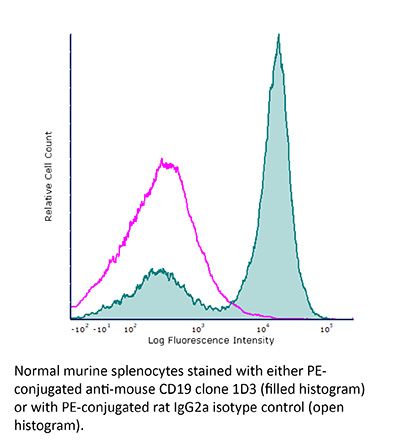
FlowMAb PE anti-mouse CD19
Product Description
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | FlowMAb PE rat IgG2a isotype control, anti-trinitrophenol |
| Conjugation | PE |
| Excitation Source | Yellow-Green 488 nm, 532 nm, 561 nm |
| Excitation Max | 496 nm, 566 nm |
| Emission Max | 576 nm |
| Immunogen | K562 cells expressing the extracellular domain of mouse CD19 |
| Reported Applications | Flow cytometry |
| Protocol Information | It is recommended that the reagent be carefully titrated for optimal performance in the assay of interest. |
| Concentration | 0.2 mg/ml |
| Formulation |
PBS, pH 7.0 Contains 0.09% Sodium Azide |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G. Conjugated with R-phycoerythrin under optimal conditions. |
| Storage | The antibody solution should be stored at the stock concentration at 4°C and protected from prolonged exposure to light. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Flow Cytometry
Dai, M., et al. (2015). "Curing mice with large tumors by locally delivering combinations of immunomodulatory antibodies" Clin Cancer Res 21(5): 1127-1138.
PubMed
PURPOSE: Immunomodulatory mAbs can treat cancer, but cures are rare except for small tumors. Our objective was to explore whether the therapeutic window increases by combining mAbs with different modes of action and injecting them into tumors. EXPERIMENTAL DESIGN: Combinations of mAbs to CD137/PD-1/CTLA-4 or CD137/PD-1/CTLA-4/CD19 were administrated intratumorally to mice with syngeneic tumors (B16 and SW1 melanoma, TC1 lung carcinoma), including tumors with a mean surface of approximately 80 mm(2). Survival and tumor growth were assessed. Immunologic responses were evaluated using flow cytometry and qRT-PCR. RESULTS: More than 50% of tumor-bearing mice had complete regression and long-term survival after tumor injection with mAbs recognizing CD137/PD-1/CTLA-4/CD19 with similar responses in three models. Intratumoral injection was more efficacious than intraperitoneal injection in causing rejection also of untreated tumors in the same mice. The three-mAb combination could also induce regression, but was less efficacious. There were few side effects, and therapy-resistant tumors were not observed. Transplanted tumor cells rapidly caused a Th2 response with increased CD19 cells. Successful therapy shifted this response to the Th1 phenotype with decreased CD19 cells and increased numbers of long-term memory CD8 effector cells and T cells making IFNgamma and TNFalpha. CONCLUSIONS: Intratumoral injection of mAbs recognizing CD137/PD-1/CTLA-4/CD19 can eradicate established tumors and reverse a Th2 response with tumor-associated CD19 cells to Th1 immunity, whereas a combination lacking anti-CD19 is less effective. There are several human cancers for which a similar approach may provide clinical benefit.
Flow Cytometry
Becker, A. M., et al. (2015). "ADAM17 limits the expression of CSF1R on murine hematopoietic progenitors" Exp Hematol 43(1): 44-52 e41-43.
PubMed
All-lymphoid progenitors (ALPs) yield few myeloid cells in vivo, but readily generate such cells in vitro. The basis for this difference remains unknown. We hypothesized that ALPs limit responsiveness to in vivo concentrations of myeloid-promoting cytokines by reducing expression of the corresponding receptors, potentially through posttranscriptional mechanisms. Consistent with such a mechanism, ALPs express higher levels of CSF1R transcripts than their upstream precursors, yet show limited cell-surface protein expression of colony-stimulating factor 1 receptor (CSF1R). All-lymphoid progenitors and other hematopoietic progenitors deficient in A disintegrin and metalloproteinase domain 17 (ADAM17), display elevated cell surface CSF1R expression. ADAM17(-/-) ALPs, however, fail to yield myeloid cells upon transplantation into irradiated recipients. Moreover, ADAM17(-/-) ALPs yield fewer macrophages in vitro than control ALPs at high concentrations of macrophage colony stimulating factor. Mice with hematopoietic-specific deletion of ADAM17 have normal numbers of myeloid and lymphoid progenitors and mature cells in vivo. These data demonstrate that ADAM17 limits CSF1R protein expression on hematopoietic progenitors, but that compensatory mechanisms prevent elevated CSF1R levels from altering lymphoid progenitor potential.
Flow Cytometry
De Obaldia, M. E., et al. (2013). "T cell development requires constraint of the myeloid regulator C/EBP-alpha by the Notch target and transcriptional repressor Hes1" Nat Immunol 14(12): 1277-1284.
PubMed
Notch signaling induces gene expression of the T cell lineage and discourages alternative fate outcomes. Hematopoietic deficiency in the Notch target Hes1 results in severe T cell lineage defects; however, the underlying mechanism is unknown. We found here that Hes1 constrained myeloid gene-expression programs in T cell progenitor cells, as deletion of the myeloid regulator C/EBP-alpha restored the development of T cells from Hes1-deficient progenitor cells. Repression of Cebpa by Hes1 required its DNA-binding and Groucho-recruitment domains. Hes1-deficient multipotent progenitor cells showed a developmental bias toward myeloid cells and dendritic cells after Notch signaling, whereas Hes1-deficient lymphoid progenitor cells required additional cytokine signaling for diversion into the myeloid lineage. Our findings establish the importance of constraining developmental programs of the myeloid lineage early in T cell development.
Flow Cytometry
Dai, M., et al. (2013). "Long-lasting complete regression of established mouse tumors by counteracting Th2 inflammation" J Immunother 36(4): 248-257.
PubMed
40% of mice with SW1 tumors remained healthy >150 days after last treatment and are probably cured. Therapeutic efficacy was associated with a systemic immune response with memory and antigen specificity, required CD4 cells and involved CD8 cells and NK cells to a less extent. The 3 mAb combination significantly decreased CD19 cells at tumor sites, increased IFN-gamma and TNF-alpha producing CD4 and CD8 T cells and mature CD86 dendritic cells (DC), and it increased the ratios of effector CD4 and CD8 T cells to CD4Foxp3 regulatory T (Treg) cells and to CD11bGr-1 myeloid suppressor cells (MDSC). This is consistent with shifting the tumor microenvironment from an immunosuppressive Th2 to an immunostimulatory Th1 type and is further supported by PCR data. Adding an anti-CD19 mAb to the 3 mAb combination in the SW1 model further increased therapeutic efficacy. Data from ongoing experiments show that intratumoral injection of a combination of mAbs to CD137PD-1CTLA4CD19 can induce complete regression and dramatically prolong survival also in the TC1 carcinoma and B16 melanoma models, suggesting that the approach has general validity.”}” data-sheets-userformat=”{“2″:14851,”3”:{“1″:0},”4”:{“1″:2,”2″:16777215},”12″:0,”14”:{“1″:2,”2″:1521491},”15″:”Roboto, sans-serif”,”16″:12}”>Mice with intraperitoneal ID8 ovarian carcinoma or subcutaneous SW1 melanoma were injected with monoclonal antibodies (mAbs) to CD137PD-1CTLA4 7-15 days after tumor initiation. Survival of mice with ID8 tumors tripled and >40% of mice with SW1 tumors remained healthy >150 days after last treatment and are probably cured. Therapeutic efficacy was associated with a systemic immune response with memory and antigen specificity, required CD4 cells and involved CD8 cells and NK cells to a less extent. The 3 mAb combination significantly decreased CD19 cells at tumor sites, increased IFN-gamma and TNF-alpha producing CD4 and CD8 T cells and mature CD86 dendritic cells (DC), and it increased the ratios of effector CD4 and CD8 T cells to CD4Foxp3 regulatory T (Treg) cells and to CD11bGr-1 myeloid suppressor cells (MDSC). This is consistent with shifting the tumor microenvironment from an immunosuppressive Th2 to an immunostimulatory Th1 type and is further supported by PCR data. Adding an anti-CD19 mAb to the 3 mAb combination in the SW1 model further increased therapeutic efficacy. Data from ongoing experiments show that intratumoral injection of a combination of mAbs to CD137PD-1CTLA4CD19 can induce complete regression and dramatically prolong survival also in the TC1 carcinoma and B16 melanoma models, suggesting that the approach has general validity.
Flow Cytometry
Purtha, W. E., et al. (2012). "Spontaneous mutation of the Dock2 gene in Irf5-/- mice complicates interpretation of type I interferon production and antibody responses" Proc Natl Acad Sci U S A 109(15): E898-904.
PubMed
Genome-wide studies have identified associations between polymorphisms in the IFN regulatory factor-5 (Irf5) gene and a variety of human autoimmune diseases. Its functional role in disease pathogenesis, however, remains unclear, as studies in Irf5(-/-) mice have reached disparate conclusions regarding the importance of this transcription factor in type I IFN production and antibody responses. We identified a spontaneous genomic duplication and frameshift mutation in the guanine exchange factor dedicator of cytokinesis 2 (Dock2) that has arisen in at least a subset of circulating Irf5(-/-) mice and inadvertently been bred to homozygosity. Retroviral expression of DOCK2, but not IRF-5, rescued defects in plasmacytoid dendritic cell and B-cell development, and Irf5(-/-) mice lacking the mutation in Dock2 exhibited normal plasmacytoid dendritic cell and B-cell development, largely intact type I IFN responses, and relatively normal antibody responses to viral infection. Thus, confirmation of the normal Dock2 genotype in circulating Irf5(-/-) mice is warranted, and our data may partly explain conflicting results in this field.
Flow Cytometry
Purtha, W. E., et al. (2011). "Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants" J Exp Med 208(13): 2599-2606.
PubMed
Memory B cells (MBCs) and long-lived plasma cells (LLPCs) persist after clearance of infection, yet the specific and nonredundant role MBCs play in subsequent protection is unclear. After resolution of West Nile virus infection in mice, we demonstrate that LLPCs were specific for a single dominant neutralizing epitope, such that immune serum poorly inhibited a variant virus that encoded a mutation at this critical epitope. In contrast, a large fraction of MBC produced antibody that recognized both wild-type (WT) and mutant viral epitopes. Accordingly, antibody produced by the polyclonal pool of MBC neutralized WT and variant viruses equivalently. Remarkably, we also identified MBC clones that recognized the mutant epitope better than the WT protein, despite never having been exposed to the variant virus. The ability of MBCs to respond to variant viruses in vivo was confirmed by experiments in which MBCs were adoptively transferred or depleted before secondary challenge. Our data demonstrate that class-switched MBC can respond to variants of the original pathogen that escape neutralization of antibody produced by LLPC without a requirement for accumulating additional somatic mutations.