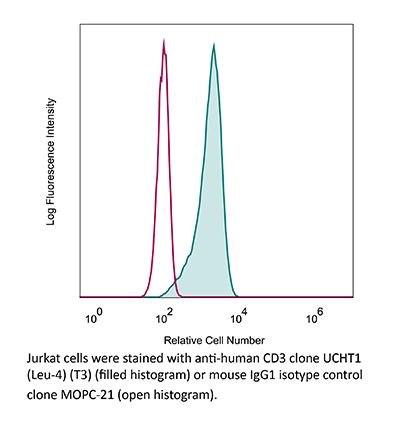
InVivoMAb anti-human CD3
Product Description
Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Human CD3ε |
| Reported Applications |
in vivo T cell depletion in humanized mice ex vivo T cell inhibition for xenographs Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687713 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo T cell depletion in humanized mice
ex vivo T cell inhibtion for xenografts
Wunderlich, M., et al (2014). "OKT3 prevents xenogeneic GVHD and allows reliable xenograft initiation from unfractionated human hematopoietic tissues" Blood 123(24): e134-144.
PubMed
Immunodeficient mice are now readily engrafted with human hematopoietic cells. However, these mice are susceptible to graft-versus-host disease (GVHD) induced by the engraftment and rapid expansion of coinjected human T cells. Therefore, highly purified sample populations must be used, adding significant time, expense, and effort. Here, we have explored in vivo and in vitro methods utilizing anti-T-cell antibodies to circumvent this problem. Intraperitoneal injection of the antibody within 48 hours prevented GVHD. Alternatively, short-term in vitro incubation of cells with antibody immediately before transplant was equally effective. Although in vitro antithymocyte globulin treatment resulted in a dramatic loss of SCID-repopulating cells (SRCs), treatment with OKT3 or UCHT1 abrogated GVHD risk and preserved engraftment potential. Leukemia samples that presented with substantial human T-cell contamination were effectively rescued from GVHD. In addition, OKT3 treatment of unfractionated cord blood resulted in robust engraftment of primary and secondary mice that was indistinguishable from grafts obtained using purified CD34(+) cells. Limiting dilution analysis of unfractionated blood demonstrated a SRC frequency of 1 in 300 to 500 CD34(+) cells, similar to that of purified hematopoietic stem and progenitor cells. This protocol streamlines xenograft studies while significantly reducing the cost and time of the procedure.
in vivo T cell depletion
Woo, J. H., et al (2010). "Pharmacology of anti-CD3 diphtheria immunotoxin in CD3 positive T-cell lymphoma trials" Methods Mol Biol 651: 157-175.
PubMed
Anti-CD3 recombinant diphtheria immunotoxin, A-dmDT(390)-bisFv(UCHT1), consists of the catalytic and translocation domains of diphtheria toxin fused to two single chain Fv fragments of an anti-CD3epsilon monoclonal antibody (UCHT1). A-dmDT(390)-bisFv(UCHT1) is capable of killing CD3(+) T-lymphoma cells and normal T cells specifically in the femtomolar concentration range. To study pharmacology of A-dmDT(390)-bisFv(UCHT1) in patients with CD3(+) T-cell lymphoma in a phase I clinical trial, (1) highly sensitive bioassay using Jurkat cells for measuring drug levels, (2) ELISA for measuring anti-DT antibody titer, and (3) 5-color FACS analysis method for measuring changes of subtype T-cell population were developed. In addition to evaluating drug efficacy and pharmacokinetics in patients, it is important to correlate pre-existing anti-DT antibody levels with maximum drug concentration in serum and extent of T-cell depletion because pre-existing anti-DT antibodies due to DPT (Diphtheria, Pertussis, and Tetanus) immunization can neutralize diphtheria immunotoxin. We observed that at the lowest treatment dose (2.5 microg/kg: twice daily for 4 days) A-dmDT(390)-bisFv(UCHT1) depletes greater than 99.0% of normal T cells in all six patients for a short period of time (2-3 days) and that there is no association of C (max) and extent of T-cell depletion with the pre-existing anti-DT antibody titer.
Flow Cytometry
Rossi, N. E., et al (2008). "Differential antibody binding to the surface alphabetaTCR.CD3 complex of CD4+ and CD8+ T lymphocytes is conserved in mammals and associated with differential glycosylation" Int Immunol 20(10): 1247-1258.
PubMed
We have previously shown that the surface alphabeta T cell antigen receptor (TCR).CD3 complex borne by human CD4(+) and CD8(+) T lymphocytes can be distinguished using mAbs. Using two unrelated sets of antibodies, we have now extended this finding to the surface alphabetaTCR.CD3 of seven additional mammalian species (six non-human primates and the mouse). We have also produced data supporting that differential glycosylation of the two main T cell subsets is involved in the observed TCR.CD3 antibody-binding differences in humans. First, we show differential lectin binding to human CD4(+) versus CD8(+) T lymphocytes, particularly with galectin 7. Second, we show that certain lectins can compete differentially with CD3 mAb binding to human primary CD4(+) and CD8(+) T lymphocytes. Third, N-glycan disruption using swainsonine was shown to increase mAb binding to the alphabetaTCR.CD3. We conclude that the differential antibody binding to the surface alphabetaTCR.CD3 complex of primary CD4(+) and CD8(+) T lymphocytes is phylogenetically conserved and associated with differential glycosylation. The differences may be exploited for therapeutic purposes, such as T cell lineage-specific immunosuppression of graft rejection. Also, the impact of glycosylation on CD3 antibody binding requires a cautious interpretation of CD3 expression levels and T cell numbers in clinical diagnosis.
Arnett, K. L., et al (2004). "Crystal structure of a human CD3-epsilon/delta dimer in complex with a UCHT1 single-chain antibody fragment" Proc Natl Acad Sci U S A 101(46): 16268-16273.
PubMed
The alpha/beta T cell receptor complex transmits signals from MHC/peptide antigens through a set of constitutively associated signaling molecules, including CD3-epsilon/gamma and CD3-epsilon/delta. We report the crystal structure at 1.9-A resolution of a complex between a human CD3-epsilon/delta ectodomain heterodimer and a single-chain fragment of the UCHT1 antibody. CD3-epsilon/delta and CD3-epsilon/gamma share a conserved interface between the Ig-fold ectodomains, with parallel packing of the two G strands. CD3-delta has a more electronegative surface and a more compact Ig fold than CD3-gamma; thus, the two CD3 heterodimers have distinctly different molecular surfaces. The UCHT1 antibody binds near an acidic region of CD3-epsilon opposite the dimer interface, occluding this region from direct interaction with the TCR. This immunodominant epitope may be a uniquely accessible surface in the TCR/CD3 complex, because there is overlap between the binding site of the UCHT1 and OKT3 antibodies. Determination of the CD3-epsilon/delta structure completes the set of TCR/CD3 globular ectodomains and contributes information about exposed CD3 surfaces.
Product Citations
-
-
Stem Cells and Developmental Biology
-
Immunology and Microbiology
Pluripotent stem cell–derived extracellular vesicles for systemic immune modulation in diabetes therapy
In Research Square on 10 June 2025 by Li, S., Zarubova, J., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
Targeting the LMP1-ALIX axis in EBV+ nasopharyngeal carcinoma inhibits immunosuppressive small extracellular vesicle secretion and boosts anti-tumor immunity.
In Cancer Commun (Lond) on 1 December 2024 by He, F., Gong, Y., et al.
PubMed
Immunotherapy has revolutionized the therapeutical regimen for nasopharyngeal carcinoma (NPC), yet its response rate remains insufficient. Programmed death-ligand 1 (PD-L1) on small extracellular vesicles (sEVs) mediates local and peripheral immunosuppression in tumors, and the mechanism of PD-L1 loading into these vesicles is garnering increasing attention. Latent membrane protein 1 (LMP1), a key viral oncoprotein expressed in Epstein-Barr virus (EBV)-positive NPC, contributes to remodeling the tumor microenvironment. However, the precise mechanisms by which LMP1 modulates tumor immunity in NPC remain unclear. Here, we aimed to investigate the roles and regulatory mechanisms of LMP1 and sEV PD-L1 in NPC immune evasion.
-
-
-
Cancer Research
-
Immunology and Microbiology
EGFR mutations induce the suppression of CD8+ T cell and anti-PD-1 resistance via ERK1/2-p90RSK-TGF-β axis in non-small cell lung cancer.
In J Transl Med on 14 July 2024 by Huang, H., Zhu, X., et al.
PubMed
Non-small cell lung cancer (NSCLC) patients with EGFR mutations exhibit an unfavorable response to immune checkpoint inhibitor (ICI) monotherapy, and their tumor microenvironment (TME) is usually immunosuppressed. TGF-β plays an important role in immunosuppression; however, the effects of TGF-β on the TME and the efficacy of anti-PD-1 immunotherapy against EGFR-mutated tumors remain unclear.
-
-
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
Circulation immune cell landscape in canonical pathogenesis of colorectal adenocarcinoma by CyTOF analysis.
In iScience on 15 March 2024 by Kong, X. X., Xu, J. S., et al.
PubMed
Current studies on the immune microenvironment of colorectal cancer (CRC) were mostly limited to the tissue level, lacking relevant studies in the peripheral blood, and failed to describe its alterations in the whole process of adenocarcinoma formation, especially of adenoma carcinogenesis. Here, we constructed a large-scale population cohort and used the CyTOF to explore the changes of various immune cell subsets in peripheral blood of CRC. We found monocytes and basophils cells were significantly higher in adenocarcinoma patients. Compared with early-stage CRC, effector CD4+T cells and naive B cells were higher in patients with lymph node metastasis, whereas the basophils were lower. We also performed random forest algorithm and found monocytes play the key role in carcinogenesis. Our study draws a peripheral blood immune cell landscape of the occurrence and development of CRC at the single-cell level and provides a reference for other researchers.
-
-
-
Flow cytometry/Cell sorting
-
Homo sapiens (Human)
-
Genetics
-
Immunology and Microbiology
-
Neuroscience
Pyrimidine de novo synthesis inhibition selectively blocks effector but not memory T cell development.
In Nat Immunol on 1 March 2023 by Scherer, S., Oberle, S. G., et al.
PubMed
Blocking pyrimidine de novo synthesis by inhibiting dihydroorotate dehydrogenase is used to treat autoimmunity and prevent expansion of rapidly dividing cell populations including activated T cells. Here we show memory T cell precursors are resistant to pyrimidine starvation. Although the treatment effectively blocked effector T cells, the number, function and transcriptional profile of memory T cells and their precursors were unaffected. This effect occurred in a narrow time window in the early T cell expansion phase when developing effector, but not memory precursor, T cells are vulnerable to pyrimidine starvation. This vulnerability stems from a higher proliferative rate of early effector T cells as well as lower pyrimidine synthesis capacity when compared with memory precursors. This differential sensitivity is a drug-targetable checkpoint that efficiently diminishes effector T cells without affecting the memory compartment. This cell fate checkpoint might therefore lead to new methods to safely manipulate effector T cell responses.
-
-
-
Binding experiments
-
Homo sapiens (Human)
-
Biochemistry and Molecular biology
Discovery of Aptamers Against Cell Surface Markers Using Ligand-Guided Selection.
In Methods Mol Biol on 27 September 2022 by Williams, N., Patel, R., et al.
PubMed
Oligonucleotide ligands (DNA, RNA, or XNA), also known as aptamers, are selected against various target molecules using an iterative, evolutionary process called systematic evolution of ligands by exponential enrichment (SELEX). To select aptamers against complex cell surface proteins in their native state, a variant of SELEX termed ligand-guided selection (LIGS) was recently introduced. The significance of LIGS is rooted in its strategy of exploiting the selection step in SELEX to identify highly specific aptamers against known cell surface markers. Thus, in LIGS, a higher-affinity secondary ligand, such as a monoclonal antibody (mAb) to a whole-cell bound to an evolved SELEX library, is introduced to outcompete sequences against the mAb targeting cell surface protein or induce a conformational switch to destabilize the aptamer-surface cell surface protein resulting in elution of the sequences. Here, we describe the detailed method of LIGS utilized in identifying aptamers against T-cell receptor cluster of differentiation three complex (TCR-CD3) expressed in human T-cells and T-cell leukemia.
-
-
-
Western Blotting
-
Homo sapiens (Human)
-
Immunology and Microbiology
Allosteric activation of T cell antigen receptor signaling by quaternary structure relaxation.
In Cell Rep on 13 July 2021 by Lanz, A. L., Masi, G., et al.
PubMed
The mechanism of T cell antigen receptor (TCR-CD3) signaling remains elusive. Here, we identify mutations in the transmembrane region of TCRβ or CD3ζ that augment peptide T cell antigen receptor (pMHC)-induced signaling not explicable by enhanced ligand binding, lateral diffusion, clustering, or co-receptor function. Using a biochemical assay and molecular dynamics simulation, we demonstrate that the gain-of-function mutations loosen the interaction between TCRαβ and CD3ζ. Similar to the activating mutations, pMHC binding reduces TCRαβ cohesion with CD3ζ. This event occurs prior to CD3ζ phosphorylation and at 0°C. Moreover, we demonstrate that soluble monovalent pMHC alone induces signaling and reduces TCRαβ cohesion with CD3ζ in membrane-bound or solubilised TCR-CD3. Our data provide compelling evidence that pMHC binding suffices to activate allosteric changes propagating from TCRαβ to the CD3 subunits, reconfiguring interchain transmembrane region interactions. These dynamic modifications could change the arrangement of TCR-CD3 boundary lipids to license CD3ζ phosphorylation and initiate signal propagation.
-
-
-
In vitro experiments
-
Homo sapiens (Human)
-
Immunology and Microbiology
-
Pharmacology
Therapeutic effect of kaempferol on atopic dermatitis by attenuation of T cell activity via interaction with multidrug resistance-associated protein 1.
In Br J Pharmacol on 1 April 2021 by Lee, H. S., Jeong, G. S., et al.
PubMed
Kaempferol is a natural flavonoid widely investigated in various fields due to its antioxidant, anti-cancer, and anti-inflammatory activities, but few studies have shown its inhibitory effect on T cell activation. This study examined the therapeutic potential of kaempferol in atopic dermatitis by modulating T cell activation.
-
-
-
Homo sapiens (Human)
-
Biochemistry and Molecular biology
-
Genetics
Ligand-Guided Selection with Artificially Expanded Genetic Information Systems against TCR-CD3ε.
In Biochemistry on 4 February 2020 by Zumrut, H., Yang, Z., et al.
PubMed
Here we are reporting, for the first time, a ligand-guided selection (LIGS) experiment using an artificially expanded genetic information system (AEGIS) to successfully identify an AEGIS-DNA aptamer against T cell receptor-CD3ε expressed on Jurkat.E6 cells. Thus, we have effectively combined the enhanced diversity of an AEGIS DNA library with LIGS to develop a superior screening platform to discover superior aptamers. Libraries of DNA molecules from highly diversified building blocks will provide better ligands due to more functional diversity and better-controlled folding. Thus, a DNA library with AEGIS components (dZ and dP) was used in LIGS experiments against TCR-CD3ε in its native state using two clinically relevant monoclonal antibodies to identify an aptamer termed JZPO-10, with nanomolar affinity. Multiple specificity assays using knockout cells, and competition experiments using monoclonal antibodies utilized in LIGS, show unprecedented specificity of JZPO-10, suggesting that the combination of LIGS with AEGIS-DNA libraries will provide a superior screening platform to discover artificial ligands against critical cellular targets.
-
-
-
Flow cytometry/Cell sorting
-
Homo sapiens (Human)
Integrating Ligand-Receptor Interactions and In Vitro Evolution for Streamlined Discovery of Artificial Nucleic Acid Ligands.
In Mol Ther Nucleic Acids on 6 September 2019 by Zumrut, H. E., Batool, S., et al.
PubMed
To discover DNA ligands against a predetermined receptor protein complex, we introduce a comprehensive version of ligand-guided selection (LIGS). LIGS is, itself, a variant of systematic evolution of ligands by exponential enrichment (SELEX). Herein, we have optimized LIGS to identify higher affinity aptamers with high specificity. In addition, we demonstrate the expandability of LIGS by performing specific aptamer elution at 25°C, utilizing multiple monoclonal antibodies (mAbs) against cultured cells and primary cells obtained from human donors expressing the same receptor. Eluted LIGS libraries obtained through Illumina high-throughput (HT) DNA sequencing were analyzed by bioinformatics tools to discover five DNA aptamers with apparent affinities ranging from 3.06 ± 0.485 nM to 325 ± 62.7 nM against the target, T cell receptor-cluster of differentiation epsilon (TCR-CD3ε) expressed on human T cells. The specificity of the aptamers was validated utilizing multiple strategies, including competitive binding analysis and a double-knockout Jurkat cell line generated by CRISPR technology. The cross-competition experiments using labeled and unlabeled aptamers revealed that all five aptamers compete for the same binding site. Collectively, the data in this report introduce a modified LIGS strategy as a universal platform to identify highly specific multiple aptamers toward multi-component receptor proteins in their native state without changing the cell-surface landscape.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
DPP8/DPP9 inhibitor-induced pyroptosis for treatment of acute myeloid leukemia.
In Nat Med on 1 August 2018 by Johnson, D. C., Taabazuing, C. Y., et al.
PubMed
Small-molecule inhibitors of the serine dipeptidases DPP8 and DPP9 (DPP8/9) induce a lytic form of cell death called pyroptosis in mouse and human monocytes and macrophages1,2. In mouse myeloid cells, Dpp8/9 inhibition activates the inflammasome sensor Nlrp1b, which in turn activates pro-caspase-1 to mediate cell death3, but the mechanism of DPP8/9 inhibitor-induced pyroptosis in human myeloid cells is not yet known. Here we show that the CARD-containing protein CARD8 mediates DPP8/9 inhibitor-induced pro-caspase-1-dependent pyroptosis in human myeloid cells. We further show that DPP8/9 inhibitors induce pyroptosis in the majority of human acute myeloid leukemia (AML) cell lines and primary AML samples, but not in cells from many other lineages, and that these inhibitors inhibit human AML progression in mouse models. Overall, this work identifies an activator of CARD8 in human cells and indicates that its activation by small-molecule DPP8/9 inhibitors represents a new potential therapeutic strategy for AML.
-