InVivoMAb anti-human EphA2
Product Description
Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
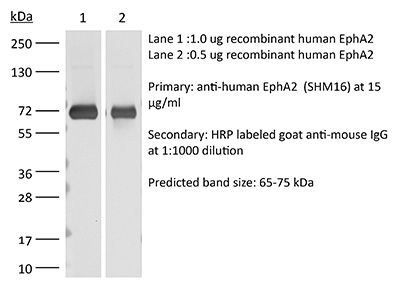
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Human melanoma cell line A375 |
| Reported Applications |
in vitro stimulation of EphA2 signaling Functional assays Immunoprecipitation Flow cytometry Immunofluorescence |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Immunofluorescence
Tweedell RE, Tao D, Hamerly T, Robinson TM, Larsen S, Grønning AGB, Norris AM, King JG, Law HCH, Baumbach J, Bergmann-Leitner ES, Dinglasan RR (2019). "The Selection of a Hepatocyte Cell Line Susceptible to Plasmodium falciparum Sporozoite Inv
PubMed
In vitro studies of liver stage (LS) development of the human malaria parasite Plasmodium falciparum are technically challenging; therefore, fundamental questions about hepatocyte receptors for invasion that can be targeted to prevent infection remain unanswered. To identify novel receptors and to further understand human hepatocyte susceptibility to P. falciparum sporozoite invasion, we created an optimized in vitro system by mimicking in vivo liver conditions and using the subcloned HC-04.J7 cell line that supports mean infection rates of 3-5% and early development of P. falciparum exoerythrocytic forms-a 3- to 5-fold improvement on current in vitro hepatocarcinoma models for P. falciparum invasion. We juxtaposed this invasion-susceptible cell line with an invasion-resistant cell line (HepG2) and performed comparative proteomics and RNA-seq analyses to identify host cell surface molecules and pathways important for sporozoite invasion of host cells. We identified and investigated a hepatocyte cell surface heparan sulfate proteoglycan, glypican-3, as a putative mediator of sporozoite invasion. We also noted the involvement of pathways that implicate the importance of the metabolic state of the hepatocyte in supporting LS development. Our study highlights important features of hepatocyte biology, and specifically the potential role of glypican-3, in mediating P. falciparum sporozoite invasion. Additionally, it establishes a simple in vitro system to study the LS with improved invasion efficiency. This work paves the way for the greater malaria and liver biology communities to explore fundamental questions of hepatocyte-pathogen interactions and extend the system to other human malaria parasite species, like P. vivax.
in vitro stimulation of EphA2 signaling
Immunoprecipitation
Flow Cytometry
Functional Assays
Agonistic Antibodies
Sakamoto A, Kato K, Hasegawa T, Ikeda S (2018). "An Agonistic Antibody to EPHA2 Exhibits Antitumor Effects on Human Melanoma Cells" Anticancer Res 38(6):3273-3282.
PubMed
Background/aim: EPH receptor A2 (EPHA2) is highly expressed in aggressive types of human cancer, and is expected to be an excellent target molecule for antibody treatments. In this study, we investigated the therapeutic potential of antibody to EPHA2 against melanoma in vitro. Materials and methods: We generated three monoclonal antibodies (mAbs) to EPHA2 and examined cell-surface expression by flow cytometry. To investigate the ability to inhibit tumor cell migration therapy with mAbs to EPHA2, we performed a wound scratch assay and invasion assay. We investigated the therapeutic effects of immunotoxins consisting of toxin-conjugated EPHA2 mAbs. Results: All human melanoma cell lines studied expressed EPHA2. Like natural ligand ephrin-A1, one of EPHA2 mAbs, SHM16, inhibited metastatic behavior of cells, such as migration and invasion. In addition, drastic growth inhibition and cytotoxicity were found using immunotoxin-conjugated SHM16. Conclusion: These observations indicate a promising role for EPHA2 as a target in antibody treatments for melanoma, and demonstrate the potential therapeutic effects of an agonistic antibody to EPHA2.