InVivoMAb anti-rat/mouse CD71 (TfR1)
Product Details
The OX-26 monoclonal antibody reacts with rat CD71 also known as transferrin receptor protein 1 (TfR1). The antibody is reported to cross-react with mouse CD71 as well. CD71 is a 170-180 kDa type II homodimeric transmembrane glycoprotein which is expressed on the surface of proliferating cells, reticulocytes, and erythroid precursors. CD71 plays a role in the control of cellular proliferation and is required for iron import from transferrin into cells by endocytosis. CD71 is expressed on malignant cells at high levels and its expression correlates with cancer progression. This high expression on malignant cells along with CD71’s ability to internalize and the necessity of iron for cancer cell proliferation makes the transferrin receptor an attractive target to exploit for the delivery of drugs into malignant cells. Upon binding to an extracellular domain of CD71, OX-26 is transferred into the blood-brain barrier (BBB) via the endogenous transferrin transport system. Under this mechanism, the OX-26 antibody is often used to transport conjugated drugs across the BBB in experimental rat models.Specifications
| Isotype | Mouse IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG2a isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | PHA-activated PVG rat lymph node cells |
| Reported Applications |
Targeted drug delivery to the brain Immunohistochemistry (frozen) Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_2894751 |
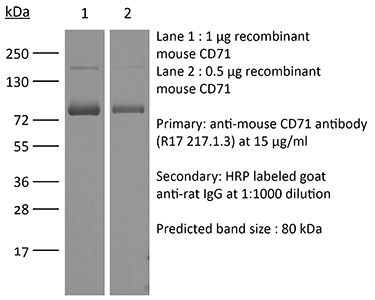
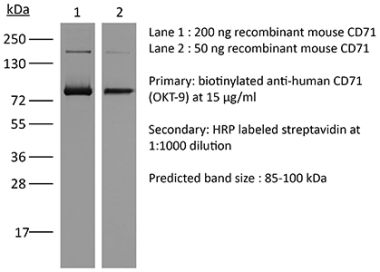
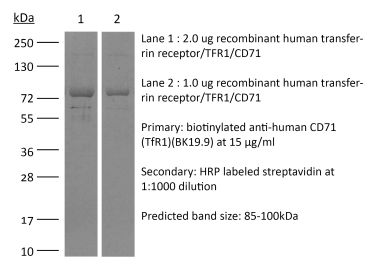
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
Targeted drug delivery to the brain
Amani, H., et al. (2019). "Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling" Sci Rep 9(1): 6044. PubMed
Ischemic cerebral stroke is a major cause of death and morbidity. Currently, no neuroprotective agents have been shown to impact the clinical outcomes in cerebral stroke cases. Here, we report therapeutic effects of Se nanoparticles on ischemic stroke in a murine model. Anti-transferrin receptor monoclonal antibody (OX26)-PEGylated Se nanoparticles (OX26-PEG-Se NPs) were designed and synthesized and their neuroprotective effects were measured using in vitro and in vivo approaches. We demonstrate that administration of the biodegradable nanoparticles leads to resolution of brain edema, protection of axons in hippocampus region, and myelination of hippocampal area after cerebral ischemic stroke. Our nanoparticle design ensures efficient targeting and minimal side effects. Hematological and biochemical analyses revealed no undesired NP-induced changes. To gain mechanistic insights into the therapeutic effects of these particles, we characterized the changes to the relevant inflammatory and metabolic signaling pathways. We assessed metabolic regulator mTOR and related signaling pathways such as hippo, Ubiquitin-proteasome system (ERK5), Tsc1/Tsc2 complex, FoxO1, wnt/beta-catenine signaling pathway. Moreover, we examined the activity of jak2/stat3 signaling pathways and Adamts1, which are critically involved in inflammation. Together, our study provides a promising treatment strategy for cerebral stroke based on Se NP induced suppression of excessive inflammation and oxidative metabolism.
Targeted drug delivery to the brain
Tang, X., et al. (2015). "Anti-transferrin receptor-modified amphotericin B-loaded PLA-PEG nanoparticles cure Candidal meningitis and reduce drug toxicity" Int J Nanomedicine 10: 6227-6241. PubMed
Fatal fungal infections in central nervous system (CNS) can occur through hematogenous spread or direct extension. At present, hydrophobic amphotericin B (AMB) is the most effective antifungal drug in clinical trials. However, AMB is hydrophobic and therefore penetrates poorly into the CNS, and therapeutic levels of AMB are hard to achieve. The transferrin receptor (TfR/CD71) located at the blood-brain barrier mediates transferrin transcytosis. In order to enhance the receptor-mediated delivery of AMB into CNS with therapeutic level, an anti-TfR antibody (OX26)-modified AMB-loaded PLA (poly[lactic acid])-PEG (polyethylene glycol)-based micellar drug delivery system was constructed. The prepared OX26-modified AMB-loaded nanoparticles (OX26-AMB-NPs) showed significant reduction of CNS fungal burden and an increase of mouse survival time. In conclusion, OX26-AMB-NPs represent a promising novel drug delivery system for intracerebral fungal infection.
Targeted drug delivery to the brain
Yue, J., et al. (2012). "Fluorescence-labeled immunomicelles: preparation, in vivo biodistribution, and ability to cross the blood-brain barrier" Macromol Biosci 12(9): 1209-1219. PubMed
Multifunctional hybrid micelles are prepared from amphiphilic mal-PEG-b-PLA and mPEG-b-P(LA-co-DHC/RhB) block copolymers. A specific anti-transferrin receptor antibody, OX26, is linked onto the surface of the micelles. ELISA indicates that the conjugated antibody preserves its activity. OX26 conjugation can increase the uptake efficiency of micelles by target cell lines (C6). Pharmacokinetics and in vivo biodistribution experiments are carried out to investigate the ability of OX26-conjugated micelles (immunomicelles) to cross the blood-brain barrier. The data show that the brain uptake of OX26-conjugated micelles is much more than that of OX26-free ones. Therefore, OX26-conjugated micelles will be promising drug carriers to cross the blood-brain barrier.
Immunohistochemistry (frozen)
Fabriek, B. O., et al. (2007). "The macrophage CD163 surface glycoprotein is an erythroblast adhesion receptor" Blood 109(12): 5223-5229. PubMed
Erythropoiesis occurs in erythroblastic islands, where developing erythroblasts closely interact with macrophages. The adhesion molecules that govern macrophage-erythroblast contact have only been partially defined. Our previous work has implicated the rat ED2 antigen, which is highly expressed on the surface of macrophages in erythroblastic islands, in erythroblast binding. In particular, the monoclonal antibody ED2 was found to inhibit erythroblast binding to bone marrow macrophages. Here, we identify the ED2 antigen as the rat CD163 surface glycoprotein, a member of the group B scavenger receptor cysteine-rich (SRCR) family that has previously been shown to function as a receptor for hemoglobin-haptoglobin (Hb-Hp) complexes and is believed to contribute to the clearance of free hemoglobin. CD163 transfectants and recombinant protein containing the extracellular domain of CD163 supported the adhesion of erythroblastic cells. Furthermore, we identified a 13-amino acid motif (CD163p2) corresponding to a putative interaction site within the second scavenger receptor domain of CD163 that could mediate erythroblast binding. Finally, CD163p2 promoted erythroid expansion in vitro, suggesting that it enhanced erythroid proliferation and/or survival, but did not affect differentiation. These findings identify CD163 on macrophages as an adhesion receptor for erythroblasts in erythroblastic islands, and suggest a regulatory role for CD163 during erythropoiesis.
Flow Cytometry
Jimenez, E., et al. (2002). "Rat peripheral CD4+CD8+ T lymphocytes are partially immunocompetent thymus-derived cells that undergo post-thymic maturation to become functionally mature CD4+ T lymphocytes" J Immunol 168(10): 5005-5013. PubMed
CD4+CD8+ double-positive (DP) T cells represent a minor subpopulation of T lymphocytes found in the periphery of adult rats. In this study, we show that peripheral DP T cells appear among the first T cells that colonize the peripheral lymphoid organs during fetal life, and represent approximately 40% of peripheral T cells during the perinatal period. Later their proportion decreases to reach the low values seen in adulthood. Most DP T cells are small size lymphocytes that do not exhibit an activated phenotype, and their proliferative rate is similar to that of the other peripheral T cell subpopulations. Only 30-40% of DP T cells expresses CD8beta chain, the remaining cells expressing CD8alphaalpha homodimers. However, both DP T cell subsets have an intrathymic origin since they appear in the recent thymic emigrant population after injection of FITC intrathymically. Functionally, although DP T cells are resistant to undergo apoptosis in response to glucocorticoids, they show poor proliferative responses upon CD3/TCR stimulation due to their inability to produce IL-2. A fraction of DP T cells are not actively synthesizing the CD8 coreceptor, and they gradually differentiate to the CD4 cell lineage in reaggregation cultures. Transfer of DP T lymphocytes into thymectomized SCID mice demonstrates that these cells undergo post-thymic maturation in the peripheral lymphoid organs and that their CD4 cell progeny is fully immunocompetent, as judged by its ability to survive and expand in peripheral lymphoid organs, to proliferate in response to CD3 ligation, and to produce IL-2 upon stimulation.