InVivoMAb anti-human EGFR
Product Description
Specifications
| Isotype | Mouse IgG2a |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG2a isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
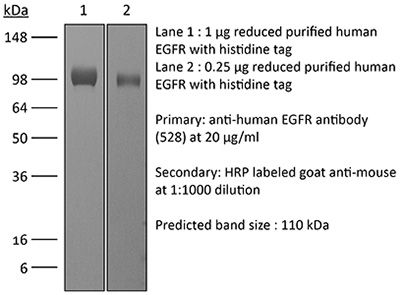
| Immunogen | Purified EGFR from A431 cells |
| Reported Applications |
in vitro EGFR blockade in vivo EGFR blockade in xenografts Western blot Functional assays Immunoprecipitation Immunohistochemistry (paraffin) Immunofluorescence Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_2687802 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored undiluted at 4°C, and protected from prolonged exposure to light. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Immunofluorescence
Dong, A., et al (2015). "Epidermal growth factor receptor (EGFR) signaling requires a specific endoplasmic reticulum thioredoxin for the post-translational control of receptor presentation to the cell surface" J Biol Chem 290(13): 8016-8027.
PubMed
The epidermal growth factor receptor (EGFR) is a well characterized receptor-tyrosine kinase that functions in development and serves a vital role in many human cancers. Understanding EGFR regulatory mechanisms, and hence approaches for clinical intervention, has focused on ligand-receptor interactions and tyrosine kinase activity. Here, we show using the NCI-H460 lung and A431 epidermoid human cancer cell lines that EGFR binding to anterior gradient homolog 2 (AGR2) in the endoplasmic reticulum is required for receptor delivery to the plasma membrane and thus EGFR signaling. Reduced AGR2 protein levels or mutation of an essential cysteine in the active site result in decreased cell surface EGFR and a concomitant decrease in signaling as reflected by AREG, EGR1, and FOS expression. Similar to previously described EGFR nulls, an AGR2 null also resulted in embryonic lethality. Consistent with its role in regulating EGFR-mediated signaling, AGR2 expression is also enhanced in many human cancers and promotes the transformed phenotype. Furthermore, EGFR-mediated signaling in NCI-H460 cells, which are resistant to the tyrosine kinase inhibitor AG1478, is also disrupted with reduced AGR2 expression. The results provide insights into why cancer prognosis or response to therapy often does not correlate with EGFR protein or RNA levels because they do not reflect delivery to the cell surface where signaling is initiated. AGR2, therefore, represents a novel post-translational regulator of EGFR-mediated signaling and a promising target for treating human cancers.
Immunoprecipitation
Functional Assays
Raimondi, F., et al (2008). "Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation" Am J Physiol Gastrointest Liver Physiol 294(4): G906-913.
PubMed
Intestinal and systemic illnesses have been linked to increased gut permeability. Bile acids, whose luminal profile can be altered in human disease, modulate intestinal paracellular permeability. We investigated the mechanism by which selected bile acids increase gut permeability using a validated in vitro model. Human intestinal Caco-2 cells were grown in monolayers and challenged with a panel of bile acids. Transepithelial electrical resistance and luminal-to-basolateral fluxes of 10-kDa Cascade blue-conjugated dextran were used to monitor paracellular permeability. Immunoprecipitation and immunoblot analyses were employed to investigate the intracellular pathway. Redistribution of tight junction proteins was studied by confocal laser microscopy. Micromolar concentrations of cholic acid, deoxycholic acid (DCA), and chenodeoxycholic acid (CDCA) but not ursodeoxycholic acid decreased transepithelial electrical resistance and increased dextran flux in a reversible fashion. Coincubation of 50 muM CDCA or DCA with EGF, anti-EGF monoclonal antibody, or specific src inhibitor 4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP-2) abolished the effect. A concentration of 50 muM of either CDCA or DCA also induced EGF receptor phosphorylation, occludin dephosphorylation, and occludin redistribution at the tight junction level in the same time frame and in a reversible fashion. We conclude that selected bile acids modulate intestinal permeability via EGF receptor autophosphorylation, occludin dephosphorylation, and rearrangement at the tight junction level. The effect is mediated by the src family kinases and is abolished by EGF treatment. These data also support the role of bile acids in the genesis of necrotizing enterocolitis and the protective effect of EGF treatment.
Flow Cytometry
Kurai, J., et al (2007). "Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines" Clin Cancer Res 13(5): 1552-1561.
PubMed
PURPOSE: Epidermal growth factor receptor (EGFR) is commonly overexpressed in lung cancer. Cetuximab is a chimeric mouse-human antibody targeted against EGFR. Compared with its inhibitory properties, its immunologic mechanisms have not been well studied. In this study, we investigated the antibody-dependent cellular cytotoxicity (ADCC) activity of cetuximab against lung cancer cell lines. EXPERIMENTAL DESIGN: We studied the correlation between EGFR expression in lung cancer cell lines and the ADCC activity of cetuximab as well as the influence of interleukin-2 and chemotherapy on the ADCC activity. EGFR expression was measured by a quantitative flow cytometric analysis and immunohistochemistry. The ADCC activity was assessed by a 4-h (51)Cr release assay. Peripheral blood mononuclear cells, purified T cells, natural killer (NK) cells, and monocytes from healthy donors or lung cancer patients were used as effector cells. RESULTS: Fresh peripheral blood mononuclear cells exhibited cetuximab-mediated ADCC activity against lung cancer cell lines at a low concentration of cetuximab (0.25 microg/mL). A logarithmic correlation was observed between the number of EGFRs and ADCC activity. Even low EGFR expression, which was weakly detectable by immunohistochemistry, was sufficient for maximum ADCC activity, and further increases in EGFR expression on the target cells had no further effect on the ADCC activity. In addition, ADCC activity was enhanced by interleukin-2 mainly through activation of NK cells and was less susceptible to immunosuppression by chemotherapy than NK activity in lung cancer patients. CONCLUSIONS: These observations suggest the importance of ADCC activity as an immunologic mechanism of cetuximab in biological therapy for lung cancer patients.
Flow Cytometry
Nakamura, T., et al (2005). "Rescue and propagation of fully retargeted oncolytic measles viruses" Nat Biotechnol 23(2): 209-214.
PubMed
Live attenuated measles viruses of the Edmonston lineage (MV-Edm) have potent anti-tumor activity but are not entirely tumor-specific owing to widespread distribution of their native receptors, CD46 and SLAM. We have therefore developed a pseudoreceptor system that allows rescue and propagation of fully retargeted viruses displaying single-chain antibody fragments. Viruses retargeted to tumor-selective CD38, epidermal growth factor receptor (EGFR) or EGFR mutant vIII (EGFRvIII) efficiently entered cells through their respective targeted receptors in vitro and in vivo, but not through CD46 and SLAM. When administered intratumorally or intravenously to mice bearing human CD38 or EGFR-positive human tumor xenografts, the targeted viruses demonstrated specific receptor-mediated anti-tumor activity. These data provide an in vivo demonstration of antibody-directed tumor destruction by retargeted oncolytic viruses.
in vivo EGFR blockade in xenografts
Perera, R. M., et al (2005). "Treatment of human tumor xenografts with monoclonal antibody 806 in combination with a prototypical epidermal growth factor receptor-specific antibody generates enhanced antitumor activity" Clin Cancer Res 11(17): 6390-6
PubMed
Monoclonal antibody (mAb) 806 is a novel epidermal growth factor receptor (EGFR) antibody with significant antitumor activity that recognizes a mutant EGFR commonly expressed in glioma known as delta2-7 EGFR (de2-7 EGFR or EGFRvIII) and a subset of the wild-type (wt) EGFR found in cells that overexpress the receptor. We have used two human xenograft mouse models to examine the efficacy of mAb 806 in combination with mAb 528, a prototypical anti-EGFR antibody with similar specificity to cetuximab. Treatment of nude mice, bearing s.c. or i.c. tumor human xenografts expressing the wt or de2-7 EGFR, with mAbs 806 and 528 in combination resulted in additive and in some cases synergistic, antitumor activity. Interestingly, mAb 528 was also effective against xenografts expressing the ligand independent de2-7 EGFR when used as a single agent, showing that its antitumor activity is not merely mediated through inhibition of ligand binding. When used as single agents, neither mAbs 806 or 528 induced down-regulation of the de2-7 EGFR either in vitro or in vivo. In contrast, the combination of antibodies produced a rapid and dramatic decrease in the total cell surface de2-7 EGFR both in vitro and in xenografts. Consistent with this decrease in total cell surface de2-7 EGFR, we observed up-regulation of the cell cycle inhibitor p27(KIP1) and a decrease in tumor cell proliferation as measured by Ki-67 immunostaining when the antibodies were used in combination in vivo. Thus, mAb 806 can synergize with other EGFR-specific antibodies thereby providing a rationale for its translation into the clinic.
Immunohistochemistry (paraffin)
Burton, E. C., et al (2002). "Aberrant p53, mdm2, and proliferation differ in glioblastomas from long-term compared with typical survivors" Clin Cancer Res 8(1): 180-187.
PubMed
PURPOSE: Glioblastoma multiforme (GBM) is a highly lethal neoplasm with a median survival of approximately 1 year. Only 2-5% of patients originally diagnosed with GBM will survive > or = 3 years. Whether tumors from these long-term survivors (LTSs) exhibit molecular genetic differences compared with typical GBM survivors is not known. EXPERIMENTAL DESIGN: Tumors from 41 patients initially diagnosed with GBM and having survival > or = 3 years (LTS) was compared with 48 GBMs from short-term survivors (STSs, survival < or = 1.5 years) for p53 aberrations (expression/mutation), epidermal growth factor receptor overexpression, mdm2 overexpression, and proliferation index. RESULTS: Nuclear p53 expression was significantly more frequent in the LTS group. However, no difference in the rate of p53 mutation was evident. Overexpression of epidermal growth factor receptor was slightly more frequent in the STS patients, but this is not statistically different. mdm2 overexpression was significantly more frequent in the STSs, and this group had a significantly higher median proliferation index. CONCLUSION: Long-term GBM survivors were more likely to have p53-overexpressing tumors, although a difference in p53 mutation rate could not be detected. They were less likely to exhibit mdm2 overexpression and had a lower proliferation rate compared with typical GBM survivors.
in vitro EGFR blockade
Immunoprecipitation
Western Blot
Keshamouni, V. G., et al (2002). "Mechanism of 17-beta-estradiol-induced Erk1/2 activation in breast cancer cells. A role for HER2 AND PKC-delta" J Biol Chem 277(25): 22558-22565.
PubMed
Activation of mitogen-activated protein kinase (Erk/MAPK) is a critical signal transduction event for estrogen (E(2))-mediated cell proliferation. Recent studies from our group and others have shown that persistent activation of Erk plays a major role in cell migration and tumor progression. The signaling mechanism(s) responsible for persistent Erk activation are not fully characterized, however. In this study, we have shown that E(2) induces a slow but persistent activation of Erk in MCF-7 breast carcinoma cells. The E(2)-induced Erk activation is dependent on new protein synthesis, suggesting that E(2)-induced growth factors play a major role in Erk activation. When MCF-7 cells were treated with E(2) in the presence of an anti-HER-2 monoclonal antibody (herceptin), 60-70% of E(2)-induced Erk activation is blocked. In addition, when untreated MCF-7 cells were exposed to conditioned medium from E(2)-treated cells, Erk activity was significantly enhanced. Furthermore Erk activity was blocked by an antibody against HER-2 or by heregulin (HRG) depletion from the conditioned medium through immunoprecipitation. In contrast, epidermal growth factor receptor (Ab528) antibody only blocked 10-20% of E(2)-induced Erk activation, suggesting that E(2)-induced Erk activation is predominantly mediated through the secretion of HRG and activation of HER-2 by an autoctine/paracrine mechanism. Inhibition of PKC-delta-mediated signaling by a dominant negative mutant or the relatively specific PKC-delta inhibitor rottlerin blocked most of the E(2)-induced Erk activation but had no effect on TGF alpha-induced Erk activation. By contrast inhibition of Ras, by inhibition of farnesyl transferase (Ftase-1) or dominant negative (N17)-Ras, significantly inhibited both E(2)- and TGF alpha-induced Erk activation. This evaluation of downstream signaling revealed that E(2)-induced Erk activation is mediated by a HRG/HER-2/PKC-delta/Ras pathway that could be crucial for E(2)-dependent growth-promoting effects in early stages of tumor progression.
in vitro EGFR blockade
Sherwood, E. R., et al (1998). "Epidermal growth factor receptor activation in androgen-independent but not androgen-stimulated growth of human prostatic carcinoma cells" Br J Cancer 77(6): 855-861.
PubMed
These studies were undertaken to assess the relative expression and autocrine activation of the epidermal growth factor receptor (EGFR) in normal and transformed prostatic epithelial cells and to determine whether EGFR activation plays a functional role in androgen-stimulated growth of prostate cancer cells in vitro. EGFR expression was determined by Western blot analysis and ELISA immunoassays. Immunoprecipitation of radiophosphorylated EGFR and evaluation of tyrosine phosphorylation was used to assess EGFR activation. The human androgen-independent prostate cancer cell lines PC3 and DU145 exhibited higher levels of EGFR expression and autocrine phosphorylation than normal human prostatic epithelial cells or the human androgen-responsive prostate cancer cell line LNCaP. PC3 and DU145 cells also showed higher levels of autonomous growth under serum-free defined conditions. Normal prostatic epithelial cells expressed EGFR but did not exhibit detectable levels of EGFR phosphorylation when cultured in the absence of exogenous EGF. Addition of EGF stimulated EGFR phosphorylation and induced proliferation of normal cells. LNCaP cells exhibited autocrine phosphorylation of EGFR but did not undergo significant proliferation when cultured in the absence of exogenous growth factors. A biphasic growth curve was observed when LNCaP cells were cultured with dihydrotestosterone (DHT). Maximum proliferation occurred at 1 nM DHT with regression of the growth response at DHT concentrations greater than 1 nM. However, neither EGFR expression nor phosphorylation was altered in LNCaP cells after androgen stimulation. In addition, DHT-stimulated growth of LNCaP cells was not inhibited by anti-EGFR. These studies show that autocrine activation of EGFR is a common feature of prostatic carcinoma cells in contrast to normal epithelial cells. However, EGFR activation does not appear to play a functional role in androgen-stimulated growth of LNCaP cells in vitro.
Functional Assays
Kawamoto, T., et al (1983). "Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody" Proc Natl Acad Sci U S A 80(5): 1337-1341.
PubMed
Epidermal growth factor (EGF) at 3 nM maximally inhibits the proliferation of A431 epidermoid carcinoma cells. We show that at lower concentrations, in the range of 3-100 pM, EGF has a mitogenic effect on A431 cells. In the presence of 100 nM anti-EGF-receptor monoclonal IgG (designated 528), which inhibits A431 cell proliferation and blocks greater than 95% of EGF binding, EGF becomes mitogenic for A431 cells at concentrations up to 3 nM. These results suggest that a minor population of high-affinity EGF receptors may be involved in stimulation of A431 cell proliferation. Saturation binding assays with 125I-labeled EGF indicate that approximately equal to 0.1-0.2% of receptors for EGF are high-affinity receptors that bind EGF with an estimated Kd of 7 X 10(-11) M. This affinity is nearly 2 orders of magnitude higher than that of the remaining EGF receptors. Although A431 cell proliferation is maximally inhibited by nonsaturating amounts of EGF (3 nM), maximal inhibition by 528 IgG (approximately equal to 70% of maximal inhibition by EGF) requires saturating concentrations of antibody (approximately equal to 15 nM). Unlike EGF, rapid down-regulation is not observed with 528 IgG. These results indicate different mechanisms of growth inhibition of A431 cells by EGF and 528 IgG.
Product Citations
-
-
Homo sapiens (Human)
Exploring the Sensitivity of Antibody-Drug Conjugate Efficacy to the Selection of Payload, Antibody, and Cell line.
In Bioconjug Chem on 17 January 2024 by Rao, M., Murali, S., et al.
PubMed
Antibody-drug conjugates (ADCs) make up a growing class of targeted therapeutics with important applications in cancer treatment. ADCs are highly modular in nature and thus can be engineered to target any cancer type, but their efficacy is strongly influenced by the specific choice of payload, antibody, and target cell. Considering the number of possible antibody-payload combinations, ADC development would benefit from an efficient method to narrow the number of ADC compositions to those with the highest and most universal potency prior to assessing pharmacokinetics and pharmacodynamics in animal models. To facilitate the identification of optimal ADC compositions, we describe the use of photoreactive antibody-binding domain-drug conjugates (known commercially as oYo-Link) to enable the site-specific labeling of off-the-shelf antibodies. This approach allows for the rapid generation of ADCs with a drug-to-antibody ratio of ∼2 with no subsequent purification required. As a demonstration of this approach, ADCs were generated with different combinations of tubulin-inhibitor drugs (DM1, DM4, VcMMAE, and VcMMAF) and anti-EGFR antibodies (cetuximab, panitumumab, anti-EGFR clone 425, and anti-EGFR clone 528) and were delivered to three EGFR-expressing cell lines (A431, A549, and MDA-MB-231). Real-time cytolysis assays indicated that the most effective antibody varied based on the choice of cell line: cetuximab was most potent against A431 cells, while 425 and 528 led to the greatest cytotoxicity against A549 and MDA-MB-231 cells. These results did not correlate with differences in measured anti-EGFR binding affinity as cetuximab had the highest affinity across all three cell lines, while 425 and 528 had the lowest affinities for all three cell lines. Panitumumab, which had the second-highest anti-EGFR affinity, exhibited the least effective cytolysis across A431, A549, and MDA-MB-231 cells. By demonstrating that ADC potency toward a given target is dependent on both the antibody and drug chosen, these findings can guide the selection of ADCs for further in vivo analysis.
-
-
Multiplexed live-cell profiling with Raman probes.
In Nat Commun on 7 June 2021 by Chen, C., Zhao, Z., et al.
PubMed
Single-cell multiparameter measurement has been increasingly recognized as a key technology toward systematic understandings of complex molecular and cellular functions in biological systems. Despite extensive efforts in analytical techniques, it is still generally challenging for existing methods to decipher a large number of phenotypes in a single living cell. Herein we devise a multiplexed Raman probe panel with sharp and mutually resolvable Raman peaks to simultaneously quantify cell surface proteins, endocytosis activities, and metabolic dynamics of an individual live cell. When coupling it to whole-cell spontaneous Raman micro-spectroscopy, we demonstrate the utility of this technique in 14-plexed live-cell profiling and phenotyping under various drug perturbations. In particular, single-cell multiparameter measurement enables powerful clustering, correlation, and network analysis with biological insights. This profiling platform is compatible with live-cell cytometry, of low instrument complexity and capable of highly multiplexed measurement in a robust and straightforward manner, thereby contributing a valuable tool for both basic single-cell biology and translation applications such as high-content cell sorting and drug discovery.
-
Single-step Enzymatic Glycoengineering for the Construction of Antibody-cell Conjugates
In bioRxiv on 10 March 2018 by Li, J., Chen, M., et al.