InVivoPlus anti-mouse/human/rat CD47 (IAP)
Product Details
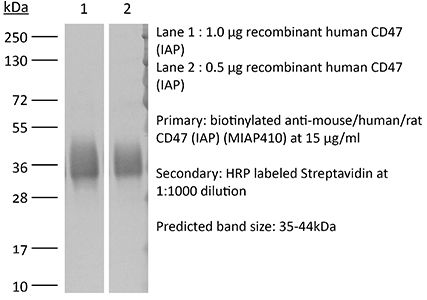
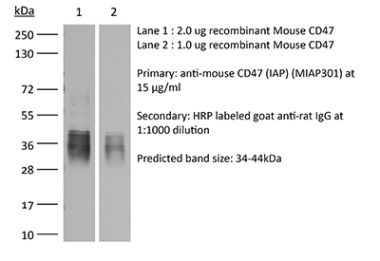
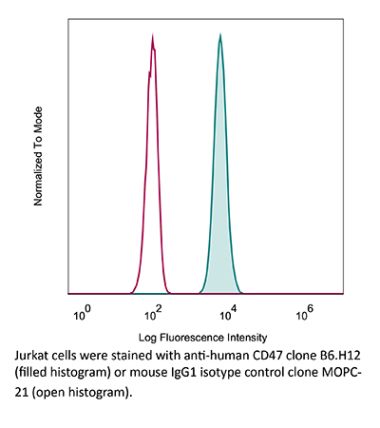
The MIAP410 monoclonal antibody reacts with mouse CD47 otherwise known as integrin-associated protein (IAP). CD47 is an approximately 50 kDa glycosylated five transmembrane protein that is ubiquitously expressed by both hematopoietic cells such as T and B lymphocytes, monocytes, platelets and erythrocytes and non-hematopoietic cells. CD47 is involved in a range of cellular processes, including apoptosis, proliferation, adhesion, and migration. Furthermore, it plays a key role in immune and angiogenic responses. CD47 is a receptor for thrombospondin-1 (TSP-1), a secreted glycoprotein that plays a role in vascular development and angiogenesis. CD47 Is has been found to be overexpressed in many different tumor cells. Because of this, anti-CD47 monoclonal antibodies have been proposed and studied as a therapeutic treatment for human cancers. The MIAP410 antibody has been shown to neutralize CD47 in vivo and in vitro.Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoPlus mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | purified human placental CD47 |
| Reported Applications |
in vivo CD47 blockade in vitro CD47 blocking Immunofluorescence |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Aggregation* |
<5% Determined by SEC |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687806 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests* |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Additional Formats
Recommended Products
in vivo CD47 blockade, in vitro CD47 blockade
Kojima, Y., et al. (2016). "CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis" Nature. DOI : 10.1038/nature18935. PubMed
Atherosclerosis is the disease process that underlies heart attack and stroke. Advanced lesions at risk of rupture are characterized by the pathological accumulation of diseased vascular cells and apoptotic cellular debris. Why these cells are not cleared remains unknown. Here we show that atherogenesis is associated with upregulation of CD47, a key anti-phagocytic molecule that is known to render malignant cells resistant to programmed cell removal, or ‘efferocytosis’. We find that administration of CD47-blocking antibodies reverses this defect in efferocytosis, normalizes the clearance of diseased vascular tissue, and ameliorates atherosclerosis in multiple mouse models. Mechanistic studies implicate the pro-atherosclerotic factor TNF-alpha as a fundamental driver of impaired programmed cell removal, explaining why this process is compromised in vascular disease. Similar to recent observations in cancer, impaired efferocytosis appears to play a pathogenic role in cardiovascular disease, but is not a fixed defect and may represent a novel therapeutic target.
in vivo CD47 blockade, in vitro CD47 blockade, Immunofluorescence
Willingham, S. B., et al. (2012). "The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors" Proc Natl Acad Sci U S A 109(17): 6662-6667. PubMed
CD47, a “don’t eat me” signal for phagocytic cells, is expressed on the surface of all human solid tumor cells. Analysis of patient tumor and matched adjacent normal (nontumor) tissue revealed that CD47 is overexpressed on cancer cells. CD47 mRNA expression levels correlated with a decreased probability of survival for multiple types of cancer. CD47 is a ligand for SIRPalpha, a protein expressed on macrophages and dendritic cells. In vitro, blockade of CD47 signaling using targeted monoclonal antibodies enabled macrophage phagocytosis of tumor cells that were otherwise protected. Administration of anti-CD47 antibodies inhibited tumor growth in orthotopic immunodeficient mouse xenotransplantation models established with patient tumor cells and increased the survival of the mice over time. Anti-CD47 antibody therapy initiated on larger tumors inhibited tumor growth and prevented or treated metastasis, but initiation of the therapy on smaller tumors was potentially curative. The safety and efficacy of targeting CD47 was further tested and validated in immune competent hosts using an orthotopic mouse breast cancer model. These results suggest all human solid tumor cells require CD47 expression to suppress phagocytic innate immune surveillance and elimination. These data, taken together with similar findings with other human neoplasms, show that CD47 is a commonly expressed molecule on all cancers, its function to block phagocytosis is known, and blockade of its function leads to tumor cell phagocytosis and elimination. CD47 is therefore a validated target for cancer therapies.
Immunofluorescence
Han, X., et al. (2000). "CD47, a ligand for the macrophage fusion receptor, participates in macrophage multinucleation" J Biol Chem 275(48): 37984-37992. PubMed
The macrophage fusion receptor (MFR), also called P84/BIT/SIRPalpha/SHPS-1, is a transmembrane glycoprotein that belongs to the superfamily of immunoglobulins. Previously, we showed that MFR expression is highly induced at the onset of fusion in macrophages, and that MFR appears to play a role in macrophage-macrophage adhesion/fusion leading to multinucleation. The recent finding that IAP/CD47 acts as a ligand for MFR led us to hypothesize that it interacts with CD47 at the onset of cell-cell fusion. CD47 is a transmembrane glycoprotein, which, like MFR, belongs to the superfamily of immunoglobulins. We show that macrophages express the hemopoietic form of CD47, the expression of which is induced at the onset of fusion, but to a lower level than MFR. A glutathione S-transferase CD47 fusion protein engineered to contain the extracellular domain of CD47, binds macrophages, associates with MFR, and prevents multinucleation. CD47 and MFR associate via their amino-terminal immunoglobulin variable domain. Of the nine monoclonal antibodies raised against the extracellular domain of CD47, three block fusion, as well as MFR-CD47 interaction, whereas the others have no effect. Together, these data suggest that CD47 is involved in macrophage multinucleation by virtue of interacting with MFR during adhesion/fusion.
- FC/FACS,
- Neuroscience
CD47 antibody blockade suppresses microglia-dependent phagocytosis and monocyte transition to macrophages, impairing recovery in EAE.
In JCI Insight on 8 November 2021 by Wang, H., Newton, G., et al.
PubMed
Experimental autoimmune encephalomyelitis (EAE) is a well-characterized animal model of multiple sclerosis. During the early phase of EAE, infiltrating monocytes and monocyte-derived macrophages contribute to T cell recruitment, especially CD4+ T cells, into the CNS, resulting in neuronal demyelination; however, in later stages, they promote remyelination and recovery by removal of myelin debris by phagocytosis. Signal regulatory protein α and CD47 are abundantly expressed in the CNS, and deletion of either molecule is protective in myelin oligodendrocyte glycoprotein-induced EAE because of failed effector T cell expansion and trafficking. Here we report that treatment with the function blocking CD47 Ab Miap410 substantially reduced the infiltration of pathogenic immune cells but impaired recovery from paresis. The underlying mechanism was by blocking the emergence of CD11chiMHCIIhi microglia at peak disease that expressed receptors for phagocytosis, scavenging, and lipid catabolism, which mediated clearance of myelin debris and the transition of monocytes to macrophages in the CNS. In the recovery phase of EAE, Miap410 Ab-treated mice had worsening paresis with sustained inflammation and limited remyelination as compared with control Ab-treated mice. In summary, Ab blockade of CD47 impaired resolution of CNS inflammation, thus worsening EAE.