InVivoMAb anti-human/rat HER2 (neu)
Product Description
Specifications
| Isotype | Mouse IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG2a isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | neu-transfected NIH 3T3 cells |
| Reported Applications |
in vivo HER2/neu inhibition in vitro HER2/neu inhibition Immunoprecipitation Immunofluorescence Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687800 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo HER2/neu inhibition
Kodumudi, K. N., et al (2019). "Sequential Anti-PD1 Therapy Following Dendritic Cell Vaccination Improves Survival in a HER2 Mammary Carcinoma Model and Identifies a Critical Role for CD4 T Cells in Mediating the Response" Front Immunol 10: 1939.
PubMed
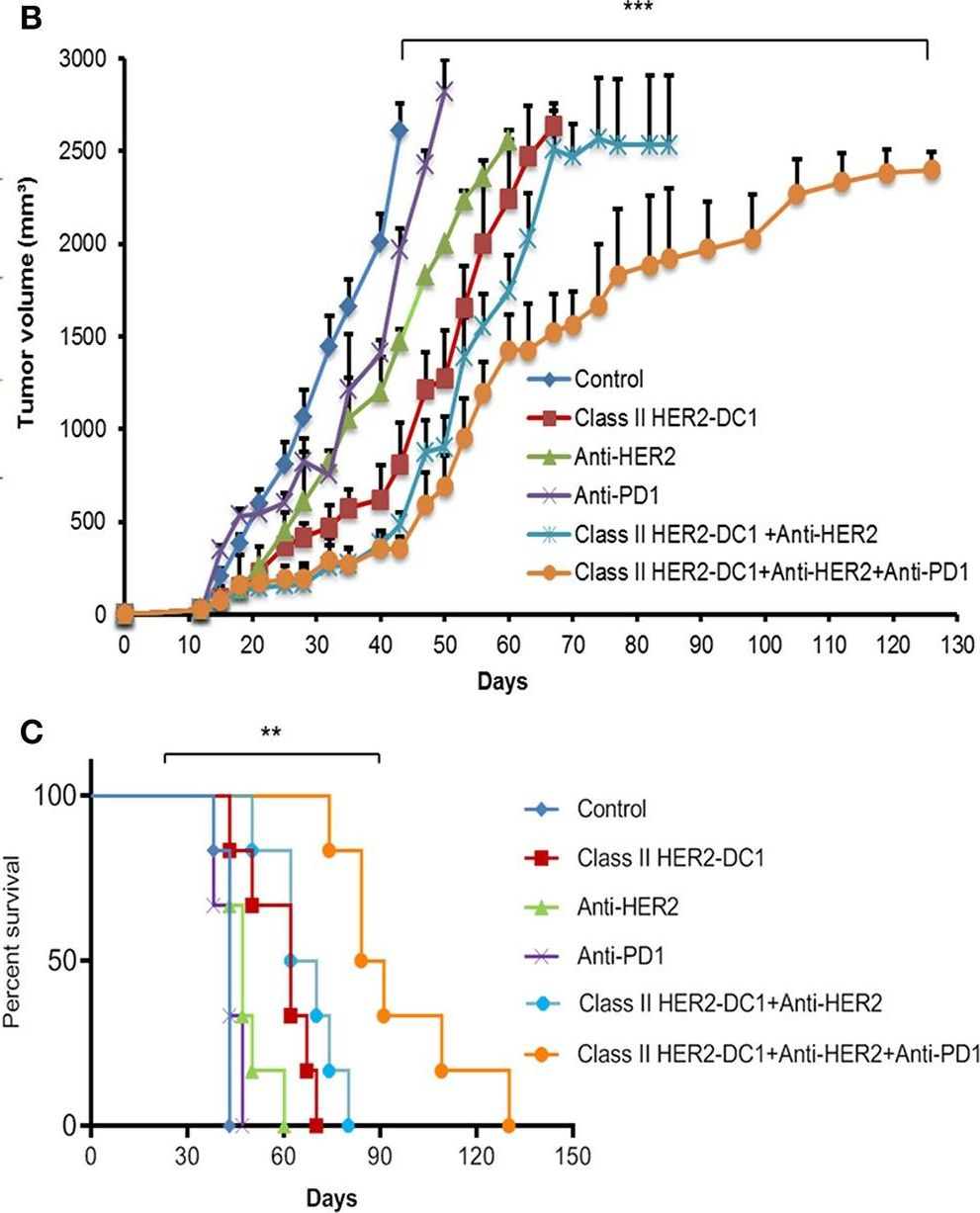
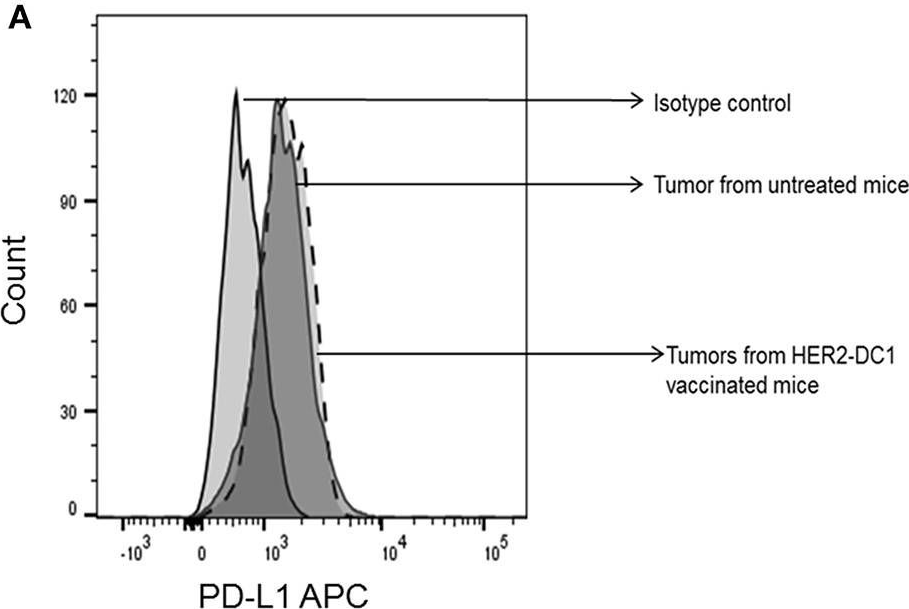
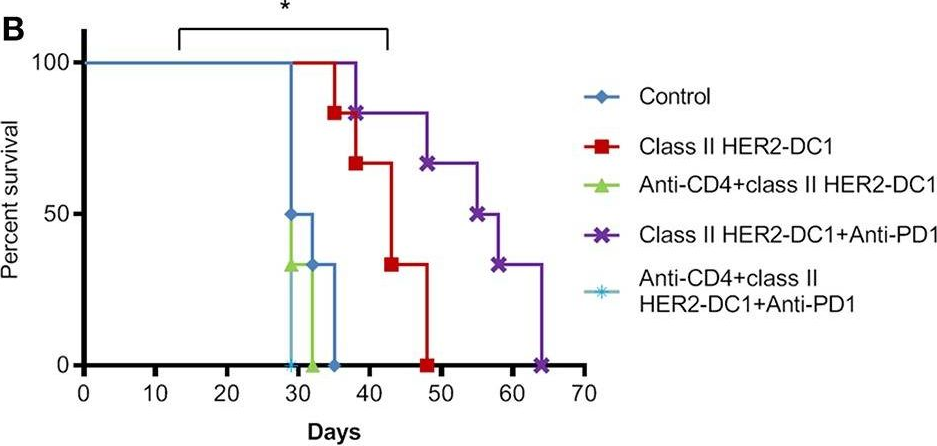
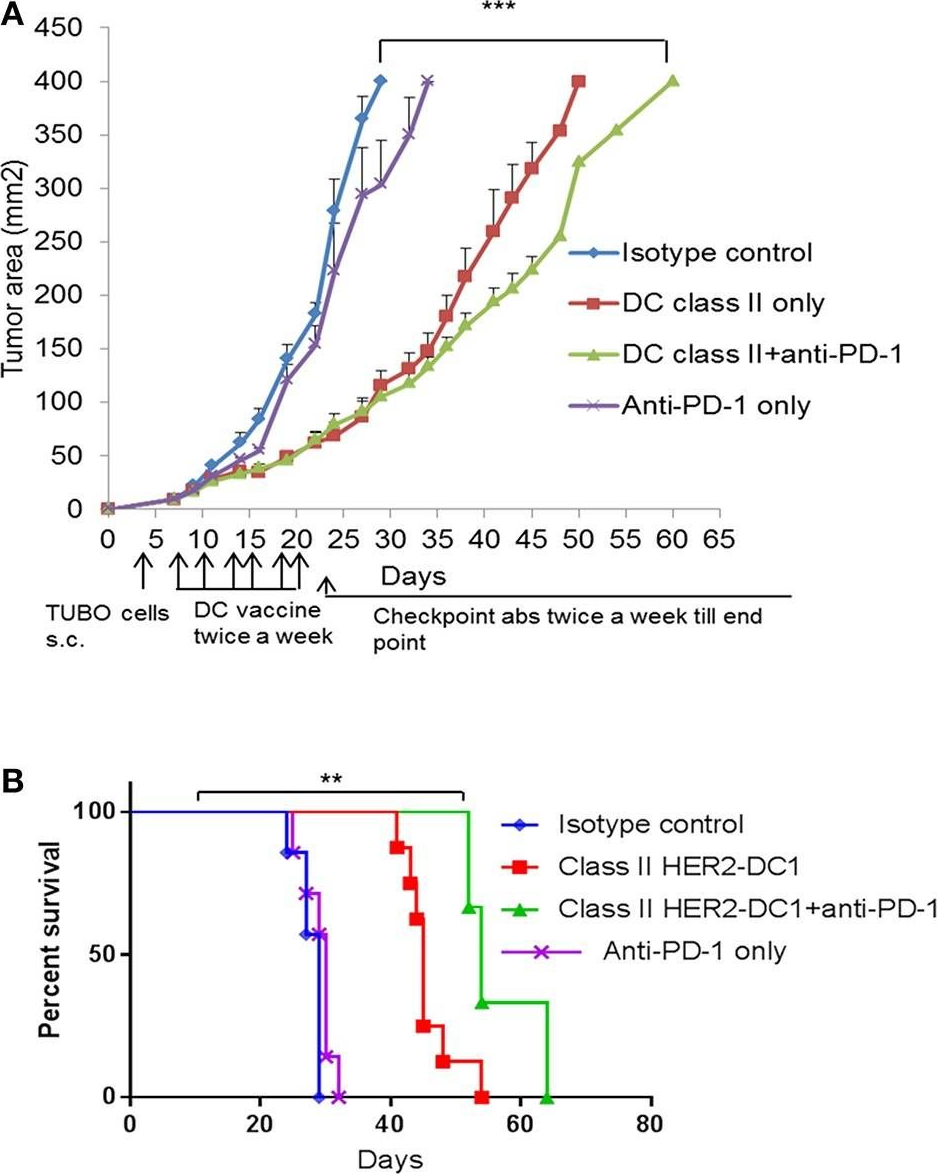
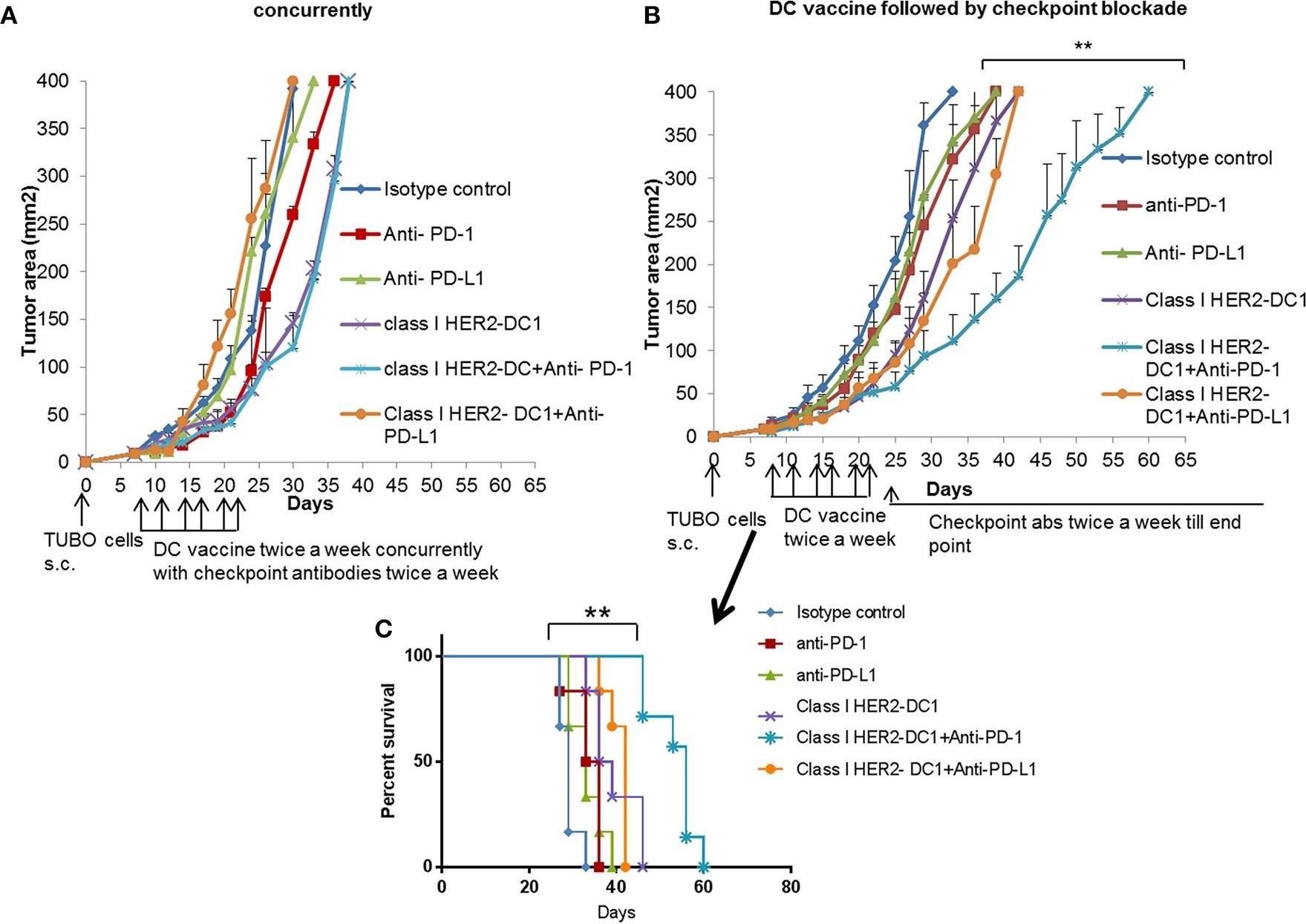
Patients with metastatic HER2 breast cancer (MBC) often become resistant to HER 2 targeted therapy and have recurrence of disease. The Panacea trial suggested that HER2 MBC patients were more likely to respond to checkpoint therapy if TIL were present or if tumor expressed PD-L1. We assessed whether type I polarized dendritic cells (DC1) could improve checkpoint therapy in a preclinical model of HER2(+) breast cancer. TUBO bearing mice were vaccinated with either MHC class I or class II HER2 peptide pulsed DC1 (class I or class II HER2-DC1) concurrently or sequentially with administration of anti-PD-1 or anti-PDL1. Infiltration of tumors by immune cells, induction of anti-HER2 immunity and response to therapy was evaluated. Class I or class II HER2-DC1 vaccinated mice generated anti-HER2 CD8 or CD4+ T cell immune responses and demonstrated delayed tumor growth. Combining both MHC class I and II HER2-pulsed DC1 did not further result in inhibition of tumor growth or enhanced survival compared to individual administration. Interestingly class II HER2-DC1 led to both increased CD4 and CD8 T cells in the tumor microenvironment while class I peptides typically resulted in only increased CD8 T cells. Anti-PD-1 but not anti-PD-L1 administered sequentially with class I or class II HER2-DC1 vaccine could improve the efficacy of HER2-DC1 vaccine as measured by tumor growth, survival, infiltration of tumors by T cells and increase in systemic anti-HER2 immune responses. Depletion of CD4+ T cells abrogated the anti-tumor efficacy of combination therapy with class II HER2-DC1 and anti-PD-1, suggesting that tumor regression was CD4 dependent. Since class II HER2-DC1 was as effective as class I, we combined class II HER2-DC1 vaccine with anti-rat neu antibodies and anti-PD-1 therapy. Combination therapy demonstrated further delay in tumor growth, and enhanced survival compared to control mice. In summary, Class II HER2-DC1 drives both a CD4 and CD8 T cell tumor infiltration that leads to increased survival, and in combination with anti-HER2 therapy and checkpoint blockade can improve survival in preclinical models of HER2 positive breast cancer and warrants exploration in patients with HER2 MBC.
in vivo HER2/neu inhibition
Wang, Q., et al (2019). "Single-cell profiling guided combinatorial immunotherapy for fast-evolving CDK4/6 inhibitor-resistant HER2-positive breast cancer" Nat Commun 10(1): 3817.
PubMed
Acquired resistance to targeted cancer therapy is a significant clinical challenge. In parallel with clinical trials combining CDK4/6 inhibitors to treat HER2+ breast cancer, we sought to prospectively model tumor evolution in response to this regimen in vivo and identify a clinically actionable strategy to combat drug resistance. Despite a promising initial response, acquired resistance emerges rapidly to the combination of anti-HER2/neu antibody and CDK4/6 inhibitor Palbociclib. Using high-throughput single-cell profiling over the course of treatments, we reveal a distinct immunosuppressive immature myeloid cell (IMC) population to infiltrate the resistant tumors. Guided by single-cell transcriptome analysis, we demonstrate that combination of IMC-targeting tyrosine kinase inhibitor cabozantinib and immune checkpoint blockade enhances anti-tumor immunity, and overcomes the resistance. Furthermore, sequential combinatorial immunotherapy enables a sustained control of the fast-evolving CDK4/6 inhibitor-resistant tumors. Our study demonstrates a translational framework for treating rapidly evolving tumors through preclinical modeling and single-cell analyses.
in vivo HER2/neu inhibition
Park, S., et al (2010). "The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity" Cancer Cell 18(2): 160-170.
PubMed
Anti-HER2/neu antibody therapy is reported to mediate tumor regression by interrupting oncogenic signals and/or inducing FcR-mediated cytotoxicity. Here, we demonstrate that the mechanisms of tumor regression by this therapy also require the adaptive immune response. Activation of innate immunity and T cells, initiated by antibody treatment, was necessary. Intriguingly, the addition of chemotherapeutic drugs, although capable of enhancing the reduction of tumor burden, could abrogate antibody-initiated immunity leading to decreased resistance to rechallenge or earlier relapse. Increased influx of both innate and adaptive immune cells into the tumor microenvironment by a selected immunotherapy further enhanced subsequent antibody-induced immunity, leading to increased tumor eradication and resistance to rechallenge. This study proposes a model and strategy for anti-HER2/neu antibody-mediated tumor clearance.
in vivo HER2/neu inhibition
in vitro HER2/neu inhibition
Knutson, K. L., et al (2004). "Neu antigen-negative variants can be generated after neu-specific antibody therapy in neu transgenic mice" Cancer Res 64(3): 1146-1151.
PubMed
Prolonged administration of HER-2/neu-specific monoclonal antibody therapy is now widely used for the treatment of HER-2/neu-overexpressing tumors in advanced-stage breast cancer patients. Monoclonal antibody therapy has the potential to promote reduced tumor expression of HER-2/neu by receptor down-modulation and/or the generation of antigen-negative variants. Loss of antigen by either mechanism could potentially impact subsequent therapeutic strategies targeting HER-2/neu. In this study, the effects of chronic neu-specific monoclonal antibody therapy on tumor growth and neu protein expression were examined in a murine model of neu-overexpressing breast cancer. Treatment of neu-overexpressing tumors with neu-specific antibody, in vitro or in vivo, resulted in significant tumor growth inhibition. When neu antibody was used to treat neu-overexpressing tumor cells both in vitro and in vivo in tumor-bearing mice, neu receptor expression was not diminished after cessation of therapy. However, in the setting of clinically undetectable disease in a fraction of animals, antigen-negative variants were generated. An understanding of the effects of monoclonal antibodies on target antigen expression is critical for the future design and testing of novel HER-2/neu-targeted therapies administered in combination with or after HER-2/neu-specific monoclonal antibody therapy.
in vitro HER2/neu inhibition
Flow Cytometry
Immunoprecipitation
Zhang, H., et al (1999). "Shared antigenic epitopes and pathobiological functions of anti-p185(her2/neu) monoclonal antibodies" Exp Mol Pathol 67(1): 15-25.
PubMed
We have studied two anti-p185 antibodies: the monoclonal antibody 7. 16.4 and rhuMAb 4D5, which were raised against the the ectodomain of rat (p185(neu)), and the human (p185(her2/neu)) homolog, respectively. Studies on the structure of these two antibodies indicate that they share structural similarity in the variable region, especially the CDR3 region, which determines the antibody-antigen interaction. Further studies by flow cytometry revealed that 7.16.4 can compete with rhuMAb4D5 for binding to the cell surface p185(her2/neu), suggesting that these two antibodies share an epitope on the p185 receptor. Furthermore, 7.16.4 can also inhibit proliferation and transformation caused by p185(her2/neu). Moreover the rhuMAb 4D5 binds to the rat p185(neu). With the observation that 7.16.4 positively stains human breast cancer tissues that overexpress p185(her2/neu), 7.16.4 may be useful for the pathological diagnosis and therapy of human tumors.
in vivo HER2/neu inhibition
Drebin, J. A., et al (1986). "Inhibition of tumor growth by a monoclonal antibody reactive with an oncogene-encoded tumor antigen" Proc Natl Acad Sci U S A 83(23): 9129-9133.
PubMed
The neu oncogene encodes a 185-kDa transmembrane glycoprotein tumor antigen, termed p185. We have recently described a monoclonal antibody reactive with a cell surface domain of the p185 molecule. In vivo treatment with this anti-p185 monoclonal antibody was able to significantly inhibit the tumorigenic growth of neu-transformed NIH 3T3 cells implanted into nude mice. Such treatment had no effect on the tumorigenic growth of Ha-ras-transformed NIH 3T3 cells. Furthermore, anti-p185 antibody treatment was able to inhibit the growth of the rat neuroblastoma cells from which the neu oncogene was initially isolated. These results demonstrate that a monoclonal antibody reactive with the extracellular domain of an oncogene-encoded protein can exert a significant antitumor effect; such antibodies may prove useful in the therapy of certain malignancies.
Flow Cytometry
Immunoprecipitation
Immunofluorescence
Drebin, J. A., et al (1984). "Monoclonal antibodies identify a cell-surface antigen associated with an activated cellular oncogene" Nature 312(5994): 545-548.
PubMed
A variety of antigens have been identified on the surface of the malignant cell. However, identical antigens are often found on non-malignant cells of the same or different histological origin, or of a different stage of embryonic development. Many of these tumour-associated antigens appear to be only incidentally expressed on neoplastic cells. Clearly, it would be of great interest to identify cell-surface antigens whose expression is associated specifically with the transformed state and linked directly with the mechanisms responsible for transformation. The detection of activated cellular oncogenes in human and animal cancer cells by the technique of DNA transfection has allowed the isolation of genetic elements which are thought to have a critical role in malignancy. Here, in an effort to identify cell-surface antigens associated with the neoplastic process, we have generated hybridomas which secrete monoclonal antibodies that react specifically with cell-surface determinants found on NIH 3T3 cells transformed by transfection with a group of rat neuroblastoma oncogenes. These antibodies bind to and immunoprecipitate a phosphoprotein of relative molecular mass 185,000 (185 K) from a DNA donor rat neuroblastoma and 13 independent rat neuroblastoma DNA transfectants. There was no antibody reactivity with normal NIH 3T3 cells or with NIH 3T3 cells transformed by various other agents.
Product Citations
-
-
Immunology and Microbiology
-
Cancer Research
Antitumor CD4+ T Helper 1 Cells Target and Control the Outgrowth of Disseminated Cancer Cells.
In Cancer Immunol Res on 2 May 2025 by Ganesan, R., Lee, M. C., et al.
PubMed
Detection of disseminated cancer cells (DCC) in the bone marrow (BM) of patients with breast cancer is a critical predictor of late recurrence and distant metastasis. Conventional therapies often fail to completely eradicate DCCs in patients. In this study, we demonstrate that intratumoral priming of antitumor CD4+ T helper 1 (Th1) cells was able to eliminate the DCC burden in distant organs and prevent overt metastasis, independent of CD8+ T cells. Intratumoral priming of tumor antigen-specific CD4+ Th1 cells enhanced their migration to the BM and distant metastatic site to selectively target DCC burden. The majority of these intratumorally activated CD4+ T cells were CD4+PD1- T cells, supporting their nonexhaustion stage. Phenotypic characterization revealed enhanced infiltration of memory CD4+ T cells and effector CD4+ T cells in the primary tumor, tumor-draining lymph node, and DCC-driven metastasis site. A robust migration of CD4+CCR7+CXCR3+ Th1 cells and CD4+CCR7-CXCR3+ Th1 cells into distant organs further revealed their potential role in eradicating DCC-driven metastasis. The intratumoral priming of antitumor CD4+ Th1 cells failed to eradicate DCC-driven metastasis in CD4- or IFN-γ knockout mice. Moreover, antitumor CD4+ Th1 cells, by increasing IFN-γ production, inhibited various molecular aspects and increased classical and nonclassical MHC molecule expression in DCCs. This reduced stemness and self-renewal while increasing immune recognition in DCCs of patients with breast cancer. These results unveil an immune basis for antitumor CD4+ Th1 cells that modulate DCC tumorigenesis to prevent recurrence and metastasis in patients.
-
-
-
Immunology and Microbiology
-
Cancer Research
XMT-2056, a HER2-Directed STING Agonist Antibody-Drug Conjugate, Induces Innate Antitumor Immune Responses by Acting on Cancer Cells and Tumor-Resident Immune Cells.
In Clin Cancer Res on 1 May 2025 by Bukhalid, R. A., Duvall, J. R., et al.
PubMed
Targeted tumor delivery may be required to potentiate the clinical benefit of innate immune modulators. The objective of the study was to apply an antibody-drug conjugate (ADC) approach to STING agonism and develop a clinical candidate.
-
-
-
Cancer Research
Pyrotinib and trastuzumab combination treatment synergistically overcomes HER2 dependency in HER2-positive breast cancer: insights from the PHILA trial.
In EBioMedicine on 1 November 2024 by Liu, S., Lan, B., et al.
PubMed
The PHILA study suggests that pyrotinib, trastuzumab, and docetaxel significantly improved progression-free survival (PFS) compared with placebo, trastuzumab, and docetaxel in patients with untreated HER2-positive metastatic breast cancer. In this study, we aimed to investigate the synergistic mechanisms of pyrotinib plus trastuzumab and provide further insights for the PHILA trial.
-
-
Targeting fatty acid oxidation enhances response to HER2-targeted therapy.
In Nat Commun on 3 August 2024 by Nandi, I., Ji, L., et al.
PubMed
Metabolic reprogramming, a hallmark of tumorigenesis, involves alterations in glucose and fatty acid metabolism. Here, we investigate the role of Carnitine palmitoyl transferase 1a (Cpt1a), a key enzyme in long-chain fatty acid (LCFA) oxidation, in ErbB2-driven breast cancers. In ErbB2+ breast cancer models, ablation of Cpt1a delays tumor onset, growth, and metastasis. However, Cpt1a-deficient cells exhibit increased glucose dependency that enables survival and eventual tumor progression. Consequently, these cells exhibit heightened oxidative stress and upregulated nuclear factor erythroid 2-related factor 2 (Nrf2) activity. Inhibiting Nrf2 or silencing its expression reduces proliferation and glucose consumption in Cpt1a-deficient cells. Combining the ketogenic diet, composed of LCFAs, or an anti-ErbB2 monoclonal antibody (mAb) with Cpt1a deficiency significantly perturbs tumor growth, enhances apoptosis, and reduces lung metastasis. Using an immunocompetent model, we show that Cpt1a inhibition promotes an antitumor immune microenvironment, thereby enhancing the efficacy of anti-ErbB2 mAbs. Our findings underscore the importance of targeting fatty acid oxidation alongside HER2-targeted therapies to combat resistance in HER2+ breast cancer patients.
-
-
Homo sapiens (Human)
Site-Specific Antibody Conjugation Using Modified Bisected N-Glycans: Method Development and Potential toward Tunable Effector Function.
In Bioconjug Chem on 20 September 2023 by Hsu, Y. P., Nourzaie, O., et al.
PubMed
Antibody-drug conjugates (ADCs) have garnered worldwide attention for disease treatment, as they possess high target specificity, a long half-life, and outstanding potency to kill or modulate the functions of targets. FDA approval of multiple ADCs for cancer therapy has generated a strong desire for novel conjugation strategies with high biocompatibility and controllable bioproperties. Herein, we present a bisecting glycan-bridged conjugation strategy that enables site-specific conjugation without the need for the oligosaccharide synthesis and genetic engineering of antibodies. Application of this method is demonstrated by conjugation of anti-HER2 human and mouse IgGs with a cytotoxic drug, monomethyl auristatin E. The glycan bridge showed outstanding stability, and the resulting ADCs eliminated HER2-expressing cancer cells effectively. Moreover, our strategy preserves the feasibility of glycan structure remodeling to fine-tune the immunogenicity and pharmacokinetic properties of ADCs through glycoengineering.
-
-
-
Cancer Research
-
Immunology and Microbiology
IFI16-dependent STING signaling is a crucial regulator of anti-HER2 immune response in HER2+ breast cancer.
In Proc Natl Acad Sci U S A on 2 August 2022 by Ong, L. T., Lee, W. C., et al.
PubMed
Relapse to anti-HER2 monoclonal antibody (mAb) therapies, such as trastuzumab in HER2+ breast cancer (BC), is associated with residual disease progression due to resistance to therapy. Here, we identify interferon-γ inducible protein 16 (IFI16)-dependent STING signaling as a significant determinant of trastuzumab responses in HER2+ BC. We show that down-regulation of immune-regulated genes (IRG) is specifically associated with poor survival of HER2+, but not other BC subtypes. Among IRG, IFI16 is identified as a direct target of EZH2, the underexpression of which leads to deficient STING activation and downstream CXCL10/11 expression in response to trastuzumab treatment. Dual inhibition of EZH2 and histone deacetylase (HDAC) significantly activates IFI16-dependent immune responses to trastuzumab. Notably, a combination of a novel histone methylation inhibitor with an HDAC inhibitor induces complete tumor eradication and long-term T cell memory in a HER2+ BC mouse model. Our findings demonstrate an epigenetic regulatory mechanism suppressing the expression of the IFI16-CXCL10/11 signaling pathway that provides a survival advantage to HER2+ BC to confer resistance to trastuzumab treatment.
-
-
-
Cancer Research
-
Immunology and Microbiology
APOBEC Mutagenesis Inhibits Breast Cancer Growth through Induction of T cell-Mediated Antitumor Immune Responses.
In Cancer Immunol Res on 1 January 2022 by DiMarco, A. V., Qin, X., et al.
PubMed
The APOBEC family of cytidine deaminases is one of the most common endogenous sources of mutations in human cancer. Genomic studies of tumors have found that APOBEC mutational signatures are enriched in the HER2 subtype of breast cancer and are associated with immunotherapy response in diverse cancer types. However, the direct consequences of APOBEC mutagenesis on the tumor immune microenvironment have not been thoroughly investigated. To address this, we developed syngeneic murine mammary tumor models with inducible expression of APOBEC3B. We found that APOBEC activity induced antitumor adaptive immune responses and CD4+ T cell-mediated, antigen-specific tumor growth inhibition. Although polyclonal APOBEC tumors had a moderate growth defect, clonal APOBEC tumors were almost completely rejected, suggesting that APOBEC-mediated genetic heterogeneity limits antitumor adaptive immune responses. Consistent with the observed immune infiltration in APOBEC tumors, APOBEC activity sensitized HER2-driven breast tumors to anti-CTLA-4 checkpoint inhibition and led to a complete response to combination anti-CTLA-4 and anti-HER2 therapy. In human breast cancers, the relationship between APOBEC mutagenesis and immunogenicity varied by breast cancer subtype and the frequency of subclonal mutations. This work provides a mechanistic basis for the sensitivity of APOBEC tumors to checkpoint inhibitors and suggests a rationale for using APOBEC mutational signatures and clonality as biomarkers predicting immunotherapy response in HER2-positive (HER2+) breast cancers.
-
-
-
Cancer Research
-
Cell Biology
-
Stem Cells and Developmental Biology
Autophagy Blockade Limits HER2+ Breast Cancer Tumorigenesis by Perturbing HER2 Trafficking and Promoting Release Via Small Extracellular Vesicles.
In Dev Cell on 8 February 2021 by Hao, M., Yeo, S. K., et al.
PubMed
Autophagy modulation is an emerging strategy for cancer therapy. By deleting an essential autophagy gene or disrupting its autophagy function, we determined a mechanism of HER2+ breast cancer tumorigenesis by directly regulating the oncogenic driver. Disruption of FIP200-mediated autophagy reduced HER2 expression on the tumor cell surface and abolished mammary tumorigenesis in MMTV-Neu mice. Decreased HER2 surface expression was due to trafficking from the Golgi to the endocytic pathways instead of the plasma membrane. Autophagy inhibition led to HER2 accumulation in early and late endosomes associated with intraluminal vesicles and released from tumor cells in small extracellular vesicles (sEVs). Increased HER2 release from sEVs correlated with reduced tumor cell surface levels. Blocking sEVs secretion rescued HER2 levels in tumor cells. Our results demonstrate a role for autophagy to promote tumorigenesis in HER2+ breast cancer. This suggests that blocking autophagy could supplement current anti-HER2 agents for treating the disease.
-
-
-
Cardiovascular biology
Nrg1/ErbB Signaling-Mediated Regulation of Fibrosis After Myocardial Infarction
In bioRxiv on 1 February 2021 by Shiraishi, M., Yamaguchi, A., et al.
-
-
Anti-HER2/neu Antibody Reduces Chemotherapy-Induced Ovarian Toxicity-From Bench to Bedside.
In Biomedicines on 7 December 2020 by Levi, M., Goshen-Lago, T., et al.
PubMed
Trastuzumab, a humanized anti-human epidermal growth factor receptor 2 (HER2/neu) antibody, is considered a standard treatment in addition to chemotherapy in the adjuvant setting for HER2/neu-positive breast cancer, yet its impact on fertility and ovarian reserve remains obscure. We aimed to study the effect of anti-HER2/neu on chemotherapy-induced ovarian toxicity in both clinical and preclinical settings.
-
Reduction of Global H3K27me3 Enhances HER2/ErbB2 Targeted Therapy.
In Cell Rep on 8 October 2019 by Hirukawa, A., Singh, S., et al.
PubMed
Monoclonal antibodies (mAbs) targeting the oncogenic receptor tyrosine kinase ERBB2/HER2, such as Trastuzumab, are the standard of care therapy for breast cancers driven by ERBB2 overexpression and activation. However, a substantial proportion of patients exhibit de novo resistance. Here, by comparing matched Trastuzumab-naive and post-treatment patient samples from a neoadjuvant trial, we link resistance with elevation of H3K27me3, a repressive histone modification catalyzed by polycomb repressor complex 2 (PRC2). In ErbB2+ breast cancer models, PRC2 silences endogenous retroviruses (ERVs) to suppress anti-tumor type-I interferon (IFN) responses. In patients, elevated H3K27me3 in tumor cells following Trastuzumab treatment correlates with suppression of interferon-driven viral defense gene expression signatures and poor response. Using an immunocompetent model, we provide evidence that EZH2 inhibitors promote interferon-driven immune responses that enhance the efficacy of anti-ErbB2 mAbs, suggesting the potential clinical benefit of epigenomic reprogramming by H3K27me3 depletion in Trastuzumab-resistant disease.
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Sequential Anti-PD1 Therapy Following Dendritic Cell Vaccination Improves Survival in a HER2 Mammary Carcinoma Model and Identifies a Critical Role for CD4 T Cells in Mediating the Response.
In Front Immunol on 3 September 2019 by Kodumudi, K. N., Ramamoorthi, G., et al.
PubMed
Patients with metastatic HER2 breast cancer (MBC) often become resistant to HER 2 targeted therapy and have recurrence of disease. The Panacea trial suggested that HER2 MBC patients were more likely to respond to checkpoint therapy if TIL were present or if tumor expressed PD-L1. We assessed whether type I polarized dendritic cells (DC1) could improve checkpoint therapy in a preclinical model of HER2+ breast cancer. TUBO bearing mice were vaccinated with either MHC class I or class II HER2 peptide pulsed DC1 (class I or class II HER2-DC1) concurrently or sequentially with administration of anti-PD-1 or anti-PDL1. Infiltration of tumors by immune cells, induction of anti-HER2 immunity and response to therapy was evaluated. Class I or class II HER2-DC1 vaccinated mice generated anti-HER2 CD8 or CD4+ T cell immune responses and demonstrated delayed tumor growth. Combining both MHC class I and II HER2-pulsed DC1 did not further result in inhibition of tumor growth or enhanced survival compared to individual administration. Interestingly class II HER2-DC1 led to both increased CD4 and CD8 T cells in the tumor microenvironment while class I peptides typically resulted in only increased CD8 T cells. Anti-PD-1 but not anti-PD-L1 administered sequentially with class I or class II HER2-DC1 vaccine could improve the efficacy of HER2-DC1 vaccine as measured by tumor growth, survival, infiltration of tumors by T cells and increase in systemic anti-HER2 immune responses. Depletion of CD4+ T cells abrogated the anti-tumor efficacy of combination therapy with class II HER2-DC1 and anti-PD-1, suggesting that tumor regression was CD4 dependent. Since class II HER2-DC1 was as effective as class I, we combined class II HER2-DC1 vaccine with anti-rat neu antibodies and anti-PD-1 therapy. Combination therapy demonstrated further delay in tumor growth, and enhanced survival compared to control mice. In summary, Class II HER2-DC1 drives both a CD4 and CD8 T cell tumor infiltration that leads to increased survival, and in combination with anti-HER2 therapy and checkpoint blockade can improve survival in preclinical models of HER2 positive breast cancer and warrants exploration in patients with HER2 MBC.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Single-cell profiling guided combinatorial immunotherapy for fast-evolving CDK4/6 inhibitor-resistant HER2-positive breast cancer.
In Nat Commun on 23 August 2019 by Wang, Q., Guldner, I. H., et al.
PubMed
Acquired resistance to targeted cancer therapy is a significant clinical challenge. In parallel with clinical trials combining CDK4/6 inhibitors to treat HER2+ breast cancer, we sought to prospectively model tumor evolution in response to this regimen in vivo and identify a clinically actionable strategy to combat drug resistance. Despite a promising initial response, acquired resistance emerges rapidly to the combination of anti-HER2/neu antibody and CDK4/6 inhibitor Palbociclib. Using high-throughput single-cell profiling over the course of treatments, we reveal a distinct immunosuppressive immature myeloid cell (IMC) population to infiltrate the resistant tumors. Guided by single-cell transcriptome analysis, we demonstrate that combination of IMC-targeting tyrosine kinase inhibitor cabozantinib and immune checkpoint blockade enhances anti-tumor immunity, and overcomes the resistance. Furthermore, sequential combinatorial immunotherapy enables a sustained control of the fast-evolving CDK4/6 inhibitor-resistant tumors. Our study demonstrates a translational framework for treating rapidly evolving tumors through preclinical modeling and single-cell analyses.
-
-
-
Cancer Research
-
Immunology and Microbiology
Single-cell profiling guided combinatorial immunotherapy for fast-evolving CDK4/6 inhibitor resistant HER2-positive breast cancer
In bioRxiv on 14 June 2019 by Wang, Q., Guldner, I. H., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
PolyI:C and CpG Synergize with Anti-ErbB2 mAb for Treatment of Breast Tumors Resistant to Immune Checkpoint Inhibitors.
In Cancer Res on 15 January 2017 by Charlebois, R., Allard, B., et al.
PubMed
Innate and adaptive immune cells play an important role in the therapeutic activity of anti-ErbB2 mAbs, such as trastuzumab. In the clinic, breast tumors poorly infiltrated with immune cells are more resistant to trastuzumab, and patients have a worse prognosis. Because type I and II IFNs are critical to the immune-mediated activity of anti-ErbB2 mAb, we investigated the effect of combining polyI:C and CpG with trastuzumab-like therapy in immunocompetent mouse models of ErbB2+ breast cancer. We demonstrated that in situ delivery of polyI:C and CpG combined to systemic anti-ErbB2 mAb triggered a potent inflammatory response in breast tumors able to induce long-lasting CD8+ T cell-dependent antitumor immunity. Remarkably, polyI:C and CpG was superior to combined PD-1/CTLA-4 blockade in sensitizing tumors to anti-ErbB2 mAb therapy. Local injection of CpG and polyI:C in a primary tumor significantly enhanced the activity of systemic anti-ErbB2 mAb against a distant untreated tumor. Type I and II IFNs, as well as natural killer cells and CD8+ T cells, were indispensible to the synergistic activity of the combination treatment. Because synthetic RNA analogues and CpG oligodeoxynucleotides have been safely used in clinical trials, our study supports combination treatments with anti-ErbB2 mAbs. Cancer Res; 77(2); 312-9. ©2016 AACR.
-