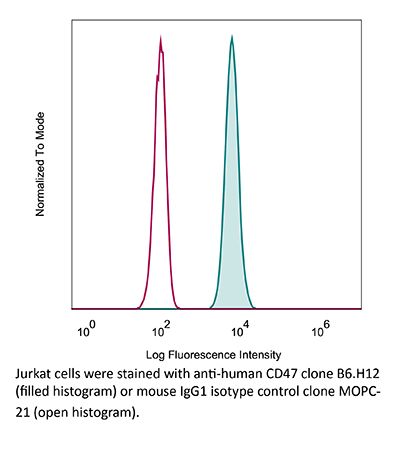
InVivoMAb anti-human CD47
Product Description
Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Intact CD47 purified from placenta |
| Reported Applications |
in vitro CD47 neutralization in vivo CD47 neutralization in human tumor xenograft models or humanized mice Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107655 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo CD47 neutralization in human tumor xenograft model
Gordon, S. R., et al (2017). "PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity" Nature 545(7655): 495-499.
PubMed
Programmed cell death protein 1 (PD-1) is an immune checkpoint receptor that is upregulated on activated T cells for the induction of immune tolerance. Tumour cells frequently overexpress the ligand for PD-1, programmed cell death ligand 1 (PD-L1), facilitating their escape from the immune system. Monoclonal antibodies that block the interaction between PD-1 and PD-L1, by binding to either the ligand or receptor, have shown notable clinical efficacy in patients with a variety of cancers, including melanoma, colorectal cancer, non-small-cell lung cancer and Hodgkin’s lymphoma. Although it is well established that PD-1-PD-L1 blockade activates T cells, little is known about the role that this pathway may have in tumour-associated macrophages (TAMs). Here we show that both mouse and human TAMs express PD-1. TAM PD-1 expression increases over time in mouse models of cancer and with increasing disease stage in primary human cancers. TAM PD-1 expression correlates negatively with phagocytic potency against tumour cells, and blockade of PD-1-PD-L1 in vivo increases macrophage phagocytosis, reduces tumour growth and lengthens the survival of mice in mouse models of cancer in a macrophage-dependent fashion. This suggests that PD-1-PD-L1 therapies may also function through a direct effect on macrophages, with substantial implications for the treatment of cancer with these agents.
in vitro CD47 neutralization
Lo, J., et al (2015). "Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice" Hepatology 62(2): 534-545.
PubMed
Sorafenib is a new standard treatment for patients with advanced hepatocellular carcinoma (HCC). However, the survival benefit of this treatment is modest, partly owing to drug resistance. Recent evidence has demonstrated the existence of tumor-initiating cells (T-ICs) as the culprit for treatment resistance. To examine whether sorafenib resistance was a result of the presence of liver T-ICs, we developed sorafenib-resistant HCC cells both in vitro and in vivo through continuous exposure to sorafenib. Using these models, we found that sorafenib-resistant clones demonstrated enhanced T-IC properties, including tumorigenicity, self-renewal, and invasiveness. In addition, several T-IC markers were found to be up-regulated, among which CD47 was found to be most significant. Using chromatin immunoprecipitation assays and expression analyses, CD47 expression was found to be regulated by nuclear factor kappa B (NF-kappaB) through a specific response element in the promoter of CD47, and the site occupancy and expression were increased and decreased upon stimulation and inhibition of NF-kappaB, respectively. Consistently, NF-kappaB was activated in sorafenib-resistant HCC cells, and this finding was confirmed in clinical HCC samples, which showed a positive correlation between NF-kappaB and CD47 expression. Functional characterization of CD47 in sorafenib-resistant HCC cells was evaluated using a lentivirus-based knockdown approach and showed increased sensitization to sorafenib upon CD47 knockdown. Furthermore, blockade of CD47 using anti-CD47 antibody (Ab) showed a similar effect. Using a patient-derived HCC xenograft mouse model, we found that anti-CD47 Ab (500 mug/mouse) in combination with sorafenib (100 mg/kg, orally) exerted synergistic effects on tumor suppression, as compared with sorafenib and anti-CD47 Ab alone. CONCLUSIONS: NF-kappaB-mediated CD47 up-regulation promotes sorafenib resistance, and targeting CD47 in combination with sorafenib is an attractive therapeutic regimen for the treatment of HCC patients.
in vivo CD47 neutralization in humanized mice
Lo, J., et al (2015). "Anti-CD47 antibody suppresses tumour growth and augments the effect of chemotherapy treatment in hepatocellular carcinoma" Liver Int. doi : 10.1111/liv.12963.
PubMed
BACKGROUND & AIMS: Hepatocellular carcinoma (HCC) is often associated with metastasis and recurrence leading to a poor prognosis. Therefore, development of novel treatment regimens is urgently needed to improve the survival of HCC patients. In this study, we aimed to investigate the in vitro and in vivo effects of anti-CD47 antibody alone and in combination with chemotherapy in HCC. METHODS: In this study, we examined the functional effects of anti-CD47 antibody (B6H12) on cell proliferation, sphere formation, migration and invasion, chemosensitivity, macrophage-mediated phagocytosis and tumourigenicity both in vitro and in vivo. The therapeutic efficacy of anti-CD47 antibody alone or in combination with doxorubicin was examined in patient-derived HCC xenograft. RESULTS: Blocking CD47 with anti-CD47 monoclonal antibody (B6H12) at 10 mug/ml could suppress self-renewal, tumourigenicity and migration and invasion abilities of MHCC-97L and Huh-7 cells. Interestingly, anti-CD47 antibody synergized the effect of HCC cells to chemotherapeutic drugs including doxorubicin and cisplatin. Blockade of CD47 by anti-CD47 antibody induced macrophage-mediated phagocytosis. Using a patient-derived HCC xenograft mouse model, we found that anti-CD47 antibody (400 mug/mouse) in combination with doxorubicin (2 mg/kg) exerted maximal effects on tumour suppression, as compared with doxorubicin and anti-CD47 antibody alone. CONCLUSIONS: Anti-CD47 antibody treatment could complement chemotherapy which may be a promising therapeutic strategy for the treatment of HCC patients.
in vivo CD47 neutralization in humanized mice
in vitro CD47 neutralization
Xiao, Z., et al (2015). "Antibody mediated therapy targeting CD47 inhibits tumor progression of hepatocellular carcinoma" Cancer Lett 360(2): 302-309.
PubMed
Human hepatocellular carcinoma (HCC) has a high rate of tumor recurrence and metastasis, resulting in shortened survival times. The efficacy of current systemic therapies for HCC is limited. In this study, we used xenograft tumor models to investigate the use of antibodies that block CD47 and inhibit HCC tumor growth. Immunostaining of tumor tissue and HCC cell lines demonstrated CD47 over-expression in HCC as compared to normal hepatocytes. Macrophage phagocytosis of HCC cells was increased after treatment with CD47 antibodies (CD47mAbs) that block CD47 binding to SIRPalpha. Further, CD47 blockade inhibited tumor growth in both heterotopic and orthotopic models of HCC, and promoted the migration of macrophages into the tumor mass. Our results demonstrate that targeting CD47 by specific antibodies has potential immunotherapeutic efficacy in human HCC.
in vivo CD47 neutralization in humanized mice
Lee, T. K., et al (2014). "Blockade of CD47-mediated cathepsin S/protease-activated receptor 2 signaling provides a therapeutic target for hepatocellular carcinoma" Hepatology 60(1): 179-191.
PubMed
Identification of therapeutic targets against tumor-initiating cells (TICs) is a priority in the development of new therapeutic paradigms against cancer. We enriched a TIC population capable of tumor initiation and self-renewal by serial passages of hepatospheres with chemotherapeutic agents. In chemoresistant hepatospheres, CD47 was found to be up-regulated, when compared with differentiated progenies. CD47 is preferentially expressed in liver TICs, which contributed to tumor initiation, self-renewal, and metastasis and significantly affected patients’ clinical outcome. Knockdown of CD47 suppressed stem/progenitor cell characteristics. CD47(+) hepatocellular carcinoma (HCC) cells preferentially secreted cathepsin S (CTSS), which regulates liver TICs through the CTSS/protease-activated receptor 2 (PAR2) loop. Suppression of CD47 by morpholino approach suppressed growth of HCC in vivo and exerted a chemosensitization effect through blockade of CTSS/PAR2 signaling. CONCLUSION: These data suggest that CD47 may be an attractive therapeutic target for HCC therapy.
in vitro CD47 neutralization
Flow Cytometry
Tseng, D., et al (2013). "Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response" Proc Natl Acad Sci U S A 110(27): 11103-11108.
PubMed
Mobilization of the T-cell response against cancer has the potential to achieve long-lasting cures. However, it is not known how to harness antigen-presenting cells optimally to achieve an effective antitumor T-cell response. In this study, we show that anti-CD47 antibody-mediated phagocytosis of cancer by macrophages can initiate an antitumor T-cell immune response. Using the ovalbumin model antigen system, anti-CD47 antibody-mediated phagocytosis of cancer cells by macrophages resulted in increased priming of OT-I T cells [cluster of differentiation 8-positive (CD8(+))] but decreased priming of OT-II T cells (CD4(+)). The CD4(+) T-cell response was characterized by a reduction in forkhead box P3-positive (Foxp3(+)) regulatory T cells. Macrophages following anti-CD47-mediated phagocytosis primed CD8(+) T cells to exhibit cytotoxic function in vivo. This response protected animals from tumor challenge. We conclude that anti-CD47 antibody treatment not only enables macrophage phagocytosis of cancer but also can initiate an antitumor cytotoxic T-cell immune response.
Product Citations
-
-
Cancer Research
Oncolytic adenovirus H101 enhances the anti-tumor effects of PD-1 blockade via CD47 downregulation in tumor cells.
In Oncol Res on 29 April 2025 by Qiao, C., Xu, Y., et al.
PubMed
To investigate the anti-tumor effects of an E1B55KD-deleted oncolytic adenovirus, H101, in combination with a humanized anti-PD-1 (Programmed cell death protein 1) monoclonal antibody, Camrelizumab.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Neuroscience
High-Throughput Screening on Primary Tumor-Associated Microglia and Macrophages Identifies HDAC Inhibitors as Enhancers of Phagocytosis and Potent Partners for Immunotherapy in Glioblastoma
In bioRxiv on 27 March 2025 by Khalaj, M., Gutierrez, M. L., et al.
-
-
-
Cell Biology
Super-resolution microscopy unveils the nanoscale organization and self-limiting clustering of CD47 in human erythrocytes.
In J Mol Cell Biol on 21 March 2025 by Yang, J., Xing, F., et al.
PubMed
The transmembrane protein CD47, an innate immune checkpoint protein, plays a pivotal role in preventing healthy erythrocytes from immune clearance. Our study utilized stochastic optical reconstruction microscopy (STORM) and single-molecule analysis to investigate the distribution of CD47 on the human erythrocyte membrane. Contrary to previous findings in mouse erythrocytes, we discovered that CD47 exists in randomly distributed monomers rather than in clusters across the human erythrocyte membrane. Using secondary antibody-induced crosslinking, we found that CD47 aggregates into stable clusters within minutes. By comparing these STORM results with those of the fully mobile protein CD59 and the cytoskeleton-bound membrane protein glycophorin C under similar conditions, as well as devising two-color STORM co-labeling and co-clustering experiments, we further quantitatively revealed an intermediate, self-limiting clustering behavior of CD47, elucidating its fractional (∼14%) attachment to the cytoskeleton. Moreover, we report reductions in both the amount of CD47 and its clustering capability in aged erythrocytes, providing new insight into erythrocyte senescence. Together, the combination of STORM and secondary antibody-based crosslinking unveils the unique self-limiting clustering behavior of CD47 due to its fractional cytoskeleton attachment.
-
-
-
Immunology and Microbiology
-
Cancer Research
A protocol for high-throughput screening for small chemicals promoting macrophage-mediated tumor cell phagocytosis in mice.
In STAR Protoc on 21 March 2025 by He, Z., Hu, Z., et al.
PubMed
Macrophage-mediated phagocytosis has emerged as a pivotal mechanism for eliminating tumor cells within the realm of cancer immunotherapy. Here, we present a protocol for identifying small molecules that enhance phagocytosis in mice using a co-culture system comprising primary macrophages, cancer cells, and a blockade of phagocytic checkpoints. We describe steps for expressing enhanced green fluorescent protein-luciferase (eGFP-Luc) and producing bone marrow-derived macrophages (BMDMs). We then detail procedures for optimizing co-culture conditions for high-throughput screen (HTS) and executing HTS chemical identification. For complete details on the use and execution of this protocol, please refer to Cao et al. 1.
-
-
-
Cancer Research
-
Immunology and Microbiology
Phosphoproteomic Profiling Reveals mTOR Signaling in Sustaining Macrophage Phagocytosis of Cancer Cells.
In Cancers (Basel) on 19 December 2024 by Wang, B., Cao, X., et al.
PubMed
Background: Macrophage-mediated cancer cell phagocytosis has demonstrated considerable therapeutic potential. While the initiation of phagocytosis, facilitated by interactions between cancer cell surface signals and macrophage receptors, has been characterized, the mechanisms underlying its sustentation and attenuation post-initiation remain poorly understood. Methods: Through comprehensive phosphoproteomic profiling, we interrogated the temporal evolution of the phosphorylation profiles within macrophages during cancer cell phagocytosis. Results: Our findings reveal that activation of the mTOR pathway occurs following the initiation of phagocytosis and is crucial in sustaining phagocytosis of cancer cells. mTOR inhibition impaired the phagocytic capacity, but not affinity, of the macrophages toward the cancer cells by delaying phagosome maturation and impeding the transition between non-phagocytic and phagocytic states of macrophages. Conclusions: Our findings delineate the intricate landscape of macrophage phagocytosis and highlight the pivotal role of the mTOR pathway in mediating this process, offering valuable mechanistic insights for therapeutic interventions.
-
-
-
Cancer Research
-
Immunology and Microbiology
CD47 predominates over CD24 as a macrophage immune checkpoint in cancer
In bioRxiv on 26 November 2024 by Allen, J., Meglan, A., et al.
-
-
-
Endocrinology and Physiology
-
Immunology and Microbiology
Enhancing anti-EGFRvIII CAR T cell therapy against glioblastoma with a paracrine SIRPγ-derived CD47 blocker.
In Nat Commun on 9 November 2024 by Martins, T. A., Kaymak, D., et al.
PubMed
A significant challenge for chimeric antigen receptor (CAR) T cell therapy against glioblastoma (GBM) is its immunosuppressive microenvironment, which is densely populated by protumoral glioma-associated microglia and macrophages (GAMs). Myeloid immune checkpoint therapy targeting the CD47-signal regulatory protein alpha (SIRPα) axis induces GAM phagocytic function, but CD47 blockade monotherapy is associated with toxicity and low bioavailability in solid tumors. In this work, we engineer a CAR T cell against epidermal growth factor receptor variant III (EGFRvIII), constitutively secreting a signal regulatory protein gamma-related protein (SGRP) with high affinity to CD47. Anti-EGFRvIII-SGRP CAR T cells eradicate orthotopic EGFRvIII-mosaic GBM in vivo, promoting GAM-mediated tumor cell phagocytosis. In a subcutaneous CD19+ lymphoma mouse model, anti-CD19-SGRP CAR T cell therapy is superior to conventional anti-CD19 CAR T. Thus, combination of CAR and SGRP eliminates bystander tumor cells in a manner that could overcome main mechanisms of CAR T cell therapy resistance, including immune suppression and antigen escape.
-
-
-
Cancer Research
In vivo perturb-seq of cancer and microenvironment cells dissects oncologic drivers and radiotherapy responses in glioblastoma.
In Genome Biol on 7 October 2024 by Liu, S. J., Zou, C., et al.
PubMed
Genetic perturbation screens with single-cell readouts have enabled rich phenotyping of gene function and regulatory networks. These approaches have been challenging in vivo, especially in adult disease models such as cancer, which include mixtures of malignant and microenvironment cells. Glioblastoma (GBM) is a fatal cancer, and methods of systematically interrogating gene function and therapeutic targets in vivo, especially in combination with standard of care treatment such as radiotherapy, are lacking.
-
-
-
Cancer Research
-
Cardiovascular biology
-
Immunology and Microbiology
Blockade of the CD47/SIRPα checkpoint axis potentiates the macrophage-mediated anti-tumor efficacy of tafasitamab.
In Haematologica on 27 June 2024 by Biedermann, A., Patra-Kneuer, M., et al.
PubMed
Macrophages are one of the key mediators of the therapeutic effects exerted by monoclonal antibodies, such as the anti-CD19 antibody tafasitamab, approved in combination with lenalidomide for the treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL). However, antibody-dependent cellular phagocytosis (ADCP) in the tumor microenvironment can be counteracted by increased expression of the inhibitory receptor SIRPα on macrophages and its ligand, the immune checkpoint molecule CD47, on tumor cells. The aim of this study was to investigate the impact of the CD47-SIRPα axis on tafasitamab- mediated phagocytosis and explore the potential of anti-CD47 blockade to enhance its antitumor activity. Elevated expression of both SIRPα and CD47 was observed in DLBCL patient-derived lymph node biopsies compared to healthy control lymph nodes. CRISPR-mediated CD47 overexpression affected tafasitamab-mediated ADCP in vitro and increased expression of SIRPα on macrophages correlated with decreased ADCP activity of tafasitamab against DLBCL cell lines. A combination of tafasitamab and an anti-CD47 blocking antibody enhanced ADCP activity of in vitro-generated macrophages. Importantly, tafasitamab-mediated phagocytosis was elevated in combination with CD47 blockade using primary DLBCL cells and patient-derived lymphoma-associated macrophages in an autologous setting. Furthermore, lymphoma cells with low CD19 expression were efficiently eliminated by the combination treatment. Finally, combined treatment of tafasitamab and an anti-CD47 antibody resulted in enhanced tumor volume reduction and survival benefit in lymphoma xenograft mouse models. These findings provide evidence that CD47 blockade can enhance the phagocytic potential of tumor-targeting immunotherapies such as tafasitamab and suggest that there is value in exploring the combination in the clinic.
-
-
Pro-phagocytic function and structural basis of GPR84 signaling.
In Nat Commun on 14 September 2023 by Zhang, X., Wang, Y., et al.
PubMed
GPR84 is a unique orphan G protein-coupled receptor (GPCR) that can be activated by endogenous medium-chain fatty acids (MCFAs). The signaling of GPR84 is largely pro-inflammatory, which can augment inflammatory response, and GPR84 also functions as a pro-phagocytic receptor to enhance phagocytic activities of macrophages. In this study, we show that the activation of GPR84 by the synthetic agonist 6-OAU can synergize with the blockade of CD47 on cancer cells to induce phagocytosis of cancer cells by macrophages. We also determine a high-resolution structure of the GPR84-Gi signaling complex with 6-OAU. This structure reveals an occluded binding pocket for 6-OAU, the molecular basis of receptor activation involving non-conserved structural motifs of GPR84, and an unusual Gi-coupling interface. Together with computational docking and simulations studies, this structure also suggests a mechanism for the high selectivity of GPR84 for MCFAs and a potential routes of ligand binding and dissociation. These results provide a framework for understanding GPR84 signaling and developing new drugs targeting GPR84.
-
-
Endocrinology and Physiology
-
Immunology and Microbiology
Enhancing anti-EGFRvIII CAR T cell therapy against glioblastoma with a paracrine SIRPγ-derived CD47 blocker
In bioRxiv on 3 September 2023 by Martins, T. A., Tatari, N., et al.
-
-
-
Cancer Research
Oncolytic adenovirus H101 enhanced antitumor effects of PD-1 blockade by downregulating CD47 on tumor cells
In Research Square on 19 April 2023 by Qiao, C., Wang, S., et al.
-
-
-
Biochemistry and Molecular biology
Blockade of CD47 function attenuates restenosis by promoting smooth muscle cell efferocytosis and inhibiting their migration and proliferation.
In J Biol Chem on 1 April 2023 by Govatati, S., Pichavaram, P., et al.
PubMed
Cluster of differentiation 47 (CD47) plays an important role in the pathophysiology of various diseases including atherosclerosis but its role in neointimal hyperplasia which contributes to restenosis has not been studied. Using molecular approaches in combination with a mouse vascular endothelial denudation model, we studied the role of CD47 in injury-induced neointimal hyperplasia. We determined that thrombin-induced CD47 expression both in human aortic smooth muscle cells (HASMCs) and mouse aortic smooth muscle cells. In exploring the mechanisms, we found that the protease-activated receptor 1-Gα protein q/11 (Gαq/11)-phospholipase Cβ3-nuclear factor of activated T cells c1 signaling axis regulates thrombin-induced CD47 expression in HASMCs. Depletion of CD47 levels using its siRNA or interference of its function by its blocking antibody (bAb) blunted thrombin-induced migration and proliferation of HASMCs and mouse aortic smooth muscle cells. In addition, we found that thrombin-induced HASMC migration requires CD47 interaction with integrin β3. On the other hand, thrombin-induced HASMC proliferation was dependent on CD47's role in nuclear export and degradation of cyclin-dependent kinase-interacting protein 1. In addition, suppression of CD47 function by its bAb rescued HASMC efferocytosis from inhibition by thrombin. We also found that vascular injury induces CD47 expression in intimal SMCs and that inhibition of CD47 function by its bAb, while alleviating injury-induced inhibition of SMC efferocytosis, attenuated SMC migration, and proliferation resulting in reduced neointima formation. Thus, these findings reveal a pathological role for CD47 in neointimal hyperplasia.
-
-
Pro-phagocytic function and structural basis of GPR84 signaling
In Research Square on 15 February 2023 by Zhang, X., Wang, Y., et al.
-
-
Cancer Research
-
Immunology and Microbiology
BND-22, a first-in-class humanized ILT2-blocking antibody, promotes antitumor immunity and tumor regression.
In J Immunother Cancer on 1 September 2022 by Mandel, I., Haves Ziv, D., et al.
PubMed
Cancer immunotherapy has revolutionized cancer treatment. However, considering the limited success of immunotherapy to only some cancer types and patient cohorts, there is an unmet need for developing new treatments that will result in higher response rates in patients with cancer. Immunoglobulin-like transcript 2 (ILT2), a LILRB family member, is an inhibitory receptor expressed on a variety of immune cells including T cells, natural killer (NK) cells and different myeloid cells. In the tumor microenvironment, binding of class I MHC (in particular HLA-G) to ILT2 on immune cells mediates a strong inhibitory effect, which manifests in inhibition of antitumor cytotoxicity of T and NK cells, and prevention of phagocytosis of the tumor cells by macrophages.
-
-
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
Immunotherapy of glioblastoma explants induces interferon-γ responses and spatial immune cell rearrangements in tumor center, but not periphery.
In Sci Adv on 1 July 2022 by Shekarian, T., Zinner, C. P., et al.
PubMed
A patient-tailored, ex vivo drug response platform for glioblastoma (GBM) would facilitate therapy planning, provide insights into treatment-induced mechanisms in the immune tumor microenvironment (iTME), and enable the discovery of biomarkers of response. We cultured regionally annotated GBM explants in perfusion bioreactors to assess iTME responses to immunotherapy. Explants were treated with anti-CD47, anti-PD-1, or their combination, and analyzed by multiplexed microscopy [CO-Detection by indEXing (CODEX)], enabling the spatially resolved identification of >850,000 single cells, accompanied by explant secretome interrogation. Center and periphery explants differed in their cell type and soluble factor composition, and responses to immunotherapy. A subset of explants displayed increased interferon-γ levels, which correlated with shifts in immune cell composition within specified tissue compartments. Our study demonstrates that ex vivo immunotherapy of GBM explants enables an active antitumoral immune response within the tumor center and provides a framework for multidimensional personalized assessment of tumor response to immunotherapy.
-
-
-
In vitro experiments
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
Dendritic cells can prime anti-tumor CD8+ T cell responses through major histocompatibility complex cross-dressing.
In Immunity on 14 June 2022 by MacNabb, B. W., Tumuluru, S., et al.
PubMed
Antigen cross-presentation, wherein dendritic cells (DCs) present exogenous antigen on major histocompatibility class I (MHC-I) molecules, is considered the primary mechanism by which DCs initiate tumor-specific CD8+ T cell responses. Here, we demonstrate that MHC-I cross-dressing, an antigen presentation pathway in which DCs acquire and display intact tumor-derived peptide:MHC-I molecules, is also important in orchestrating anti-tumor immunity. Cancer cell MHC-I expression was required for optimal CD8+ T cell activation in two subcutaneous tumor models. In vivo acquisition of tumor-derived peptide:MHC-I molecules by DCs was sufficient to induce antigen-specific CD8+ T cell priming. Transfer of tumor-derived human leukocyte antigen (HLA) molecules to myeloid cells was detected in vitro and in human tumor xenografts. In conclusion, MHC-I cross-dressing is crucial for anti-tumor CD8+ T cell priming by DCs. In addition to quantitatively enhancing tumor antigen presentation, MHC cross-dressing might also enable DCs to more faithfully and efficiently mirror the cancer cell peptidome.
-
-
-
Cancer Research
-
Pharmacology
-
Immunohistochemistry
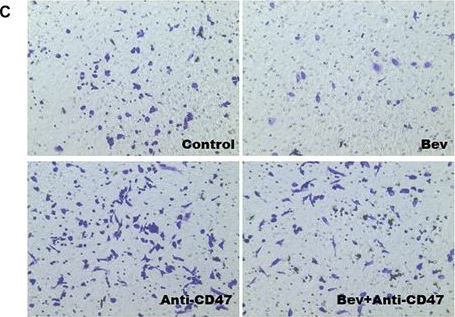
Blocking CD47 Shows Superior Anti-tumor Therapeutic Effects of Bevacizumab in Gastric Cancer.
In Front Pharmacol on 14 June 2022 by Shi, C., Li, J., et al.
PubMed
Background: Bevacizumab (Avastin®), a humanized antiangiogenic monoclonal antibody, is widely used in the clinical treatment of tumour diseases. However, recent research has shown that the beneficial antiangiogenic effects of these agents have been limited in a number of patients due to complex immunosuppressive mechanisms. Here, we report a synergistic antitumour strategy through simultaneous blockade of VEGF and CD47 signalling to enhance the curative effect of advanced gastric cancer. Method: A BGC-823 gastric tumour model was chosen to evaluate antitumour efficacy. Macrophage migration and phagocytosis were evaluated to determine immune-related resistance to bevacizumab therapy. Synergistic antitumour activity was observed on the basis of tumour volume, tumour weight, tumour inhibition rate, tumour angiogenesis and tumour metastasis when bevacizumab was combined with an anti-CD47 monoclonal antibody. Results: Our study demonstrated that synergistic therapy targeting CD47 and VEGF reversed macrophage migration and phagocytosis, which were inhibited by antiangiogenic therapy and enhanced antitumour effects. Moreover, blockade of CD47 induced by antiangiogenic therapy inhibited tumour metastasis. Conclusion: Our data provide an effective strategy to attenuate resistance to bevacizumab therapy, promoting clinical cancer treatment with antiangiogenic drugs in combination with CD47-targeting inhibitors.
-
-
-
Immunology and Microbiology
Preclinical characterization of the novel anti-SIRPα antibody BR105 that targets the myeloid immune checkpoint.
In J Immunother Cancer on 1 March 2022 by Wu, Z. H., Li, N., et al.
PubMed
The CD47-SIRPα pathway acts as an important myeloid cell immune checkpoint and targeting the CD47/SIRPα axis represents a promising strategy to promote antitumor immunity. Several CD47-targeting agents show encouraging early activity in clinical trials. However, due to ubiquitous expression of CD47, the antigen sink and hematologic toxicity, such as anemia and thrombocytopenia, are main problems for developing CD47-targeting therapies. Considering the limited expression of SIRPα, targeting SIRPα is an alternative approach to block the CD47-SIRPα pathway, which may result in differential efficacy and safety profiles.
-
-
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
Immunotherapy of glioblastoma explants induces interferon-γ responses and spatial immune cell rearrangements in tumor center, but not periphery
In bioRxiv on 21 January 2022 by Shekarian, T., Zinner, C. P., et al.
-