InVivoMAb anti-human Ganglioside GD2
Product Description
Specifications
| Isotype | Mouse IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG2a isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Neuroblastoma cell line LAN-1 |
| Reported Applications |
in vitro induction of apoptosis in GD2+ cells in vivo inhibition of GD2+ tumor cell growth |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2819045 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vitro induction of apoptosis in GD2+ cells
Durbas, M., et al (2018). "GD2 ganglioside-binding antibody 14G2a and specific aurora A kinase inhibitor MK-5108 induce autophagy in IMR-32 neuroblastoma cells" Apoptosis 23(9-10): 492-511.
PubMed
The process of autophagy and its role in survival of human neuroblastoma cell cultures was studied upon addition of an anti-GD2 ganglioside (GD2) 14G2a mouse monoclonal antibody (14G2a mAb) and an aurora A kinase specific inhibitor, MK-5108. It was recently shown that combination of these agents significantly potentiates cytotoxicity against IMR-32 and CHP-134 neuroblastoma cells in vitro, as compared to the inhibitor used alone. In this study we gained mechanistic insights on autophagy in the observed cytotoxic effects exerted by both agents using cytotoxicity assays, RT-qPCR, immunoblotting, and autophagy detection methods. Enhancement of the autophagy process in the 14G2a mAb- and MK-5108-treated IMR-32 cells was documented by assessing autophagic flux. Application of a lysosomotropic agent-chloroquine (CQ) affected the 14G2a mAb- and MK-5108-stimulated autophagic flux. It is our conclusion that the 14G2a mAb (40 mug/ml) and MK-5108 inhibitor (0.1 muM) induce autophagy in IMR-32 cells. Moreover, the combinatorial treatment of IMR-32 cells with the 14G2a mAb and CQ significantly potentiates cytotoxic effect, as compared to CQ used alone. Most importantly, we showed that interfering with autophagy at its early and late step augments the 14G2a mAb-induced apoptosis, therefore we can conclude that inhibition of autophagy is the primary mechanism of the CQ-mediated sensitization to the 14G2a mAb-induced apoptosis. Although, there was no virtual stimulation of autophagy in the 14G2a mAb-treated CHP-134 neuroblastoma cells, we were able to show that PHLDA1 protein positively regulates autophagy and this process exists in a mutually exclusive manner with apoptosis in PHLDA1-silenced CHP-134 cells.
in vitro induction of apoptosis in GD2+ cells
Kowalczyk, A., et al (2009). "The GD2-specific 14G2a monoclonal antibody induces apoptosis and enhances cytotoxicity of chemotherapeutic drugs in IMR-32 human neuroblastoma cells" Cancer Lett 281(2): 171-182.
PubMed
Neuroblastoma (NB) is the most common extracranial solid tumor of childhood. The majority of children suffers from high risk neuroblastoma and has disseminated disease at the time of diagnosis. Despite recent advances in chemotherapy, the prognoses for children with high risk NB remain poor. Therefore, new treatment modalities are urgently needed. GD2 ganglioside is an antigen that is highly expressed on NB cells with only limited distribution on healthy tissues. Consequently, it appears to be an ideal target for both active and passive immunotherapy. The immunological effector mechanisms mediated by anti-GD2 monoclonal antibodies (mAbs) have been already well characterized. However, a growing number of reports suggest that GD2-specific antibodies may exhibit anti-proliferative effects without the immune system involvement. Here, we have shown that anti-GD2 14G2a mAb is capable of decreasing survival of IMR-32 human neuroblastoma cells in a dose-dependent manner. Death induced by this antibody exhibited several characteristics typical for apoptosis such as increased number of Annexin V- and propidium iodide-positive cells, cleavage of caspase 3 and prominent rise in caspase activity. The use of a pan caspase inhibitor Z-VAD-fmk suggested that the killing potential of this mAb is partially caspase-dependent. 14G2a mAb was rapidly endocytosed upon antigen binding. Employment of chloroquine, an inhibitor of lysosomal degradation, did not rescue IMR-32 cells from antibody-induced cell death suggesting lack of ceramide involvement in the observed effect. Most importantly, our studies showed that at particular drug concentrations 14G2a mAb exerts a synergistic effect with doxorubicin and topotecan, as well as an additive effect with carboplatin in killing IMR-32 cells in vitro. Our results provide guidance regarding how to best combine GD2-specific 14G2a antibody with existing cancer therapeutic agents to improve available treatment modalities for neuroblastoma.
in vivo induction of apoptosis in GD2+ cells
Mujoo, K., et al (1989). "Functional properties and effect on growth suppression of human neuroblastoma tumors by isotype switch variants of monoclonal antiganglioside GD2 antibody 14.18" Cancer Res 49(11): 2857-2861.
PubMed
A complete family of IgG isotype switch variant hybridomas was generated from the anti-GD2 monoclonal IgG3-producing hybridoma, 14.18, with the aid of the fluorescence-activated cell sorter. The IgG1, IgG2b, and IgG2a monoclonal antibodies (Mabs) produced by respective isotype switch variant hybridomas 14G1, 14G2b, or 14G2a, have binding activities for the biochemically defined GD2 antigen and GD2-expressing neuroblastoma target cell lines identical to that of IgG3 Mabs produced by the 14.18 parent cell line. This permitted us to examine the relative in vitro and in vivo cytotoxic capacities of each of the anti-GD2 antibodies for GD2-expressing neuroblastoma cells independent of antibody binding affinity or specificity. Mabs produced by 14.18, 14G2a, or 14G2b, but not 14G1, can direct efficient complement-dependent cytotoxicity against neuroblastoma tumor cells in the presence of human complement. Mabs produced by the parent 14.18 or by 14G2a are more efficient in directing antibody-dependent cell-mediated cytotoxicity than Mabs produced by 14G2b, and Mabs of 14G1 are inactive. However, despite these noted in vitro differences, antibodies produced by each member of this switch variant family suppress the growth of human neuroblastoma tumor cells in BALB/c athymic nu/nu mice. These studies suggest that a mechanism(s) other than Fc-directed complement-dependent cytotoxicity or antibody-dependent cell-mediated cytotoxicity may account for the in vivo antitumor effects of these particular antibodies.
Product Citations
-
-
Cancer Research
Conditional Activation of c-MYC in Distinct Catecholaminergic Cells Drives Development of Neuroblastoma or Somatostatinoma.
In Cancer Res on 1 February 2025 by Wang, T., Liu, L., et al.
PubMed
c-MYC is an important driver of high-risk neuroblastoma. A lack of c-MYC-driven genetically engineered mouse models (GEMM) has hampered the ability to better understand mechanisms of neuroblastoma oncogenesis and to develop effective therapies. In this study, we showed that conditional c-MYC induction via Cre recombinase driven by a tyrosine hydroxylase promoter led to a preponderance of PDX1+ somatostatinoma, a type of pancreatic neuroendocrine tumor. However, c-MYC activation via an improved Cre recombinase driven by a dopamine β-hydroxylase promoter resulted in neuroblastoma development. The c-MYC murine neuroblastoma tumors recapitulated the pathologic and genetic features of human neuroblastoma and responded to anti-GD2 immunotherapy and difluoromethylornithine, an FDA-approved inhibitor targeting the MYC transcriptional target ODC1. Thus, c-MYC overexpression results in different but related tumor types depending on the targeted cell. The GEMMs represent valuable tools for testing immunotherapies and targeted therapies for these diseases. Significance: The development of c-MYC-driven genetically engineered neuroblastoma and somatostatinoma mouse models provides useful tools for understanding the tumor cell origin and investigating treatment strategies.
-
-
-
Radioactive-immunoassays
-
Mus musculus (Mouse)
Detection properties of indium-111 and IRDye800CW for intraoperative molecular imaging use across tissue phantom models.
In J Biomed Opt on 1 January 2025 by Sever, R. E., Rosenblum, L. T., et al.
PubMed
Intraoperative molecular imaging (IMI) enables the detection and visualization of cancer tissue using targeted radioactive or fluorescent tracers. While IMI research has rapidly expanded, including the recent Food and Drug Administration approval of a targeted fluorophore, the limits of detection have not been well-defined.
-
-
-
Immunohistochemistry-immunofluorescence
-
Cancer Research
Copper chelation redirects neutrophil function to enhance anti-GD2 antibody therapy in neuroblastoma.
In Nat Commun on 12 December 2024 by Rouaen, J. R. C., Salerno, A., et al.
PubMed
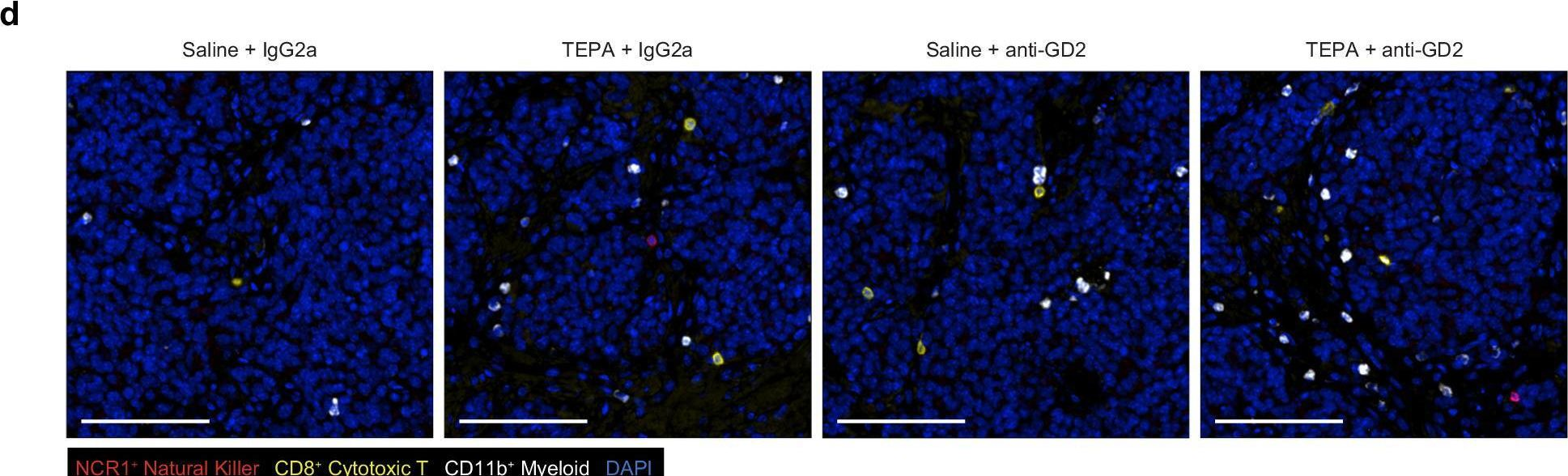
Anti-disialoganglioside (GD2) antibody therapy has provided clinical benefit to patients with neuroblastoma however efficacy is likely impaired by the immunosuppressive tumor microenvironment. We have previously defined a link between intratumoral copper levels and immune evasion. Here, we report that adjuvant copper chelation potentiates anti-GD2 antibody therapy to confer durable tumor control in immunocompetent models of neuroblastoma. Mechanistic studies reveal copper chelation creates an immune-primed tumor microenvironment through enhanced infiltration and activity of Fc-receptor-bearing cells, specifically neutrophils which are emerging as key effectors of antibody therapy. Moreover, we report copper sequestration by neuroblastoma attenuates neutrophil function which can be successfully reversed using copper chelation to increase pro-inflammatory effector functions. Importantly, we repurpose the clinically approved copper chelating agent Cuprior as a non-toxic, efficacious immunomodulatory strategy. Collectively, our findings provide evidence for the clinical testing of Cuprior as an adjuvant to enhance the activity of anti-GD2 antibody therapy and improve outcomes for patients with neuroblastoma.
-
-
-
Cancer Research
Dual-labeled anti-GD2 targeted probe for intraoperative molecular imaging of neuroblastoma.
In J Transl Med on 15 October 2024 by Rosenblum, L. T., Sever, R. E., et al.
PubMed
Surgical resection is integral for the treatment of neuroblastoma, the most common extracranial solid malignancy in children. Safely locating and resecting primary tumor and remote deposits of disease remains a significant challenge, resulting in high rates of complications and incomplete surgery, worsening outcomes. Intraoperative molecular imaging (IMI) uses targeted radioactive or fluorescent tracers to identify and visualize tumors intraoperatively. GD2 was selected as an IMI target, as it is highly overexpressed in neuroblastoma and minimally expressed in normal tissue.
-
-
-
Cancer Research
-
Endocrinology and Physiology
Conditional c-MYC activation in catecholaminergic cells drives distinct neuroendocrine tumors: neuroblastoma vs somatostatinoma
In bioRxiv on 14 March 2024 by Wang, T., Liu, L., et al.
-
-
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
Integrative analysis of neuroblastoma by single-cell RNA sequencing identifies the NECTIN2-TIGIT axis as a target for immunotherapy.
In Cancer Cell on 12 February 2024 by Wienke, J., Visser, L. L., et al.
PubMed
Pediatric patients with high-risk neuroblastoma have poor survival rates and urgently need more effective treatment options with less side effects. Since novel and improved immunotherapies may fill this need, we dissect the immunoregulatory interactions in neuroblastoma by single-cell RNA-sequencing of 24 tumors (10 pre- and 14 post-chemotherapy, including 5 pairs) to identify strategies for optimizing immunotherapy efficacy. Neuroblastomas are infiltrated by natural killer (NK), T and B cells, and immunosuppressive myeloid populations. NK cells show reduced cytotoxicity and T cells have a dysfunctional profile. Interaction analysis reveals a vast immunoregulatory network and identifies NECTIN2-TIGIT as a crucial immune checkpoint. Combined blockade of TIGIT and PD-L1 significantly reduces neuroblastoma growth, with complete responses (CR) in vivo. Moreover, addition of TIGIT+PD-L1 blockade to standard relapse treatment in a chemotherapy-resistant Th-ALKF1174L/MYCN 129/SvJ syngeneic model induces CR. In conclusion, our integrative analysis provides promising targets and a rationale for immunotherapeutic combination strategies.
-