that differential expression of IL-2 and IL-15 receptor subunits on cycling T cells in vivo may direct activated T cells to respond to IL-2 or IL-15, thereby regulating the homeostasis of T-cell response in vivo. By observing in vivo T-cell divisions and expression of IL-2 and IL-15 receptor subunits, we demonstrate that IL-15 is a critical growth factor in initiating T cell divisions in vivo, whereas IL-2 limits continued T-cell expansion via downregulation of the gamma-c expression. Decreased gamma-c expression on cycling T cells reduced sustained Bcl-2 expression and rendered cells susceptible to apoptotic cell death. Our study provides data that IL-2 and IL-15 regulate distinct aspects of primary T-cell expansion in vivo.
in vivo γc blockade
Malek, T. R., et al (1998). "Monoclonal antibodies to the common gamma-chain as cytokine receptor antagonists in vivo: effect on intrathymic and intestinal intraepithelial T lymphocyte development" J Leukoc Biol 63(6): 643-649.
PubMed
Mice lacking a functional gamma c subunit of cytokine receptors exhibit profound defects in the development of multiple lymphoid lineages. To investigate the role of gamma c-dependent cytokines in T cell development, the phenotype of developing T cells was compared in interleukin (IL)-7Ralpha-deficient mice and anti-gamma c mAb-treated chimeric mice reconstituted with adult bone marrow cells or subsets of pro-T cells. These studies indicate that gamma c contributes to T cell development at multiple stages of pro-T cell maturation and that IL-7/IL-7R is the primary cytokine for thymic-dependent T cell development. However, our data also implicate other gamma c-dependent cytokines during thymic T cell development. By contrast, substantial intestinal intraepithelial lymphocytes (IEL) development was observed in the intestinal intraepithelium in both types of mice. Analysis of IL-7Ralpha-deficient mice indicates that the IL-7/IL-7R system is critical only for the development of TCR gammadelta+ IEL. However, the inhibitory activity of the anti-deltac mAb in the chimeric mouse model suggests that additional gamma cutilizing cytokines regulate the development of the remaining subsets of IEL.
in vivo γc blockade
He, Y. W., et al (1995). "Expression and function of the gamma c subunit of the IL-2, IL-4, and IL-7 receptors. Distinct interaction of gamma c in the IL-4 receptor" J Immunol 154(4): 1596-1605.
PubMed
IL-2R, IL-4R, and IL-7R share a common subunit referred to as gamma c and the IL-13R has been proposed to contain gamma c as a subunit. In this report we have used two novel mAbs (3E12 and 4G3) to distinct epitopes of mouse gamma c to determine its lymphoid cell distribution and to examine whether gamma c uses similar epitopes to interact with different cytokines and cytokine receptors. FACS analysis revealed that gamma c is expressed in most lymphocytes, myeloid cells, embryonic thymocytes, and lymphoid cell lines. Results from radiolabeled ligand binding studies, biochemical analysis of ligand-receptor cross-linked complexes, and cytokine bioassays indicate that the epitope defined by mAb 4G3 closely defines the IL-7 binding region of gamma c and overlaps the IL-2 binding region of gamma c. These studies also indicate that gamma c interacts with IL-4 in the context of the IL-4R in a manner that is distinct from its role in the IL-2R and IL-7R and suggest that the 3E12 epitope defines a region of gamma c that intimately interacts with the IL-4R. The B9 plasmacytoma, which proliferates in response to IL-4 and IL-13, was shown to not express gamma c. Thus, at least in some circumstances, gamma c is dispensable for signaling via the IL-4R and is not a required subunit of the IL-13R.
Functional Assays
Flow Cytometry
He, Y. W., et al (1995). "Blockade of T- and B-lymphocyte development by antibody to the gamma c subunit of the receptors for interleukins 2, 4, and 7" Proc Natl Acad Sci U S A 92(12): 5689-5693.
PubMed
Cytokines are important regulators of hematopoesis. Mutations in gamma c, which is a subunit shared by the receptors for interleukin (IL) 2, IL-4, and IL-7, have been causally associated with human X chromosome-linked severe combined immunodeficiency disease. This finding indicates a mandatory role for cytokine receptor signaling at one or more stages of lymphocyte development. To evaluate the cellular level at which gamma c is critical for lymphopoiesis, the effect of monoclonal antibodies to gamma c on the capacity of syngeneic bone marrow cells to reconstitute the hematopoietic compartment of lethally irradiated recipient mice was examined. We show that monoclonal antibody to gamma c blocked lymphocyte development at or before the appearance of pro-B cells and prior to or at the seeding of the thymus by precursor cells while erythromyeloid cell development was normal. These results suggest that one level of lymphocyte development that requires gamma c is a point in hematopoietic cell differentiation near the divergence of lymphopoiesis and erythromyelopoesis.
Product Citations
-
-
Immunology and Microbiology
Monocyte/macrophage-derived interleukin-15 mediates the pro-inflammatory phenotype of CD226+ B cells in type 1 diabetes.
In EBioMedicine on 1 October 2025 by Li, J., Liang, X., et al.
PubMed
Type 1 diabetes (T1D) is characterised by the autoimmune-mediated destruction of pancreatic β-cells. Although traditionally viewed as a disease dominated by T cells, recent studies have emphasised the crucial role of B cells in the development of T1D. Genome-wide association studies (GWAS) have revealed that CD226 is related to susceptibility to several autoimmune diseases, including T1D. Our recent work identified a pathogenic role of CD226+ CD8+ T cells in T1D. However, the involvement of CD226+ B cells in T1D development remains unclear.
-
-
-
Immunology and Microbiology
-
Cancer Research
PGE2 limits effector expansion of tumour-infiltrating stem-like CD8+ T cells.
In Nature on 1 May 2024 by Lacher, S. B., Dörr, J., et al.
PubMed
Cancer-specific TCF1+ stem-like CD8+ T cells can drive protective anticancer immunity through expansion and effector cell differentiation1-4; however, this response is dysfunctional in tumours. Current cancer immunotherapies2,5-9 can promote anticancer responses through TCF1+ stem-like CD8+ T cells in some but not all patients. This variation points towards currently ill-defined mechanisms that limit TCF1+CD8+ T cell-mediated anticancer immunity. Here we demonstrate that tumour-derived prostaglandin E2 (PGE2) restricts the proliferative expansion and effector differentiation of TCF1+CD8+ T cells within tumours, which promotes cancer immune escape. PGE2 does not affect the priming of TCF1+CD8+ T cells in draining lymph nodes. PGE2 acts through EP2 and EP4 (EP2/EP4) receptor signalling in CD8+ T cells to limit the intratumoural generation of early and late effector T cell populations that originate from TCF1+ tumour-infiltrating CD8+ T lymphocytes (TILs). Ablation of EP2/EP4 signalling in cancer-specific CD8+ T cells rescues their expansion and effector differentiation within tumours and leads to tumour elimination in multiple mouse cancer models. Mechanistically, suppression of the interleukin-2 (IL-2) signalling pathway underlies the PGE2-mediated inhibition of TCF1+ TIL responses. Altogether, we uncover a key mechanism that restricts the IL-2 responsiveness of TCF1+ TILs and prevents anticancer T cell responses that originate from these cells. This study identifies the PGE2-EP2/EP4 axis as a molecular target to restore IL-2 responsiveness in anticancer TILs to achieve cancer immune control.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Impact of MyD88, Microbiota, and Location on Type 1 and Type 3 Innate Lymphoid Cells during Toxoplasma gondii Infection.
In Immunohorizons on 12 September 2022 by Snyder, L. M., Belmares-Ortega, J., et al.
PubMed
Toxoplasma gondii induces strong IFN-γ-based immunity. Innate lymphoid cells (ILC), in particular ILC1, are an important innate source of this protective cytokine during infection. Our objective was to determine how MyD88-dependent signaling influences ILC function during peroral compared with i.p. infection with T. gondii. MyD88+/+ and MyD88-/- mice were orally inoculated with ME49 cysts, and small intestinal lamina propria ILC were assessed using flow cytometry. We observed T-bet+ ILC1, retinoic acid-related orphan receptor γt+ ILC3, and a population of T-bet+retinoic acid-related orphan receptor γt+ double-positive ILC. In MyD88-/- mice, IFN-γ-producing T-bet+ ILC1 frequencies were reduced compared with wild-type. Treatment of MyD88-/- mice with an antibiotic mixture to deplete microflora reduced IFN-γ+ ILC1 frequencies. To examine ILC responses outside of the mucosal immune system, peritoneal exudate cells were collected from wild-type and knockout mice after i.p. inoculation with ME49 cysts. In this compartment, ILC were highly polarized to the ILC1 subset that increased significantly and became highly positive for IFN-γ over the course of infection. Increased ILC1 was associated with expression of the Ki67 cell proliferation marker, and the response was driven by IL-12p40. In the absence of MyD88, IFN-γ expression by ILC1 was not maintained, but proliferation remained normal. Collectively, these data reveal new aspects of ILC function that are influenced by location of infection and shaped further by MyD88-dependent signaling.
-