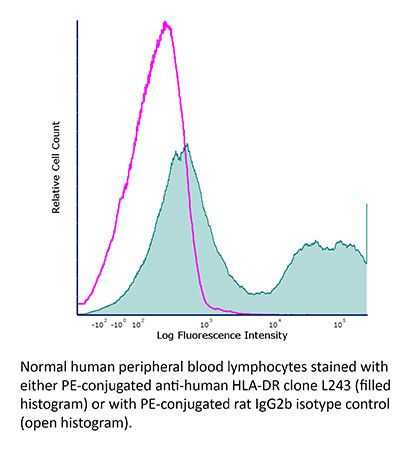
InVivoMAb anti-human/monkey MHC class II (HLA-DR)
Product Description
Specifications
| Isotype | Mouse IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG2a isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Human lymphoblastoid B cell line RPMI 8866.9 |
| Reported Applications |
in vitro blocking of MHC class II HLA-DR HLA class II binding assay in vitro MHC class II HLA-DR expressing cell negative selection Western blot Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2736986 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vitro blocking of MHC class II HLA-DR
Brentville, V. A., et al (2016). "Citrullinated Vimentin Presented on MHC-II in Tumor Cells Is a Target for CD4+ T-Cell-Mediated Antitumor Immunity" Cancer Res 76(3): 548-560.
PubMed
Stressful conditions in the harsh tumor microenvironment induce autophagy in cancer cells as a mechanism to promote their survival. However, autophagy also causes post-translational modification of proteins that are recognized by the immune system. In particular, modified self-antigens can trigger CD4(+) T-cell responses that might be exploited to boost antitumor immune defenses. In this study, we investigated the ability of CD4 cells to target tumor-specific self-antigens modified by citrullination, which converts arginine residues in proteins to citrulline. Focusing on the intermediate filament protein vimentin, which is frequently citrullinated in cells during epithelial-to-mesenchymal transition of metastasizing epithelial tumors, we generated citrullinated vimentin peptides for immunization experiments in mice. Immunization with these peptides induced IFNgamma- and granzyme B-secreting CD4 T cells in response to autophagic tumor targets. Remarkably, a single immunization with modified peptide, up to 14 days after tumor implant, resulted in long-term survival in 60% to 90% of animals with no associated toxicity. This antitumor response was dependent on CD4 cells and not CD8(+) T cells. These results show how CD4 cells can mediate potent antitumor responses against modified self-epitopes presented on tumor cells, and they illustrate for the first time how the citrullinated peptides may offer especially attractive vaccine targets for cancer therapy.
HLA class II binding assay
Hirasawa, M., et al (2015). "The Possible Mechanism of Idiosyncratic Lapatinib-Induced Liver Injury in Patients Carrying Human Leukocyte Antigen-DRB1*07:01" PLoS One 10(6): e0130928.
PubMed
Idiosyncratic lapatinib-induced liver injury has been reported to be associated with human leukocyte antigen (HLA)-DRB1*07:01. In order to investigate its mechanism, interaction of lapatinib with HLA-DRB1*07:01 and its ligand peptide derived from tetanus toxoid, has been evaluated in vitro. Here we show that lapatinib enhances binding of the ligand peptide to HLA-DRB1*07:01. Furthermore in silico molecular dynamics analysis revealed that lapatinib could change the beta chain helix in the HLA-DRB1*07:01 specifically to form a tightly closed binding groove structure and modify a large part of the binding groove. These results indicate that lapatinib affects the ligand binding to HLA-DRB1*07:01 and idiosyncratic lapatinib-induced liver injury might be triggered by this mechanism. This is the first report showing that the clinically available drug can enhance the binding of ligand peptide to HLA class II molecules in vitro and in silico.
Flow Cytometry
Kho, S., et al (2015). "Preserved dendritic cell HLA-DR expression and reduced regulatory T cell activation in asymptomatic Plasmodium falciparum and P. vivax infection" Infect Immun 83(8): 3224-3232.
PubMed
Clinical illness with Plasmodium falciparum or Plasmodium vivax compromises the function of dendritic cells (DC) and expands regulatory T (Treg) cells. Individuals with asymptomatic parasitemia have clinical immunity, restricting parasite expansion and preventing clinical disease. The role of DC and Treg cells during asymptomatic Plasmodium infection is unclear. During a cross-sectional household survey in Papua, Indonesia, we examined the number and activation of blood plasmacytoid DC (pDC), CD141(+), and CD1c(+) myeloid DC (mDC) subsets and Treg cells using flow cytometry in 168 afebrile children (of whom 15 had P. falciparum and 36 had P. vivax infections) and 162 afebrile adults (of whom 20 had P. falciparum and 20 had P. vivax infections), alongside samples from 16 patients hospitalized with uncomplicated malaria. Unlike DC from malaria patients, DC from children and adults with asymptomatic, microscopy-positive P. vivax or P. falciparum infection increased or retained HLA-DR expression. Treg cells in asymptomatic adults and children exhibited reduced activation, suggesting increased immune responsiveness. The pDC and mDC subsets varied according to clinical immunity (asymptomatic or symptomatic Plasmodium infection) and, in asymptomatic infection, according to host age and parasite species. In conclusion, active control of asymptomatic infection was associated with and likely contingent upon functional DC and reduced Treg cell activation.
in vitro blocking of MHC class II HLA-DR
Kalka-Moll, W. M., et al (2002). "Zwitterionic polysaccharides stimulate T cells by MHC class II-dependent interactions" J Immunol 169(11): 6149-6153.
PubMed
Polysaccharides of pathogenic extracellular bacteria commonly have negatively charged groups or no charged groups at all. These molecules have been considered classic T cell-independent Ags that do not elicit cell-mediated immune responses in mice. However, bacterial polysaccharides with a zwitterionic charge motif (ZPSs), such as the capsular polysaccharides of many strains of Bacteroides fragilis, Staphylococcus aureus, and Streptococcus pneumoniae type 1 elicit potent CD4(+) T cell responses in vivo and in vitro. The cell-mediated response to ZPS depends on the presence of both positively charged and negatively charged groups on each repeating unit of the polysaccharide. In this study, we define some of the requirements for the presentation of ZPS to CD4(+) T cells. We provide evidence that direct interactions of T cells with APCs are essential for T cell activation by ZPS. Monocytes, dendritic cells, and B cells are all able to serve as APCs for ZPS-mediated T cell activation. APCs lacking MHC class II molecules do not support this activity. Furthermore, mAb to HLA-DR specifically blocks ZPS-mediated T cell activation, while mAbs to other MHC class II and class I molecules do not. Immunoprecipitation of lysates of MHC class II-expressing cells following incubation with ZPS shows binding of ZPS and HLA-DR. Electron microscopy reveals colocalization of ZPS with HLA-DR on the cell surface and in compartments of the endocytic pathway. These results indicate that MHC class II molecules expressing HLA-DR on professional APCs are required for ZPS-induced T cell activation. The implication is that binding of ZPS to HLA-DR may be required for T cell activation.
Western Blot
Esser, M. T., et al (2001). "Differential incorporation of CD45, CD80 (B7-1), CD86 (B7-2), and major histocompatibility complex class I and II molecules into human immunodeficiency virus type 1 virions and microvesicles: implications for viral pathog
PubMed
Human immunodeficiency virus (HIV) infection results in a functional impairment of CD4(+) T cells long before a quantitative decline in circulating CD4(+) T cells is evident. The mechanism(s) responsible for this functional unresponsiveness and eventual depletion of CD4(+) T cells remains unclear. Both direct effects of cytopathic infection of CD4(+) cells and indirect effects in which uninfected “bystander” cells are functionally compromised or killed have been implicated as contributing to the immunopathogenesis of HIV infection. Because T-cell receptor engagement of major histocompatibility complex (MHC) molecules in the absence of costimulation mediated via CD28 binding to CD80 (B7-1) or CD86 (B7-2) can lead to anergy or apoptosis, we determined whether HIV type 1 (HIV-1) virions incorporated MHC class I (MHC-I), MHC-II, CD80, or CD86. Microvesicles produced from matched uninfected cells were also evaluated. HIV infection increased MHC-II expression on T- and B-cell lines, macrophages, and peripheral blood mononclear cells (PBMC) but did not significantly alter the expression of CD80 or CD86. HIV virions derived from all MHC-II-positive cell types incorporated high levels of MHC-II, and both virions and microvesicles preferentially incorporated CD86 compared to CD80. CD45, expressed at high levels on cells, was identified as a protein present at high levels on microvesicles but was not detected on HIV-1 virions. Virion-associated, host cell-derived molecules impacted the ability of noninfectious HIV virions to trigger death in freshly isolated PBMC. These results demonstrate the preferential incorporation or exclusion of host cell proteins by budding HIV-1 virions and suggest that host cell proteins present on HIV-1 virions may contribute to the overall pathogenesis of HIV-1 infection.
in vitro MHC class II HLA-DR expressing cell negative selection
Goodier, M. R. and M. Londei (2000). "Lipopolysaccharide stimulates the proliferation of human CD56+CD3- NK cells: a regulatory role of monocytes and IL-10" J Immunol 165(1): 139-147.
PubMed
NK cells recognize and kill tumor cells and normal cells, and these play an important role in immune defense in cancer, infectious disease, and autoimmunity. NK killing is regulated by positive or negative signals derived from the interaction of surface receptors with ligands on the target cells. However, the mechanisms controlling the proliferation and maintenance of NK cells in normal human individuals are less clearly defined. In this study, using an entirely autologous system, we demonstrate that human peripheral blood CD3-CD56+, killer cell-inhibitory receptor (KIR)-expressing cells proliferate and expand in response to LPS. These responses are enhanced in the presence of anti-IL-10 receptor-blocking Abs or on the removal of CD14+ cells from the cultures. This enhancement is also reflected in substantial increases in cytolytic activity and IFN-gamma production. The negative effect of CD14+ cells may also be IL-10 mediated, IL-10 being lost from the culture supernatants of CD14-depleted PBMC and rIL-10 reversing the effect of this depletion. On the other hand, mRNA for the p35 and p40 subunits of IL-12 is still induced in CD14-depleted cultures. The expansion of CD3-CD56+ cells was also inhibited by CTLA4-Ig, indicating a role for CD80/86. B lymphocytes were not required for the expansion of CD3-CD56+ cells, whereas removal of MHC class II+ cells from CD14-depleted cultures resulted in a complete abrogation of these responses. Expansion of CD3-CD56+ cells was reconstituted in MHC class II-depleted cell cultures by adding back monocyte-derived dendritic cells. These results indicate that the responses of CD3-CD56+ NK cells to LPS may be driven by a MHC class II+ B7+ CD14- peripheral population, most likely blood dendritic cells.
Product Citations
-
-
Immunology and Microbiology
-
Cell Biology
Single-Cell Sequencing Reveals That CD4+ T Cells Eliminate Senescent Prostate Epithelium to Delay Progression of Benign Prostatic Hyperplasia.
In Aging Cell on 1 October 2025 by Li, Z., Wang, X., et al.
PubMed
Benign prostatic hyperplasia (BPH) is an age-related condition characterized by progressive prostate enlargement driven in part by the accumulation of senescent epithelial cells and their pro-inflammatory secretome. Using human single-cell RNA sequencing and laser capture microdissection, we identified C-X-C Motif Chemokine Ligand 13 (CXCL13) as a key chemokine secreted by senescent prostate epithelial cells. CXCL13 recruits CD4+ T cells via the C-X-C Chemokine Receptor Type 5 (CXCR5) receptor, facilitating immune recognition through human leukocyte antigen-DR isotype (HLA-DR) and promoting senescent cell clearance. Functional assays revealed that CD4+ cytotoxic T lymphocytes (CTLs) mediate this clearance, while regulatory T cells (Tregs) suppress it, forming a functional dichotomy. Immunohistochemistry, transwell migration, and co-culture assays confirmed this CXCL13-CXCR5-HLA-DR axis. In a testosterone-induced BPH mouse model, CXCL13 treatment enhanced CD4+ T cell infiltration and reduced epithelial senescence, while CD4+ T cell depletion reversed these effects. Single-cell transcriptomics in mice further validated increased CXCL13 expression and CD4+ T cell engagement. These findings uncover a critical immune surveillance mechanism in BPH and suggest that targeting the CXCL13-CD4+ T cell axis may offer a novel therapeutic strategy for age-related prostate enlargement.
-
-
-
Immunology and Microbiology
ALS-associated TDP-43 aggregates drive innate and adaptive immune cell activation.
In iScience on 20 June 2025 by Evangelista, B. A., Ragusa, J. V., et al.
PubMed
Amyotrophic lateral sclerosis (ALS) is the most common and fatal motor neuron disease. Approximately 90% of ALS patients exhibit pathology of the master RNA regulator, transactive response DNA binding protein (TDP-43). Despite the prevalence TDP-43 pathology in ALS motor neurons, recent findings suggest immune dysfunction is a determinant of disease progression in patients. Whether TDP-43 aggregates elicit immune responses remains underexplored. In this study, we demonstrate that TDP-43 aggregates are internalized by antigen-presenting cell populations, cause vesicle rupture, and drive innate and adaptive immune cell activation by way of antigen presentation. Using a multiplex imaging platform, we observed enrichment of activated microglia/macrophages in ALS white matter that correlated with phosphorylated TDP-43 accumulation, CD8 T cell infiltration, and major histocompatibility complex expression. Taken together, this study sheds light on a novel cellular response to TDP-43 aggregates through an immunological lens.
-
-
-
Immunology and Microbiology
-
Cancer Research
Interferon-γ-stimulated antigen-presenting cancer-associated fibroblasts hinder neoadjuvant chemoimmunotherapy efficacy in lung cancer.
In Cell Rep Med on 18 March 2025 by Cao, Z., Meng, Z., et al.
PubMed
Conventional neoadjuvant chemotherapy provides limited benefit for patients with resectable non-small cell lung cancer (NSCLC). Recently, neoadjuvant chemoimmunotherapy (NCIT) has transformed the perioperative management of NSCLC by priming systemic anti-tumor immunity before surgery, yet it remains ineffective for at least 50% of patients. Through single-cell sequencing analysis of our NCIT cohort, we identify that antigen-presenting cancer-associated fibroblasts (apCAFs) can impede the efficacy of NCIT. Using a custom cancer-associated fibroblast biobank, we uncover that interferon (IFN)-γ stimulates apCAF expansion via the JAK1/2-STAT1-IFI6/27 pathway. Mechanistically, apCAFs significantly contribute to PD-L2 expression in the tumor microenvironment (TME), triggering the accumulation of FOXP1+regulatory T cells (Tregs) through the PD-L2-RGMB axis. Reprogramming apCAFs by inhibiting the IFN-γ pathway or blocking the PD-L2-RGMB axis substantially mitigates apCAFs-mediated FOXP1+Tregs' expansion. In summary, we reveal the role of apCAFs in compromising NCIT efficacy and propose applications for anti-PD-L2/RGMB regimens to synergize with anti-PD1 therapies by targeting apCAFs.
-
-
Characterizations of a neutralizing antibody broadly reactive to multiple gluten peptide:HLA-DQ2.5 complexes in the context of celiac disease.
In Nat Commun on 22 December 2023 by Okura, Y., Ikawa-Teranishi, Y., et al.
PubMed
In human celiac disease (CeD) HLA-DQ2.5 presents gluten peptides to antigen-specific CD4+ T cells, thereby instigating immune activation and enteropathy. Targeting HLA-DQ2.5 with neutralizing antibody for treating CeD may be plausible, yet using pan-HLA-DQ antibody risks affecting systemic immunity, while targeting selected gluten peptide:HLA-DQ2.5 complex (pHLA-DQ2.5) may be insufficient. Here we generate a TCR-like, neutralizing antibody (DONQ52) that broadly recognizes more than twenty-five distinct gluten pHLA-DQ2.5 through rabbit immunization with multi-epitope gluten pHLA-DQ2.5 and multidimensional optimization. Structural analyses show that the proline-rich and glutamine-rich motif of gluten epitopes critical for pathogenesis is flexibly recognized by multiple tyrosine residues present in the antibody paratope, implicating the mechanisms for the broad reactivity. In HLA-DQ2.5 transgenic mice, DONQ52 demonstrates favorable pharmacokinetics with high subcutaneous bioavailability, and blocks immunity to gluten while not affecting systemic immunity. Our results thus provide a rationale for clinical testing of DONQ52 in CeD.
-
-
Immunology and Microbiology
Cytotoxic CD4+ T cells eliminate senescent cells by targeting cytomegalovirus antigen.
In Cell on 30 March 2023 by Hasegawa, T., Oka, T., et al.
PubMed
Senescent cell accumulation has been implicated in the pathogenesis of aging-associated diseases, including cancer. The mechanism that prevents the accumulation of senescent cells in aging human organs is unclear. Here, we demonstrate that a virus-immune axis controls the senescent fibroblast accumulation in the human skin. Senescent fibroblasts increased in old skin compared with young skin. However, they did not increase with advancing age in the elderly. Increased CXCL9 and cytotoxic CD4+ T cells (CD4 CTLs) recruitment were significantly associated with reduced senescent fibroblasts in the old skin. Senescent fibroblasts expressed human leukocyte antigen class II (HLA-II) and human cytomegalovirus glycoprotein B (HCMV-gB), becoming direct CD4 CTL targets. Skin-resident CD4 CTLs eliminated HCMV-gB+ senescent fibroblasts in an HLA-II-dependent manner, and HCMV-gB activated CD4 CTLs from the human skin. Collectively, our findings demonstrate HCMV reactivation in senescent cells, which CD4 CTLs can directly eliminate through the recognition of the HCMV-gB antigen.
-
-
-
Immunocytochemistry
-
Immunology and Microbiology
Cytotoxic CD4+T cells eliminate senescent cells by targeting cytomegalovirus antigen
In bioRxiv on 8 March 2023 by Hasegawa, T., Oka, T., et al.
-
-
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
Therapy-induced senescence upregulates antigen presentation machinery and triggers anti-tumor immunity in Acute Myeloid Leukemia
In bioRxiv on 17 November 2022 by Gilioli, D., Fusco, S., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
Unmasking the suppressed immunopeptidome of EZH2-mutated diffuse large B-cell lymphomas through combination drug treatment.
In Blood Adv on 26 July 2022 by Bourne, C. M., Mun, S. S., et al.
PubMed
Exploring the repertoire of peptides presented on major histocompatibility complexes (MHCs) helps identify targets for immunotherapy in many hematologic malignancies. However, there is a paucity of such data for diffuse large B-cell lymphomas (DLBCLs), which might be explained by the profound downregulation of MHC expression in many DLBCLs, and in particular in the enhancer of zeste homolog 2 (EZH2)-mutated subgroup. Epigenetic drug treatment, especially in the context of interferon-γ (IFN-γ), restored MHC expression in DLBCL. In DLBCL, peptides presented on MHCs were identified via mass spectrometry after treatment with tazemetostat or decitabine alone or in combination with IFN-γ. Such treatment synergistically increased the expression of MHC class I surface proteins up to 50-fold and the expression of class II surface proteins up to threefold. Peptides presented on MHCs increased to a similar extent for both class I and class II MHCs. Overall, these treatments restored the diversity of the immunopeptidome to levels described in healthy B cells for 2 of 3 cell lines and allowed the systematic search for new targets for immunotherapy. Consequently, we identified multiple MHC ligands from the regulator of G protein signaling 13 (RGS13) and E2F transcription factor 8 (E2F8) on different MHC alleles, none of which have been described in healthy tissues and therefore represent tumor-specific MHC ligands that are unmasked only after drug treatment. Overall, our results show that EZH2 inhibition in combination with decitabine and IFN-γ can expand the repertoire of MHC ligands presented on DLBCLs by revealing suppressed epitopes, thus allowing the systematic analysis and identification of new potential immunotherapy targets.
-
-
-
Cancer Research
-
Immunology and Microbiology
Unmasking the cryptic immunopeptidome of EZH2 mutated diffuse large B-cell lymphomas through combination drug treatment
In bioRxiv on 3 September 2021 by Bourne, C. M., Mun, S. S., et al.
-
-
-
Immunology and Microbiology
Immunopeptidomics for Dummies: Detailed Experimental Protocols and Rapid, User-Friendly Visualization of MHC I and II Ligand Datasets with MhcVizPipe
In bioRxiv on 3 November 2020 by Kovalchik, K. A., Wessling, L., et al.
-
-
-
Immunology and Microbiology
Immunopeptidomic Data Integration to Artificial Neural Networks Enhances Protein-Drug Immunogenicity Prediction.
In Front Immunol on 14 July 2020 by Barra, C., Ackaert, C., et al.
PubMed
Recombinant DNA technology has, in the last decades, contributed to a vast expansion of the use of protein drugs as pharmaceutical agents. However, such biological drugs can lead to the formation of anti-drug antibodies (ADAs) that may result in adverse effects, including allergic reactions and compromised therapeutic efficacy. Production of ADAs is most often associated with activation of CD4 T cell responses resulting from proteolysis of the biotherapeutic and loading of drug-specific peptides into major histocompatibility complex (MHC) class II on professional antigen-presenting cells. Recently, readouts from MHC-associated peptide proteomics (MAPPs) assays have been shown to correlate with the presence of CD4 T cell epitopes. However, the limited sensitivity of MAPPs challenges its use as an immunogenicity biomarker. In this work, MAPPs data was used to construct an artificial neural network (ANN) model for MHC class II antigen presentation. Using Infliximab and Rituximab as showcase stories, the model demonstrated an unprecedented performance for predicting MAPPs and CD4 T cell epitopes in the context of protein-drug immunogenicity, complementing results from MAPPs assays and outperforming conventional prediction models trained on binding affinity data.
-
-
-
In vitro experiments
-
Immunology and Microbiology
The Possible Mechanism of Idiosyncratic Lapatinib-Induced Liver Injury in Patients Carrying Human Leukocyte Antigen-DRB1*07:01.
In PLoS One on 23 June 2015 by Hirasawa, M., Hagihara, K., et al.
PubMed
Idiosyncratic lapatinib-induced liver injury has been reported to be associated with human leukocyte antigen (HLA)-DRB1*07:01. In order to investigate its mechanism, interaction of lapatinib with HLA-DRB1*07:01 and its ligand peptide derived from tetanus toxoid, has been evaluated in vitro. Here we show that lapatinib enhances binding of the ligand peptide to HLA-DRB1*07:01. Furthermore in silico molecular dynamics analysis revealed that lapatinib could change the β chain helix in the HLA-DRB1*07:01 specifically to form a tightly closed binding groove structure and modify a large part of the binding groove. These results indicate that lapatinib affects the ligand binding to HLA-DRB1*07:01 and idiosyncratic lapatinib-induced liver injury might be triggered by this mechanism. This is the first report showing that the clinically available drug can enhance the binding of ligand peptide to HLA class II molecules in vitro and in silico.
-
-
-
Immunology and Microbiology
H7N9 T-cell epitopes that mimic human sequences are less immunogenic and may induce Treg-mediated tolerance.
In Hum Vaccin Immunother on 20 June 2015 by Liu, R., Moise, L., et al.
PubMed
Avian-origin H7N9 influenza is a novel influenza A virus (IAV) that emerged in humans in China in 2013. Using immunoinformatics tools, we identified several H7N9 T cell epitopes with T cell receptor (TCR)-facing residues identical to those of multiple epitopes from human proteins. We hypothesized that host tolerance to these peptides may impair T helper response and contribute to the low titer, weak hemagglutination inhibiting (HI) antibody responses and diminished seroconversion rates that have been observed in human H7N9 infections and vaccine trials. We found that the magnitude of human T effector responses to individual H7N9 peptides was inversely correlated with the peptide's resemblance to self. Furthermore, a promiscuous T cell epitope from the hemagglutinin (HA) protein suppressed responses to other H7N9 peptides when co-administered in vitro. Along with other highly 'human-like' peptides from H7N9, this peptide was also shown to expand FoxP3(+) regulatory T cells (Tregs). Thus, H7N9 may be camouflaged from effective human immune response by T cell epitope sequences that avert or regulate effector T cell responses through host tolerance.
-