InVivoMAb anti-human CD40
Product Description
Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Human tonsillar lymphocytes |
| Reported Applications |
in vitro CD40 stimulation Functional assays Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_10950314 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vitro CD40 stimulation
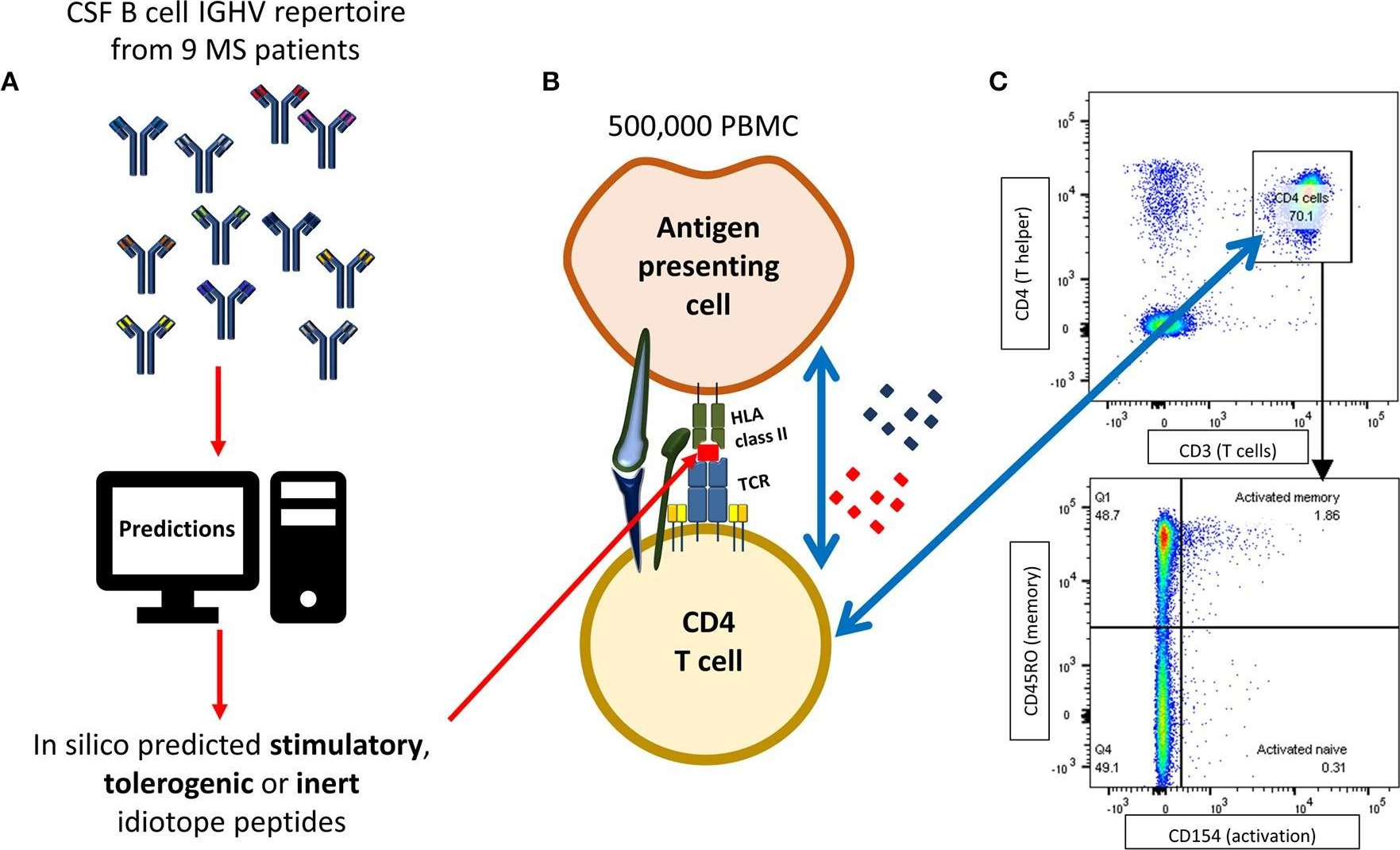
Høglund, R. A., et al (2020). "CD4(+) T Cells in the Blood of MS Patients Respond to Predicted Epitopes From B cell Receptors Found in Spinal Fluid" Front Immunol 11: 598.
PubMed
B cells are important pathogenic players in multiple sclerosis (MS), but their exact role is not known. We have previously demonstrated that B cells from cerebrospinal fluid (CSF) of MS patients can activate T cells that specifically recognize antigenic determinants (idiotopes) from their B cell receptors (BCRs). The aim of this study was to evaluate whether in silico prediction models could identify antigenic idiotopes of immunoglobulin heavy-chain variable (IGHV) transcriptomes in MS patients. We utilized a previously assembled dataset of CSF IGHV repertoires from MS patients. To guide selection of potential antigenic idiotopes, we used in silico predicted HLA-DR affinity, endosomal processing, as well as transcript frequency from nine MS patients. Idiotopes with predicted low affinity and low likelihood of cathepsins cleavage were inert controls. Peripheral blood mononuclear cells from these patients were stimulated with the selected idiotope peptides in presence of anti-CD40 for 12 h. T cells were then labeled for activation status with anti-CD154 antibodies and CD3(+)CD4(+) T cells phenotyped as memory (CD45RO(+)) or naïve (CD45RO(-)), with potential for brain migration (CXCR3 and/or CCR6 expression). Anti-CD14 and -CD8 were utilized to exclude monocytes and CD8(+) T cells. Unstimulated cells or insulin peptides were negative controls, and EBNA-1 peptides or CD3/CD28 beads were positive controls. The mean proportion of responding memory CD4(+) T cells from all nine MS patients was significantly higher for idiotope peptides with predicted high HLA-DR affinity and high likelihood of cathepsin cleavage, than toward predicted inert peptides. Responses were mainly observed toward peptides affiliated with the CDR3 region. Activated memory CD4(+) T cells expressed the chemokine receptor CCR6, affiliated with a Th17 phenotype and allowing passage into the central nervous system (CNS). This in vitro study suggests that that antigenic properties of BCR idiotopes can be identified in silico using HLA affinity and endosomal processing predictions. It further indicates that MS patients have a memory T cell repertoire capable of recognizing frequent BCR idiotopes found in endogenous CSF, and that these T cells express chemokine receptors allowing them to reach the CSF B cells expressing these idiotopes.
in vitro CD40 stimulation
Bankert, K. C., et al (2015). "Induction of an altered CD40 signaling complex by an antagonistic human monoclonal antibody to CD40" J Immunol 194(9): 4319-4327.
PubMed
Blocking the interaction of CD40 with its ligand CD154 is a desirable goal of therapies for preventing and/or ameliorating autoimmune diseases and transplant rejection. CD154-blocking mAbs used in human clinical trials resulted in unanticipated vascular complications, leading to heightened interest in the therapeutic potential of antagonist mAbs specific for human CD40. Abs that do not require physical competition with CD154 to inhibit CD40 signaling have particular therapeutic promise. In this study, we demonstrate that the antagonist anti-human CD40 mAb PG102 fails to trigger CD40-mediated activation, as well as impairs CD154-mediated CD40 activation, via a distinct nonstimulatory CD40 signaling mechanism. PG102 did not induce early CD40-induced signaling events, and it inhibited early kinase and transcription factor activation by CD154 or agonist anti-CD40 mAbs. However, PG102 stimulated normal CD40-mediated TNFR-associated factor (TRAF)2 and TRAF3 degradation. PG102 induced the formation of a CD40 signaling complex that contained decreased amounts of both TRAF2 and TRAF3 and TRAF2-associated signaling proteins. Additionally, PG102-induced CD40 signaling complexes failed to recruit TRAF6 to detergent-insoluble membrane fractions. Fab fragments of PG102, while retaining CD40 binding, did not induce TRAF degradation, nor could they inhibit CD154-stimulated B cell signaling, indicating that CD40 aggregation is required for the signaling inhibition induced by PG102. The antagonistic impact of PG102 on CD40 signaling reveals that the manner of CD40 ligation can determine sharply different outcomes for CD40 signaling and suggests that such information can be used to therapeutically manipulate these outcomes.
in vitro CD40 stimulation
Cooley, L. F., et al (2015). "Increased B Cell ADAM10 in Allergic Patients and Th2 Prone Mice" PLoS One 10(5): e0124331.
PubMed
ADAM10, as the sheddase of the low affinity IgE receptor (CD23), promotes IgE production and thus is a unique target for attenuating allergic disease. Herein, we describe that B cell levels of ADAM10, specifically, are increased in allergic patients and Th2 prone WT mouse strains (Balb/c and A/J). While T cell help augments ADAM10 expression, Balb WT B cells exhibit increased ADAM10 in the naive state and even more dramatically increased ADAM10 after anti-CD40/IL4 stimulation compared C57 (Th1 prone) WT B cells. Furthermore, ADAM17 and TNF are reduced in allergic patients and Th2 prone mouse strains (Balb/c and A/J) compared to Th1 prone controls. To further understand this regulation, ADAM17 and TNF were studied in C57Bl/6 and Balb/c mice deficient in ADAM10. C57-ADAM10B-/- were more adept at increasing ADAM17 levels and thus TNF cleavage resulting in excess follicular TNF levels and abnormal secondary lymphoid tissue architecture not noted in Balb-ADAM10B-/-. Moreover, the level of B cell ADAM10 as well as Th context is critical for determining IgE production potential. Using a murine house dust mite airway hypersensitivity model, we describe that high B cell ADAM10 level in a Th2 context (Balb/c WT) is optimal for disease induction including bronchoconstriction, goblet cell metaplasia, mucus, inflammatory cellular infiltration, and IgE production. Balb/c mice deficient in B cell ADAM10 have attenuated lung and airway symptoms compared to Balb WT and are actually most similar to C57 WT (Th1 prone). C57-ADAM10B-/- have even further reduced symptomology. Taken together, it is critical to consider both innate B cell levels of ADAM10 and ADAM17 as well as Th context when determining host susceptibility to allergic disease. High B cell ADAM10 and low ADAM17 levels would help diagnostically in predicting Th2 disease susceptibility; and, we provide support for the use ADAM10 inhibitors in treating Th2 disease.
Flow Cytometry
Okimura, K., et al (2014). "Characterization of ASKP1240, a fully human antibody targeting human CD40 with potent immunosuppressive effects" Am J Transplant 14(6): 1290-1299.
PubMed
Blocking the CD40-CD154 interaction is reported to be effective for transplantation management and autoimmune disease models in rodents and nonhuman primates. However, clinical trials with anti-CD154 mAbs were halted because of high incidence of thromboembolic complications. Thus, we generated and characterized a fully human anti-CD40 mAb ASKP1240, as an alternative to anti-CD154 mAb. In vitro ASKP1240 concentration-dependently inhibited human peripheral blood mononuclear cell proliferation induced by soluble CD154. In addition, ASKP1240 did not destabilize platelet thrombi under physiological high shear conditions while mouse anti-human CD154 mAb (mu5C8) did. And ASKP1240 itself did not activate platelet and endothelial cells. In vivo administration of ASKP1240 (1 or 10 mg/kg, intravenously) to cynomolgus monkeys, weekly for 3 weeks, significantly attenuated both delayed-type hypersensitivity and specific antibody formation evoked by tetanus toxoid. The immunosuppressive effect was well correlated with the CD40 receptor saturation. Thus, these results suggest that ASKP1240 is immunosuppressive but not prothromboembolic, and as such appears to be a promising therapeutic candidate for the management of solid organ transplant rejection and autoimmune diseases therapy.
in vitro CD40 stimulation
Dumas, G., et al (2013). "CD40 pathway activation reveals dual function for macrophages in human endometrial cancer cell survival and invasion" Cancer Immunol Immunother 62(2): 273-283.
PubMed
Reproductive malignancies are a major cause of cancer death in women worldwide. CD40 is a TNF receptor family member, which upon activation may mediate tumor regression. However, despite the great potential of CD40 agonists, their use as a therapeutic option for reproductive cancers has never been investigated. Because CD40 ligation is a potent pathway of macrophage activation, an in vitro model of pro-inflammatory type-1 (Mvarphi-1) and anti-inflammatory type-2 (Mvarphi-2) macrophages was developed to determine whether and how macrophage CD40 pathway activation might influence endometrial tumor cell behavior. Analysis of tumor growth kinetic in the endometrial cancer xenograft model indicates that, when injected once into the growing tumors, CD40-activated Mvarphi-1 greatly reduced, while CD40-activated Mvarphi-2 increased tumor size when compared to control isotype-activated Mvarphi-1 and Mvarphi-2, respectively. In vitro assays indicated that CD40-activated Mvarphi-2 increased cell viability but failed to promote cell invasion. CD40-activated Mvarphi-1, in contrast, decreased cell survival but greatly increased cell invasion in tumor cells less susceptible to cell death by apoptosis; they also induced the expression of some pro-inflammatory genes, such as IL-6, LIF, and TNF-alpha, known to be involved in tumor promotion and metastasis. The presence of IFN-gamma is minimally required for CD40-activated Mvarphi-1 to promote tumor cell invasion, a process that is mediated in part through the activation of the PI3K/Akt2 signaling pathway in tumor cells. From these results, we speculate that some functions of CD40 in tumor-associated Mvarphis might limit the therapeutic development of CD40 agonists in endometrial cancer malignancies.
Flow Cytometry
Frentsch, M., et al (2013). "CD40L expression permits CD8+ T cells to execute immunologic helper functions" Blood 122(3): 405-412.
PubMed
CD8(+) T cells play an essential role in immunity against intracellular pathogens, with cytotoxicity being considered their major effector mechanism. However, we here demonstrate that a major part of central and effector memory CD8(+) T cells expresses CD40L, one key molecule for CD4(+) T-cell-mediated help. CD40L(+) CD8(+) T cells are detectable among human antigen-specific immune responses, including pathogens such as influenza and yellow fever virus. CD40L(+) CD8(+) T cells display potent helper functions in vitro and in vivo, such as activation of antigen-presenting cells, and exhibit a cytokine expression signature similar to CD4(+) T cells and unrelated to cytotoxic CD8(+) T cells. The broad occurrence of CD40L(+) CD8(+) T cells in cellular immunity implicates that helper functions are not only executed by major histocompatibility complex (MHC) class II-restricted CD4(+) helper T cells but are also a common feature of MHC class I-restricted CD8(+) T cell responses. Due to their versatile functional capacities, human CD40L(+) CD8(+) T cells are promising candidate cells for immune therapies, particularly when CD4(+) T-cell help or pathogen-associated molecular pattern signals are limited.
in vitro CD40 stimulation
Price, A. M., et al (2012). "Analysis of Epstein-Barr virus-regulated host gene expression changes through primary B-cell outgrowth reveals delayed kinetics of latent membrane protein 1-mediated NF-kappaB activation" J Virol 86(20): 11096-11106.
PubMed
Epstein-Barr virus (EBV) is an oncogenic human herpesvirus that dramatically reorganizes host gene expression to immortalize primary B cells. In this study, we analyzed EBV-regulated host gene expression changes following primary B-cell infection, both during initial proliferation and through transformation into lymphoblastoid cell lines (LCLs). While most EBV-regulated mRNAs were changed during the transition from resting, uninfected B cells through initial B-cell proliferation, a substantial number of mRNAs changed uniquely from early proliferation through LCL outgrowth. We identified constitutively and dynamically EBV-regulated biological processes, protein classes, and targets of specific transcription factors. Early after infection, genes associated with proliferation, stress responses, and the p53 pathway were highly enriched. However, the transition from early to long-term outgrowth was characterized by genes involved in the inhibition of apoptosis, the actin cytoskeleton, and NF-kappaB activity. It was previously thought that the major viral protein responsible for NF-kappaB activation, latent membrane protein 1 (LMP1), is expressed within 2 days after infection. Our data indicate that while this is true, LCL-level LMP1 expression and NF-kappaB activity are not evident until 3 weeks after primary B-cell infection. Furthermore, heterologous NF-kappaB activation during the first week after infection increased the transformation efficiency, while early NF-kappaB inhibition had no effect on transformation. Rather, inhibition of NF-kappaB was not toxic to EBV-infected cells until LMP1 levels and NF-kappaB activity were high. These data collectively highlight the dynamic nature of EBV-regulated host gene expression and support the notion that early EBV-infected proliferating B cells have a fundamentally distinct growth and survival phenotype from that of LCLs.
in vitro CD40 stimulation
D’Souza, B. N., et al (2004). "Nuclear factor kappa B-dependent activation of the antiapoptotic bfl-1 gene by the Epstein-Barr virus latent membrane protein 1 and activated CD40 receptor" J Virol 78(4): 1800-1816.
PubMed
Suppression of the cellular apoptotic program by the oncogenic herpesvirus Epstein-Barr virus (EBV) is central to both the establishment of latent infection and the development of EBV-associated malignancies. We have previously shown that expression of the EBV latent membrane protein 1 (LMP1) in Burkitt’s lymphoma cell lines leads to increased mRNA levels from the cellular antiapoptotic bfl-1 gene (also known as A1). Furthermore, ectopic expression of Bfl-1 in an EBV-positive cell line exhibiting a latency type 1 infection protects against apoptosis induced by growth factor deprivation (B. N. D’Souza, M. Rowe, and D. Walls, J. Virol. 74:6652-6658, 2000). We now report that LMP1 drives bfl-1 promoter activity through interactions with components of the tumor necrosis factor receptor (TNFR)/CD40 signaling pathway. We present evidence that this process is NF-kappa B dependent, involves the recruitment of TNFR-associated factor 2, and is mediated to a greater extent by the carboxyl-terminal activating region 2 (CTAR2) relative to the CTAR1 domain of LMP1. Activation of CD40 receptor also led to increased bfl-1 mRNA levels and an NF-kappa B-dependent increase in bfl-1 promoter activity in Burkitt’s lymphoma-derived cell lines. We have delineated a 95-bp region of the promoter that functions as an LMP1-dependent transcriptional enhancer in this cellular context. This sequence contains a novel NF-kappa B-like binding motif that is essential for transactivation of bfl-1 by LMP1, CD40, and the NF-kappa B subunit protein p65. These findings highlight the role of LMP1 as a mediator of EBV-host cell interactions and may indicate an important route by which it exerts its cellular growth transforming properties.
in vitro CD40 stimulation
Francisco, J. A., et al (2000). "Agonistic properties and in vivo antitumor activity of the anti-CD40 antibody SGN-14" Cancer Res 60(12): 3225-3231.
PubMed
Ligation of CD40 is essential for primary B-cell activation and expansion and yet has suppressive or apoptotic effects on some CD40-expressing neoplasia. SGN-14 is a monoclonal antibody that binds to the human CD40 receptor. Here we report that SGN-14, in the presence of interleukin 4, provided a modest level of stimulation of peripheral blood B cells, as measured by proliferation. Stimulation was greatly enhanced in the presence of nonproliferating CD40 ligand-expressing cells. The enhanced agonistic activity could be attributed to a dose-dependent increase in CD40L binding to CD40 in the presence of SGN-14. In contrast to its proliferative effect on primary B cells, SGN-14 inhibited the growth of B-cell-derived tumor lines in vitro, and this growth inhibition was enhanced in the presence of CD40L-expressing cells. In vivo, SGN-14 showed significant antitumor activity in treating human B-cell lymphoma and multiple myeloma xenografted severe combined immunodeficient mice. Antitumor activity was not diminished by blunting murine natural killer activity, suggesting that CD40 ligation contributes to the antitumor efficacy of SGN-14. On the basis of these activities, SGN-14 is being pursued for therapeutic use in treating patients with CD40-expressing hematological malignancies.
Product Citations
-
-
In Vitro
-
Homo sapiens (Human)
-
Cardiovascular biology
-
Immunology and Microbiology
-
Neuroscience
CD4+ T Cells in the Blood of MS Patients Respond to Predicted Epitopes From B cell Receptors Found in Spinal Fluid.
In Frontiers in Immunology on 25 April 2020 by Høglund, R. A., Bremel, R. D., et al.
PubMed
B cells are important pathogenic players in multiple sclerosis (MS), but their exact role is not known. We have previously demonstrated that B cells from cerebrospinal fluid (CSF) of MS patients can activate T cells that specifically recognize antigenic determinants (idiotopes) from their B cell receptors (BCRs). The aim of this study was to evaluate whether in silico prediction models could identify antigenic idiotopes of immunoglobulin heavy-chain variable (IGHV) transcriptomes in MS patients. We utilized a previously assembled dataset of CSF IGHV repertoires from MS patients. To guide selection of potential antigenic idiotopes, we used in silico predicted HLA-DR affinity, endosomal processing, as well as transcript frequency from nine MS patients. Idiotopes with predicted low affinity and low likelihood of cathepsins cleavage were inert controls. Peripheral blood mononuclear cells from these patients were stimulated with the selected idiotope peptides in presence of anti-CD40 for 12 h. T cells were then labeled for activation status with anti-CD154 antibodies and CD3+CD4+ T cells phenotyped as memory (CD45RO+) or naïve (CD45RO-), with potential for brain migration (CXCR3 and/or CCR6 expression). Anti-CD14 and -CD8 were utilized to exclude monocytes and CD8+ T cells. Unstimulated cells or insulin peptides were negative controls, and EBNA-1 peptides or CD3/CD28 beads were positive controls. The mean proportion of responding memory CD4+ T cells from all nine MS patients was significantly higher for idiotope peptides with predicted high HLA-DR affinity and high likelihood of cathepsin cleavage, than toward predicted inert peptides. Responses were mainly observed toward peptides affiliated with the CDR3 region. Activated memory CD4+ T cells expressed the chemokine receptor CCR6, affiliated with a Th17 phenotype and allowing passage into the central nervous system (CNS). This in vitro study suggests that that antigenic properties of BCR idiotopes can be identified in silico using HLA affinity and endosomal processing predictions. It further indicates that MS patients have a memory T cell repertoire capable of recognizing frequent BCR idiotopes found in endogenous CSF, and that these T cells express chemokine receptors allowing them to reach the CSF B cells expressing these idiotopes. Copyright © 2020 Høglund, Bremel, Homan, Torsetnes, Lossius and Holmøy.
-
-
-
Genetics
-
Immunology and Microbiology
Genetic engineering in primary human B cells with CRISPR-Cas9 ribonucleoproteins.
In Journal of Immunological Methods on 1 June 2018 by Wu, C. M., Roth, T. L., et al.
PubMed
Genome editing in human cells with targeted nucleases now enables diverse experimental and therapeutic genome engineering applications, but extension to primary human B cells remains limited. Here we report a method for targeted genetic engineering in primary human B cells, utilizing electroporation of CRISPR-Cas9 ribonucleoproteins (RNPs) to introduce gene knockout mutations at protein-coding loci with high efficiencies that in some cases exceeded 80%. Further, we demonstrate knock-in editing of targeted nucleotides with efficiency exceeding 10% through co-delivery of oligonucleotide templates for homology directed repair. We delivered Cas9 RNPs in two distinct in vitro culture systems to achieve editing in both undifferentiated B cells and activated B cells undergoing differentiation, reflecting utility in diverse experimental conditions. In summary, we demonstrate a powerful and scalable research tool for functional genetic studies of human B cell biology that may have further applications in engineered B cell therapeutics. Copyright © 2018 Elsevier B.V. All rights reserved.
-
-
-
Immunology and Microbiology
Inflammatory arthritis immune related adverse events represent a unique autoimmune disease entity primarily driven by T cells, but likely not autoantibodies
In medRxiv on 6 June 2025 by Zhu, X., Yu, Y., et al.
-
-
-
Cell Culture
-
Homo sapiens (Human)
-
Immunology and Microbiology
TFEB activation hallmarks antigenic experience of B lymphocytes and directs germinal center fate decisions.
In Nat Commun on 14 August 2024 by Münchhalfen, M., Goerg, R., et al.
PubMed
Ligation of the B cell antigen receptor (BCR) initiates humoral immunity. However, BCR signaling without appropriate co-stimulation commits B cells to death rather than to differentiation into immune effector cells. How BCR activation depletes potentially autoreactive B cells while simultaneously primes for receiving rescue and differentiation signals from cognate T lymphocytes remains unknown. Here, we use a mass spectrometry-based proteomic approach to identify cytosolic/nuclear shuttling elements and uncover transcription factor EB (TFEB) as a central BCR-controlled rheostat that drives activation-induced apoptosis, and concurrently promotes the reception of co-stimulatory rescue signals by supporting B cell migration and antigen presentation. CD40 co-stimulation prevents TFEB-driven cell death, while enhancing and prolonging TFEB's nuclear residency, which hallmarks antigenic experience also of memory B cells. In mice, TFEB shapes the transcriptional landscape of germinal center B cells. Within the germinal center, TFEB facilitates the dark zone entry of light-zone-residing centrocytes through regulation of chemokine receptors and, by balancing the expression of Bcl-2/BH3-only family members, integrates antigen-induced apoptosis with T cell-provided CD40 survival signals. Thus, TFEB reprograms antigen-primed germinal center B cells for cell fate decisions.
-
-
-
Immunology and Microbiology
-
COVID-19
SARS-CoV-2 Vaccine-Elicited Immunity after B Cell Depletion in Multiple Sclerosis.
In Immunohorizons on 1 March 2024 by Baxter, R. M., Cabrera-Martinez, B., et al.
PubMed
The impact of B cell deficiency on the humoral and cellular responses to SARS-CoV2 mRNA vaccination remains a challenging and significant clinical management question. We evaluated vaccine-elicited serological and cellular responses in 1) healthy individuals who were pre-exposed to SARS-CoV-2 (n = 21), 2) healthy individuals who received a homologous booster (mRNA, n = 19; or Novavax, n = 19), and 3) persons with multiple sclerosis on B cell depletion therapy (MS-αCD20) receiving mRNA homologous boosting (n = 36). Pre-exposure increased humoral and CD4 T cellular responses in immunocompetent individuals. Novavax homologous boosting induced a significantly more robust serological response than mRNA boosting. MS-α CD20 had an intact IgA mucosal response and an enhanced CD8 T cell response to mRNA boosting compared with immunocompetent individuals. This enhanced cellular response was characterized by the expansion of only effector, not memory, T cells. The enhancement of CD8 T cells in the setting of B cell depletion suggests a regulatory mechanism between B and CD8 T cell vaccine responses.
-
-
-
Cancer Research
TRAIL-dependent apoptosis of peritoneal mesothelial cells by NK cells promotes ovarian cancer invasion.
In iScience on 15 December 2023 by Steitz, A. M., Schröder, C., et al.
PubMed
A crucial requirement for metastasis formation in ovarian high-grade serous carcinoma (HGSC) is the disruption of the protective peritoneal mesothelium. Using co-culture systems of primary human cells, we discovered that tumor-associated NK cells induce TRAIL-dependent apoptosis in mesothelial cells via death receptors DR4 and DR5 upon encounter with activated T cells. Upregulation of TRAIL expression in NK cells concomitant with enhanced cytotoxicity toward mesothelial cells was driven predominantly by T-cell-derived TNFα, as shown by affinity proteomics-based analysis of the T cell secretome in conjunction with functional studies. Consistent with these findings, we detected apoptotic mesothelial cells in the peritoneal fluid of HGSC patients. In contrast to mesothelial cells, HGSC cells express negligible levels of both DR4 and DR5 and are TRAIL resistant, indicating cell-type-selective killing by NK cells. Our data point to a cooperative action of T and NK in breaching the mesothelial barrier in HGSC patients.
-
-
-
Immunology and Microbiology
Peptidylarginine deiminase 2 citrullinates MZB1 and promotes the secretion of IgM and IgA.
In Front Immunol on 14 December 2023 by Geary, B., Sun, B., et al.
PubMed
MZB1 is an endoplasmic reticulum residential protein preferentially expressed in plasma cells, marginal zone and B1 B cells. Recent studies on murine B cells show that it interacts with the tail piece of IgM and IgA heavy chain and promotes the secretion of these two classes of immunoglobulin. However, its role in primary human B cells has yet to be determined and how its function is regulated is still unknown. The conversion of peptidylarginine to peptidylcitrulline, also known as citrullination, by peptidylarginine deiminases (PADs) can critically influence the function of proteins in immune cells, such as neutrophils and T cells; however, the role of PADs in B cells remains to be elucidated.
-
-
-
In vitro experiments
-
Homo sapiens (Human)
-
Cancer Research
Neutrophil-activating therapy for the treatment of cancer.
In Cancer Cell on 13 February 2023 by Linde, I. L., Prestwood, T. R., et al.
PubMed
Despite their cytotoxic capacity, neutrophils are often co-opted by cancers to promote immunosuppression, tumor growth, and metastasis. Consequently, these cells have received little attention as potential cancer immunotherapeutic agents. Here, we demonstrate in mouse models that neutrophils can be harnessed to induce eradication of tumors and reduce metastatic seeding through the combined actions of tumor necrosis factor, CD40 agonist, and tumor-binding antibody. The same combination activates human neutrophils in vitro, enabling their lysis of human tumor cells. Mechanistically, this therapy induces rapid mobilization and tumor infiltration of neutrophils along with complement activation in tumors. Complement component C5a activates neutrophils to produce leukotriene B4, which stimulates reactive oxygen species production via xanthine oxidase, resulting in oxidative damage and T cell-independent clearance of multiple tumor types. These data establish neutrophils as potent anti-tumor immune mediators and define an inflammatory pathway that can be harnessed to drive neutrophil-mediated eradication of cancer.
-
-
-
Chromatin immunoprecipitation
-
Homo sapiens (Human)
Ectopic Lymphoid Follicle Formation and Human Seasonal Influenza Vaccination Responses Recapitulated in an Organ-on-a-Chip.
In Adv Sci (Weinh) on 1 May 2022 by Goyal, G., Prabhala, P., et al.
PubMed
Lymphoid follicles (LFs) are responsible for generation of adaptive immune responses in secondary lymphoid organs and form ectopically during chronic inflammation. A human model of ectopic LF formation will provide a tool to understand LF development and an alternative to non-human primates for preclinical evaluation of vaccines. Here, it is shown that primary human blood B- and T-lymphocytes autonomously assemble into ectopic LFs when cultured in a 3D extracellular matrix gel within one channel of a two-channel organ-on-a-chip microfluidic device. Superfusion via a parallel channel separated by a microporous membrane is required for LF formation and prevents lymphocyte autoactivation. These germinal center-like LFs contain B cells expressing Activation-Induced Cytidine Deaminase and exhibit plasma cell differentiation upon activation. To explore their utility for seasonal vaccine testing, autologous monocyte-derived dendritic cells are integrated into LF Chips. The human LF chips demonstrate improved antibody responses to split virion influenza vaccination compared to 2D cultures, which are enhanced by a squalene-in-water emulsion adjuvant, and this is accompanied by increases in LF size and number. When inoculated with commercial influenza vaccine, plasma cell formation and production of anti-hemagglutinin IgG are observed, as well as secretion of cytokines similar to vaccinated humans over clinically relevant timescales.
-
-
-
Cell Culture
-
Homo sapiens (Human)
-
Immunology and Microbiology
CRAC Channel Controls the Differentiation of Pathogenic B Cells in Lupus Nephritis.
In Front Immunol on 9 November 2021 by Li, X., Zeng, Q., et al.
PubMed
Store-operated Ca2+ release-activated Ca2+ (CRAC) channel is the main Ca2+ influx pathway in lymphocytes and is essential for immune response. Lupus nephritis (LN) is an autoimmune disease characterized by the production of autoantibodies due to widespread loss of immune tolerance. In this study, RNA-seq analysis revealed that calcium transmembrane transport and calcium channel activity were enhanced in naive B cells from patients with LN. The increased expression of ORAI1, ORAI2, and STIM2 in naive B cells from patients with LN was confirmed by flow cytometry and Western blot, implying a role of CRAC channel in B-cell dysregulation in LN. For in vitro study, CRAC channel inhibition by YM-58483 or downregulation by ORAI1-specific small-interfering RNA (siRNA) decreased the phosphorylation of Ca2+/calmodulin-dependent protein kinase2 (CaMK2) and suppressed Blimp-1 expression in primary human B cells, resulting in decreased B-cell differentiation and immunoglobulin G (IgG) production. B cells treated with CaMK2-specific siRNA showed defects in plasma cell differentiation and IgG production. For in vivo study, YM-58483 not only ameliorated the progression of LN but also prevented the development of LN. MRL/lpr lupus mice treated with YM-58483 showed lower percentage of plasma cells in the spleen and reduced concentration of anti-double-stranded DNA antibodies in the sera significantly. Importantly, mice treated with YM-58483 showed decreased immune deposition in the glomeruli and alleviated kidney damage, which was further confirmed in NZM2328 lupus mice. Collectively, CRAC channel controlled the differentiation of pathogenic B cells and promoted the progression of LN. This study provides insights into the pathogenic mechanisms of LN and that CRAC channel could serve as a potential therapeutic target for LN.
-
-
-
Immunology and Microbiology
Clickable Vitamins as a New Tool to Track Vitamin A and Retinoic Acid in Immune Cells.
In Front Immunol on 27 July 2021 by Bos, A. V., Erkelens, M. N., et al.
PubMed
The vitamin A derivative, retinoid acid (RA) is key player in guiding adaptive mucosal immune responses. However, data on the uptake and metabolism of vitamin A within human immune cells has remained largely elusive because retinoids are small, lipophilic molecules which are difficult to detect. To overcome this problem and to be able to study the effect of vitamin A metabolism in human immune cell subsets, we have synthesized novel bio-orthogonal retinoid-based probes (clickable probes), which are structurally and functionally indistinguishable from vitamin A. The probes contain a functional group (an alkyne) to conjugate to a fluorogenic dye to monitor retinoid molecules in real-time in immune cells. We demonstrate, by using flow cytometry and microscopy, that multiple immune cells have the capacity to internalize retinoids to varying degrees, including human monocyte-derived dendritic cells (DCs) and naïve B lymphocytes. We observed that naïve B cells lack the enzymatic machinery to produce RA, but use exogenous retinoic acid to enhance CD38 expression. Furthermore, we showed that human DCs metabolize retinal into retinoic acid, which in co-culture with naïve B cells led to of the induction of CD38 expression. These data demonstrate that in humans, DCs can serve as an exogenous source of RA for naïve B cells. Taken together, through the use of clickable vitamins our data provide valuable insight in the mechanism of vitamin A metabolism and its importance for human adaptive immunity.
-
-
-
Genetics
-
Immunology and Microbiology
Frontline Science: CD40 signaling restricts RNA virus replication in Mϕs, leading to rapid innate immune control of acute virus infection.
In J Leukoc Biol on 1 February 2021 by Rogers, K. J., Shtanko, O., et al.
PubMed
Many acute viral infections target tissue Mϕs, yet the mechanisms of Mϕ-mediated control of viruses are poorly understood. Here, we report that CD40 expressed by peritoneal Mϕs restricts early infection of a broad range of RNA viruses. Loss of CD40 expression enhanced virus replication as early as 12-24 h of infection and, conversely, stimulation of CD40 signaling with an agonistic Ab blocked infection. With peritoneal cell populations infected with the filovirus, wild-type (WT) Ebola virus (EBOV), or a BSL2 model virus, recombinant vesicular stomatitis virus encoding Ebola virus glycoprotein (rVSV/EBOV GP), we examined the mechanism conferring protection. Here, we demonstrate that restricted virus replication in Mϕs required CD154/CD40 interactions that stimulated IL-12 production through TRAF6-dependent signaling. In turn, IL-12 production resulted in IFN-γ production, which induced proinflammatory polarization of Mϕs, protecting the cells from infection. These CD40-dependent events protected mice against virus challenge. CD40-/- mice were exquisitely sensitive to intraperitoneal challenge with a dose of rVSV/EBOV GP that was sublethal to CD40+/+ mice, exhibiting viremia within 12 h of infection and rapidly succumbing to infection. This study identifies a previously unappreciated role for Mϕ-intrinsic CD40 signaling in controlling acute virus infection.
-
-
-
Chromatin immunoprecipitation
Lymphoid follicle formation and human vaccination responses recapitulated in an organ-on-a-chip
In bioRxiv on 16 October 2019 by Goyal, G., Prabhala, P., et al.
-
-
-
Cardiovascular biology
-
Immunology and Microbiology
-
Neuroscience
B-cell composition in the blood and cerebrospinal fluid of multiple sclerosis patients treated with dimethyl fumarate.
In Mult Scler Relat Disord on 1 November 2018 by Høglund, R. A., Polak, J., et al.
PubMed
B cells may contribute to the immunopathogenesis of multiple sclerosis (MS). Dimethyl fumarate (DMF) has recently been shown to reduce the frequency of memory B cells in blood, but it is not known whether the drug influences the cellular composition in the cerebrospinal fluid (CSF).
-