InVivoMAb anti-mouse IFNγ
Product Details
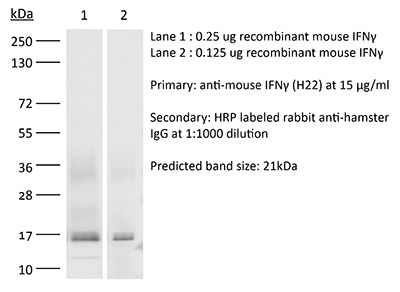
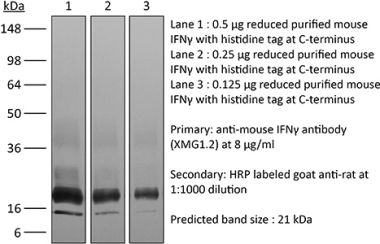
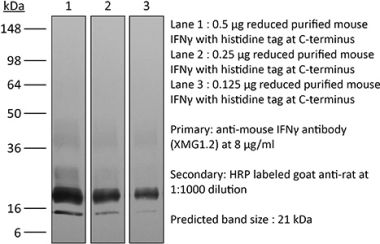
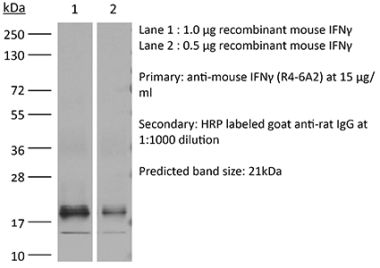
The H22 monoclonal antibody reacts with mouse IFNγ (interferon gamma) a 20 kDa soluble pleiotropic cytokine and the sole member of the type II class of interferons. IFNγ is primarily produced by activated lymphocytes including T, B, NK cells, and ILCs. IFNγ exerts immunoregulatory, anti-proliferative, anti-viral, and proinflammatory activities and plays an important role in activation, growth, and differentiation of T and B lymphocytes, macrophages, NK cells and other non-hematopoietic cell types. Additionally, IFNγ induces the production of cytokines, Fc receptor, and adhesion molecules and up-regulates MHC class I and II antigen expression by antigen presenting cells during an immune response. IFNγ has also been shown to modulate macrophage effector functions, influence isotype switching and induce the secretion of immunoglobulins by B cells. IFNγ signals through the IFN gamma receptor which exists as a heterodimer composed of CD119 (IFN gamma receptor 1) and AF-1 (IFN gamma receptor 2). The IFNγ receptor is expressed ubiquitously on almost all cell types with the exception of mature erythrocytes. The H22 antibody is a neutralizing antibody.Specifications
| Isotype | Armenian hamster IgG |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb polyclonal Armenian hamster IgG |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Recombinant full-length murine IFNγ |
| Reported Applications |
in vivo IFNγ neutralization in vitro IFNγ neutralization |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2736992 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
in vivo IFNγ neutralization
Slaney, C. Y., et al. (2017). "Dual-specific Chimeric Antigen Receptor T Cells and an Indirect Vaccine Eradicate a Variety of Large Solid Tumors in an Immunocompetent, Self-antigen Setting" Clin Cancer Res 23(10): 2478-2490. PubMed
Purpose: While adoptive transfer of T cells bearing a chimeric antigen receptor (CAR) can eliminate substantial burdens of some leukemias, the ultimate challenge remains the eradication of large solid tumors for most cancers. We aimed to develop an immunotherapy approach effective against large tumors in an immunocompetent, self-antigen preclinical mouse model.Experimental Design: In this study, we generated dual-specific T cells expressing both a CAR specific for Her2 and a TCR specific for the melanocyte protein (gp100). We used a regimen of adoptive cell transfer incorporating vaccination (ACTIV), with recombinant vaccinia virus expressing gp100, to treat a range of tumors including orthotopic breast tumors and large liver tumors.Results: ACTIV therapy induced durable complete remission of a variety of Her2(+) tumors, some in excess of 150 mm(2), in immunocompetent mice expressing Her2 in normal tissues, including the breast and brain. Vaccinia virus induced extensive proliferation of T cells, leading to massive infiltration of T cells into tumors. Durable tumor responses required the chemokine receptor CXCR3 and exogenous IL2, but were independent of IFNgamma. Mice were resistant to tumor rechallenge, indicating immune memory involving epitope spreading. Evidence of limited neurologic toxicity was observed, associated with infiltration of cerebellum by T cells, but was only transient.Conclusions: This study supports a view that it is possible to design a highly effective combination immunotherapy for solid cancers, with acceptable transient toxicity, even when the target antigen is also expressed in vital tissues. Clin Cancer Res; 23(10); 2478-90. (c)2016 AACR.
in vivo IFNγ neutralization
Nair, S., et al. (2017). "Interferon regulatory factor-1 (IRF-1) protects against chikungunya virus induced immunopathology by restricting infection in muscle cells" J Virol . PubMed
The innate immune system protects cells against viral pathogens in part through the autocrine and paracrine actions of interferons (IFN)-alpha/beta (type I), -gamma (type II), and -lambda (type III). The transcription factor interferon regulatory factor (IRF)-1 has a demonstrated role in shaping innate and adaptive antiviral immunity by inducing the expression of IFN stimulated genes (ISGs) and mediating signals downstream of IFN-gamma. Although ectopic expression experiments have suggested an inhibitory function of IRF-1 against infection of alphaviruses in cell culture, its role in vivo remains unknown. Here, we infected Irf1(-/-) mice with two distantly related arthritogenic alphaviruses, chikungunya (CHIKV) and Ross River (RRV), and assessed the early antiviral functions of IRF-1 prior to induction of adaptive B and T cell responses. IRF-1 expression limited CHIKV-induced foot swelling in joint-associated tissues and prevented dissemination of CHIKV and RRV at early time points. Virological and histological analysis revealed greater infection of muscle tissues in Irf1(-/-) compared to wild-type mice. The antiviral actions of IRF-1 appeared independent of the induction of type I IFN or effects of type II and III IFNs but were associated with altered local pro-inflammatory cytokine and chemokine responses and differential infiltration of myeloid cell subsets. Collectively, our in vivo experiments suggest that IRF-1 restricts CHIKV and RRV infection in stromal cells, especially muscle cells, and this controls local inflammation and joint-associated swelling.IMPORTANCE Interferon regulatory factor (IRF)-1 is a transcription factor that regulates the expression of a broad range of antiviral host defense genes. In this study, using Irf1(-/-) mice, we investigated the role of IRF-1 in modulating pathogenesis of two related arthritogenic alphaviruses, chikungunya and Ross River viruses. Our studies show that IRF-1 controlled alphavirus replication and swelling in joint-associated tissues within days of infection. Detailed histopathological and virological analyses revealed that IRF-1 preferentially restricted CHIKV infection in cells of non-hematopoietic lineage, including muscle cells. The antiviral actions of IRF-1 resulted in decreased local inflammatory responses in joint associated tissues, which prevented immunopathology.
in vivo IFNγ neutralization
Noguchi, T., et al. (2017). "Temporally Distinct PD-L1 Expression by Tumor and Host Cells Contributes to Immune Escape" Cancer Immunol Res 5(2): 106-117. PubMed
Antibody blockade of programmed death-1 (PD-1) or its ligand, PD-L1, has led to unprecedented therapeutic responses in certain tumor-bearing individuals, but PD-L1 expression’s prognostic value in stratifying cancer patients for such treatment remains unclear. Reports conflict on the significance of correlations between PD-L1 on tumor cells and positive clinical outcomes to PD-1/PD-L1 blockade. We investigated this issue using genomically related, clonal subsets from the same methylcholanthrene-induced sarcoma: a highly immunogenic subset that is spontaneously eliminated in vivo by adaptive immunity and a less immunogenic subset that forms tumors in immunocompetent mice, but is sensitive to PD-1/PD-L1 blockade therapy. Using CRISPR/Cas9-induced loss-of-function approaches and overexpression gain-of-function techniques, we confirmed that PD-L1 on tumor cells is key to promoting tumor escape. In addition, the capacity of PD-L1 to suppress antitumor responses was inversely proportional to tumor cell antigenicity. PD-L1 expression on host cells, particularly tumor-associated macrophages (TAM), was also important for tumor immune escape. We demonstrated that induction of PD-L1 on tumor cells was IFNgamma-dependent and transient, but PD-L1 induction on TAMs was of greater magnitude, only partially IFNgamma dependent, and was stable over time. Thus, PD-L1 expression on either tumor cells or host immune cells could lead to tumor escape from immune control, indicating that total PD-L1 expression in the immediate tumor microenvironment may represent a more accurate biomarker for predicting response to PD-1/PD-L1 blockade therapy, compared with monitoring PD-L1 expression on tumor cells alone. Cancer Immunol Res; 5(2); 106-17. (c)2017 AACR.
in vivo IFNγ neutralization
Wu, L. L., et al. (2014). "Commensal bacterial endocytosis in epithelial cells is dependent on myosin light chain kinase-activated brush border fanning by interferon-gamma" Am J Pathol 184(8): 2260-2274. PubMed
Abnormal bacterial adherence and internalization in enterocytes have been documented in Crohn disease, celiac disease, surgical stress, and intestinal obstruction and are associated with low-level interferon (IFN)-gamma production. How commensals gain access to epithelial soma through densely packed microvilli rooted on the terminal web (TW) remains unclear. We investigated molecular and ultrastructural mechanisms of bacterial endocytosis, focusing on regulatory roles of IFN-gamma and myosin light chain kinase (MLCK) in TW myosin phosphorylation and brush border fanning. Mouse intestines were sham operated on or obstructed for 6 hours by loop ligation with intraluminally administered ML-7 (a MLCK inhibitor) or Y27632 (a Rho-associated kinase inhibitor). After intestinal obstruction, epithelial endocytosis and extraintestinal translocation of bacteria were observed in the absence of tight junctional damage. Enhanced TW myosin light chain phosphorylation, arc formation, and brush border fanning coincided with intermicrovillous bacterial penetration, which were inhibited by ML-7 and neutralizing anti-IFN-gamma but not Y27632. The phenomena were not seen in mice genetically deficient for long MLCK-210 or IFN-gamma. Stimulation of human Caco-2BBe cells with IFN-gamma caused MLCK-dependent TW arc formation and brush border fanning, which preceded caveolin-mediated bacterial internalization through cholesterol-rich lipid rafts. In conclusion, epithelial MLCK-activated brush border fanning by IFN-gamma promotes adherence and internalization of normally noninvasive enteric bacteria. Transcytotic commensal penetration may contribute to initiation or relapse of chronic inflammation.
in vivo IFNγ neutralization, in vitro IFNγ neutralization
Steed, A. L., et al. (2006). "Gamma interferon blocks gammaherpesvirus reactivation from latency" J Virol 80(1): 192-200. PubMed
Establishment of latent infection and reactivation from latency are critical aspects of herpesvirus infection and pathogenesis. Interfering with either of these steps in the herpesvirus life cycle may offer a novel strategy for controlling herpesvirus infection and associated disease pathogenesis. Prior studies show that mice deficient in gamma interferon (IFN-gamma) or the IFN-gamma receptor have elevated numbers of cells reactivating from murine gammaherpesvirus 68 (gammaHV68) latency, produce infectious virus after the establishment of latency, and develop large-vessel vasculitis. Here, we demonstrate that IFN-gamma is a powerful inhibitor of reactivation of gammaHV68 from latency in tissue culture. In vivo, IFN-gamma controls viral gene expression during latency. Importantly, depletion of IFN-gamma in latently infected mice results in an increased frequency of cells reactivating virus. This demonstrates that IFN-gamma is important for immune surveillance that limits reactivation of gammaHV68 from latency.
in vivo IFNγ neutralization
Leiby, D. A., et al. (1992). "In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies" Infect Immun 60(1): 84-89. PubMed
The role(s) of gamma interferon (IFN-gamma), tumor necrosis factor alpha (TNF-alpha), and interleukin-4 (IL-4) in establishment and maintenance of protective immunity to Francisella tularensis LVS in mice (C3H/HeN) was examined by selective removal of these cytokines in vivo with neutralizing antibodies. The 50% lethal dose (LD50) for mice infected intradermally with F. tularensis alone was 136,000 CFU; treatment of mice with anti-IFN-gamma or anti-TNF-alpha at the time of infection significantly reduced (P much less than 0.05) the LD50 to 2 and 5 CFU, respectively. Abrogation of protective immunity, however, was effective only when anti-IFN-gamma or anti-TNF-alpha was administered prior to day 3 postinfection. In contrast, the LD50 for mice treated with anti-IL-4 was repeatedly higher (555,000 CFU) than for controls; this difference, however, was not significant (P greater than 0.05). Thus, IL-4 may be detrimental, while IFN-gamma and TNF-alpha were clearly crucial to the establishment of protective immunity to F. tularensis during a primary infection. The importance of IFN-gamma and TNF-alpha during a secondary immune response to F. tularensis was also investigated. Spleen cells from immune mice passively transfer protective immunity to recipient mice in the absence of confounding antibody-mediated immunity. This passive transfer of immunity, however, was abrogated by treatment of recipient mice with anti-IFN-gamma or anti-TNF-alpha at the time of challenge infection. That anticytokines effectively abrogate protective immunity very early in the course of infection with F. tularensis suggests that T-cell-dependent activation of macrophages for microbicidal activity is unlikely. These T-cell-independent events early in the course of infection may suppress bacterial replication until a T-cell-dependent response ultimately clears the bacteria.
in vitro IFNγ neutralization
Schreiber, R. D., et al. (1985). "Monoclonal antibodies to murine gamma-interferon which differentially modulate macrophage activation and antiviral activity" J Immunol 134(3): 1609-1618. PubMed
Four monoclonal IgG antibodies to purified, recombinant murine gamma-interferon (rIFN-gamma) have been produced by fusion of immune hamster splenocytes with HAT-sensitive murine myeloma cells. Specificity was confirmed either with an enzyme-linked immunosorbent assay (ELISA) that used immobilized rIFN-gamma or with a radioimmunoassay that employed soluble 125I-rIFN-gamma and heat-killed, fixed Staphylococcus aureus-bearing Protein A. Competition binding experiments suggested that the monoclonal antibodies (MoAb) displayed two distinct epitope specificities: one displayed by H1 and H2, and the other displayed by H21 and H22. By using murine-human recombinant IFN-gamma hybrid molecules, the H1/H2 epitope was shown to depend on the amino-terminus of IFN-gamma, whereas the H21/H22 epitope was formed by the carboxy-terminal amino acid sequence. The MoAb also reacted with natural IFN-gamma. When bound to a surface, all four MoAb, but not normal hamster IgG, removed 100% of the antiviral and MAF activities present in supernatants of cultures of the murine 24/G1 T cell hybridoma. In free solution, all four antibodies inhibited IFN-gamma dependent antiviral activity, but with different efficiencies. Soluble H21/H22 also blocked all of the 24/G1-derived activity that induces nonspecific tumoricidal activity in macrophages (MAF) while H1/H2 enhanced MAF activity. The differential inhibitory or enhancing activities of H21 or H1 reflected their ability to inhibit or enhance binding of 125I-rIFN-gamma to macrophages, respectively. Soluble H21/H22 and solid-phase H1/H2 inhibited 100% of the MAF, microbicidal, and Ia-inducing activities from lymphokine preparations produced by mitogen stimulation of normal murine splenic cells. These results help to establish definitive structure-function relationships for the IFN-gamma molecule, and indicate that IFN-gamma is the primary lymphokine responsible for inducing nonspecific tumoricidal activity and Ia antigen expression, and for enhancing microbicidal activity in macrophages.
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
IFNγ is a central node of cancer immune equilibrium.
In Cell Reports on 28 March 2023 by Walsh, M. J., Stump, C. T., et al.
PubMed
Tumors in immune equilibrium are held in balance between outgrowth and destruction by the immune system. The equilibrium phase defines the duration of clinical remission and stable disease, and escape from equilibrium remains a major clinical problem. Using a non-replicating HSV-1 vector expressing interleukin-12 (d106S-IL12), we developed a mouse model of therapy-induced immune equilibrium, a phenomenon previously seen only in humans. This immune equilibrium was centrally reliant on interferon-γ (IFNγ). CD8+ T cell direct recognition of MHC class I, perforin/granzyme-mediated cytotoxicity, and extrinsic death receptor signaling such as Fas/FasL were all individually dispensable for equilibrium. IFNγ was critically important and played redundant roles in host and tumor cells such that IFNγ sensing in either compartment was sufficient for immune equilibrium. We propose that these redundant mechanisms of action are integrated by IFNγ to protect from oncogenic or chronic viral threats and establish IFNγ as a central node in therapy-induced immune equilibrium. Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
- Immunology and Microbiology,
- Mus musculus (House mouse)
Galectin-3 expression in donor T cells reduces GvHD severity and lethality after allogeneic hematopoietic cell transplantation.
In Cell Reports on 28 March 2023 by Mohammadpour, H., Tsuji, T., et al.
PubMed
Abundant donor cytotoxic T cells that attack normal host organs remain a major problem for patients receiving allogeneic hematopoietic cell transplantation (allo-HCT). Despite an increase in our knowledge of the pathobiology of acute graft versus host disease (aGvHD), the mechanisms regulating the proliferation and function of donor T cells remain unclear. Here, we show that activated donor T cells express galectin-3 (Gal-3) after allo-HCT. In both major and minor histocompatibility-mismatched models of murine aGvHD, expression of Gal-3 is associated with decreased T cell activation and suppression of the secretion of effector cytokines, including IFN-γ and GM-CSF. Mechanistically, Gal-3 results in activation of NFAT signaling, which can induce T cell exhaustion. Gal-3 overexpression in human T cells prevents severe disease by suppressing cytotoxic T cells in xenogeneic aGvHD models. Together, these data identify the Gal-3-dependent regulatory pathway in donor T cells as a critical component of inflammation in aGvHD. Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
- Cancer Research,
- Immunology and Microbiology,
- Mus musculus (House mouse)
B cells imprint adoptively transferred CD8+ T cells with enhanced tumor immunity.
In Journal for Immunotherapy of Cancer on 1 January 2022 by Smith, A. S., Knochelmann, H. M., et al.
PubMed
Adoptive T cell transfer (ACT) therapy improves outcomes in patients with advanced malignancies, yet many individuals relapse due to the infusion of T cells with poor function or persistence. Toll-like receptor (TLR) agonists can invigorate antitumor T cell responses when administered directly to patients, but these responses often coincide with toxicities. We posited that TLR agonists could be repurposed ex vivo to condition T cells with remarkable potency in vivo, circumventing TLR-related toxicity. In this study we investigated how tumor-specific murine CD8+ T cells and human tumor infiltrating lymphocytes (TILs) are impacted when expanded ex vivo with the TLR9 agonist CpG. Herein we reveal a new way to reverse the tolerant state of adoptively transferred CD8+ T cells against tumors using TLR-activated B cells. We repurposed the TLR9 agonist, CpG, commonly used in the clinic, to bolster T cell-B cell interactions during expansion for ACT. T cells expanded ex vivo from a CpG-treated culture demonstrated potent antitumor efficacy and prolonged persistence in vivo. This antitumor efficacy was accomplished without in vivo administration of TLR agonists or other adjuvants of high-dose interleukin (IL)-2 or vaccination, which are classically required for effective ACT therapy. CpG-conditioned CD8+ T cells acquired a unique proteomic signature hallmarked by an IL-2RαhighICOShighCD39low phenotype and an altered metabolic profile, all reliant on B cells transiently present in the culture. Likewise, human TILs benefitted from expansion with CpG ex vivo, as they also possessed the IL-2RαhighICOShighCD39low phenotype. CpG fostered the expansion of potent CD8+ T cells with the signature phenotype and antitumor ability via empowering a direct B-T cell interaction. Isolated B cells also imparted T cells with the CpG-associated phenotype and improved tumor immunity without the aid of additional antigen-presenting cells or other immune cells in the culture. Our results demonstrate a novel way to use TLR agonists to improve immunotherapy and reveal a vital role for B cells in the generation of potent CD8+ T cell-based therapies. Our findings have immediate implications in the clinical treatment of advanced solid tumors. © Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
- Cancer Research,
- Immunology and Microbiology,
- Mus musculus (House mouse)
HOIP limits anti-tumor immunity by protecting against combined TNF and IFN-gamma-induced apoptosis.
In EMBO Reports on 4 November 2021 by Freeman, A. J., Vervoort, S. J., et al.
PubMed
The success of cancer immunotherapy is limited to a subset of patients, highlighting the need to identify the processes by which tumors evade immunity. Using CRISPR/Cas9 screening, we reveal that melanoma cells lacking HOIP, the catalytic subunit of LUBAC, are highly susceptible to both NK and CD8+ T-cell-mediated killing. We demonstrate that HOIP-deficient tumor cells exhibit increased sensitivity to the combined effect of the inflammatory cytokines, TNF and IFN-γ, released by NK and CD8+ T cells upon target recognition. Both genetic deletion and pharmacological inhibition of HOIP augment tumor cell sensitivity to combined TNF and IFN-γ. Together, we unveil a protective regulatory axis, involving HOIP, which limits a transcription-dependent form of cell death that engages both intrinsic and extrinsic apoptotic machinery upon exposure to TNF and IFN-γ. Our findings highlight HOIP inhibition as a potential strategy to harness and enhance the killing capacity of TNF and IFN-γ during immunotherapy. © 2021 The Authors.
- Cancer Research,
- Immunology and Microbiology
MAIT cells regulate NK cell-mediated tumor immunity.
In Nature Communications on 6 August 2021 by Petley, E. V., Koay, H. F., et al.
PubMed
The function of MR1-restricted mucosal-associated invariant T (MAIT) cells in tumor immunity is unclear. Here we show that MAIT cell-deficient mice have enhanced NK cell-dependent control of metastatic B16F10 tumor growth relative to control mice. Analyses of this interplay in human tumor samples reveal that high expression of a MAIT cell gene signature negatively impacts the prognostic significance of NK cells. Paradoxically, pre-pulsing tumors with MAIT cell antigens, or activating MAIT cells in vivo, enhances anti-tumor immunity in B16F10 and E0771 mouse tumor models, including in the context of established metastasis. These effects are associated with enhanced NK cell responses and increased expression of both IFN-γ-dependent and inflammatory genes in NK cells. Importantly, activated human MAIT cells also promote the function of NK cells isolated from patient tumor samples. Our results thus describe an activation-dependent, MAIT cell-mediated regulation of NK cells, and suggest a potential therapeutic avenue for cancer treatment. © 2021. Crown.
- Immunology and Microbiology,
- Mus musculus (House mouse)
Inflammation-Induced Lactate Leads to Rapid Loss of Hepatic Tissue-Resident NK Cells.
In Cell Reports on 7 July 2020 by Dodard, G., Tata, A., et al.
PubMed
The liver harbors two main innate lymphoid cell (ILC) populations: conventional NK (cNK) cells and tissue-resident NK (trNK) cells. Using the MCMV model of infection, we find that, in contrast to liver cNK cells, trNK cells initially undergo a contraction phase followed by a recovery phase to homeostatic levels. The contraction is MCMV independent because a similar phenotype is observed following poly(I:C)/CpG or α-GalCer injection. The rapid contraction phase is due to apoptosis, whereas the recovery phase occurs via proliferation in situ. Interestingly, trNK cell apoptosis is not mediated by fratricide and not induced by liver lymphocytes or inflammatory cytokines. Instead, we find that trNK cell apoptosis is the consequence of an increased sensitivity to lactic acid. Mechanistic analysis indicates that trNK cell sensitivity to lactate is linked to impaired mitochondrial function. These findings underscore the distinctive properties of the liver-resident NK cell compartment. Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
- Cell Biology,
- Immunology and Microbiology
Defective early innate immune response to ectromelia virus in the draining lymph nodes of aged mice due to impaired dendritic cell accumulation.
In Aging Cell on 1 July 2020 by Stotesbury, C., Wong, E. B., et al.
PubMed
It is known that aging decreases natural resistance to viral diseases due to dysfunctional innate and adaptive immune responses, but the nature of these dysfunctions, particularly in regard to innate immunity, is not well understood. We have previously shown that C57BL/6J (B6) mice lose their natural resistance to footpad infection with ectromelia virus (ECTV) due to impaired maturation and recruitment of natural killer (NK) cells to the draining popliteal lymph node (dLN). More recently, we have also shown that in young B6 mice infected with ECTV, the recruitment of NK cells is dependent on a complex cascade whereby migratory dendritic cells (mDCs) traffic from the skin to the dLN, where they produce CCL2 and CCL7 to recruit inflammatory monocytes (iMOs). In the dLN, mDCs also upregulate NKG2D ligands to induce interferon gamma (IFN-γ) expression by group 1 innate lymphoid cells (G1-ILCs), mostly NK in cells but also some ILC1. In response to the IFN-γ, the incoming uninfected iMOs secret CXCL9 to recruit the critical NK cells. Here, we show that in aged B6 mice, the trafficking of mDCs to the dLN in response to ECTV is decreased, resulting in impaired IFN-γ expression by G1-ILCs, reduced accumulation of iMOs, and attenuated CXCL9 production by iMOs, which likely contributes to decrease in NK cell recruitment. Together, these data indicate that defects in the mDC response to viral infection during aging result in a reduced innate immune response in the dLN and contribute to increased susceptibility to viral disease in the aged. © 2020 The Authors. Aging Cell published by Anatomical Society and John Wiley Sons Ltd.
- Cardiovascular biology
Host-Derived Serine Protease Inhibitor 6 Provides Granzyme B-Independent Protection of Intestinal Epithelial Cells in Murine Graft-versus-Host Disease.
In Biology of Blood and Marrow Transplantation : Journal of the American Society for Blood and Marrow Transplantation on 1 December 2018 by Mohammadpour, H., Du, W., et al.
PubMed
Graft-versus-host disease (GVHD) is a serious complication after allogeneic hematopoietic cell transplantation (allo-HCT) that limits the therapeutic potential of this treatment. Host antigen-presenting cells (APCs) play a vital role in activating donor T cells that subsequently use granzyme B (GzmB) and other cytotoxic molecules to damage host normal tissues. Serine protease inhibitor 6 (Spi6), known as the sole endogenous inhibitor of GzmB, has been implicated in protecting T cells and APCs against GzmB-inflicted damage. In this study we used murine models to examine the previously unknown role of host-derived Spi6 in GVHD pathogenesis. Our results indicated that host Spi6 deficiency exacerbated GVHD as evidenced by significantly increased lethality and clinical and histopathologic scores. Using bone marrow chimera system, we found that Spi6 in nonhematopoietic tissue played a dominant role in protecting against GVHD and was significantly upregulated in intestinal epithelial cells after allo-HCT, whereas Spi6 in hematopoietic APCs surprisingly suppressed alloreactive T cell response. Interestingly, the protective effect of Spi6 and its expression in intestinal epithelial cells appeared to be independent of donor-derived GzmB. We used in silico modeling to explore potential targets of Spi6. Interaction tested in silico demonstrated that Spi6 could inhibit caspase-3 and caspase-8 with the same functional loop that inhibits GzmB but was not capable of forming stable interaction with caspase-1 or granzyme A. Using an in vitro co-culture system, we further identified that donor T cell-derived IFN-γ was important for inducing Spi6 expression in an intestinal epithelial cell line. Altogether, our data indicate that host Spi6 plays a novel, GzmB-independent role in regulating alloreactive T cell response and protecting intestinal epithelial cells. Therefore, enhancing host-derived Spi6 function has the potential to reduce GVHD. Copyright © 2018. Published by Elsevier Inc.
- Cancer Research,
- Immunology and Microbiology
Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease.
In Cancer Discovery on 1 December 2016 by Liu, J., Blake, S. J., et al.
PubMed
Immunotherapy has recently entered a renaissance phase with the approval of multiple agents for the treatment of cancer. Immunotherapy stands ready to join traditional modalities, including surgery, chemotherapy, radiation, and hormone therapy, as a pillar of cancer treatment. Although immunotherapy has begun to have success in advanced cancer treatment, its scheduling and efficacy with surgery to treat earlier stages of cancer and prevent distant metastases have not been systematically examined. Here, we have used two models of spontaneously metastatic breast cancers in mice to illustrate the significantly greater therapeutic power of neoadjuvant, compared with adjuvant, immunotherapies in the context of primary tumor resection. Elevated and sustained peripheral tumor-specific immune responses underpinned the outcome, and blood sampling of tumor-specific CD8+ T cells immediately prior to and post surgery may provide a predictor of outcome. These data now provide a strong rationale to extensively test and compare neoadjuvant immunotherapy in humans. We demonstrate the significantly greater therapeutic efficacy of neoadjuvant, compared with adjuvant, immunotherapies to eradicate distant metastases following primary tumor resection. Elevated and sustained peripheral tumor-specific immune responses underpinned the outcome, and blood sampling of tumor-specific CD8+ T cells immediately prior to and post surgery may provide a predictor of outcome. Cancer Discov; 6(12); 1382-99. ©2016 AACR.See related commentary by Melero et al., p. 1312This article is highlighted in the In This Issue feature, p. 1293. ©2016 American Association for Cancer Research.