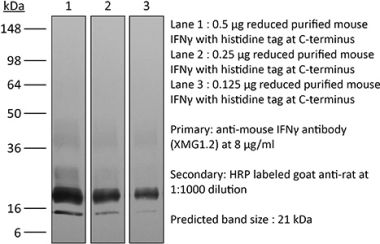
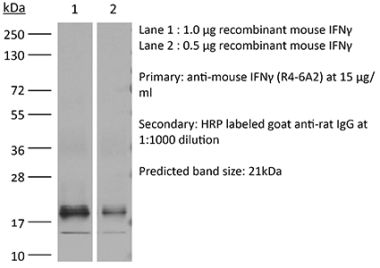
InVivoMAb anti-human IFNγ
Product Details
The B133.5 monoclonal antibody reacts with human IFNγ (interferon gamma) a 20 kDa soluble pleiotropic cytokine and the sole member of the type II class of interferons. IFNγ is primarily produced by activated lymphocytes including T, B, NK cells, and ILCs. IFNγ exerts immunoregulatory, anti-proliferative, anti-viral, and proinflammatory activities and plays an important role in activation, growth, and differentiation of T and B lymphocytes, macrophages, NK cells and other non-hematopoietic cell types. Additionally, IFNγ induces the production of cytokines, Fc receptor, and adhesion molecules and up-regulates MHC class I and II antigen expression by antigen presenting cells during an immune response. IFNγ has also been shown to modulate macrophage effector functions, influence isotype switching and induce the secretion of immunoglobulins by B cells. IFNγ signals through the IFN gamma receptor which exists as a heterodimer composed of CD119 (IFN gamma receptor 1) and AF-1 (IFN gamma receptor 2). The IFNγ receptor is expressed ubiquitously on almost all cell types with the exception of mature erythrocytes. The B133.5 antibody is a neutralizing antibody.Specifications
| Isotype | Mouse IgG1 |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Recombinant human IFNγ |
| Reported Applications |
in vivo IFNγ neutralization in humanized mice in vitro IFNγ neutralization |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
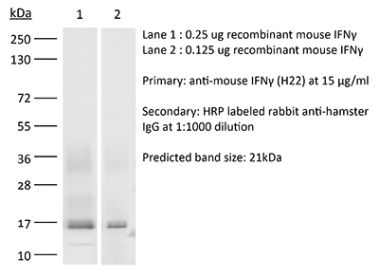
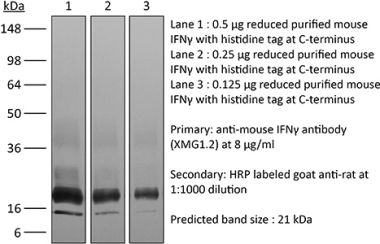
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_2687717 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
in vivo IFNγ neutralization in humanized mice
Cremasco, F., et al. (2021). "Cross-linking of T cell to B cell lymphoma by the T cell bispecific antibody CD20-TCB induces IFNγ/CXCL10-dependent peripheral T cell recruitment in humanized murine model" PLoS One 16(1): e0241091. PubMed
Diffuse large B cell lymphomas (DLBCL) are a highly heterogeneous subtype of Non Hodgkin Lymphoma (NHL), accounting for about 25% of NHL. Despite an increased progression-free survival upon therapy, 40-50% of patients develop relapse/refractory disease, therefore there remains an important medical need. T cell recruiting therapies, such as the CD20xCD3 T cell bi-specific antibody CD20-TCB (RG6026 or glofitamab), represent a novel approach to target all stages of DLBCL, especially those that fail to respond to multiple lines of treatment. We aimed for a better understanding of the molecular features related to the mode of action (MoA) of CD20-TCB in inducing Target/T cell synapse formation and human T cell recruitment to the tumor. To directly evaluate the correlation between synapse, cytokine production and anti-tumor efficacy using CD20-TCB, we developed an innovative preclinical human DLBCL in vivo model that allowed tracking in vivo human T cell dynamics by multiphoton intravital microscopy (MP-IVM). By ex vivo and in vivo approaches, we revealed that CD20-TCB is inducing strong and stable synapses between human T cell and tumor cells, which are dependent on the dose of CD20-TCB and on LFA-1 activity but not on FAS-L. Moreover, despite CD20-TCB being a large molecule (194.342 kDa), we observed that intra-tumor CD20-TCB-mediated human T cell-tumor cell synapses occur within 1 hour upon CD20-TCB administration. These tight interactions, observed for at least 72 hours post TCB administration, result in tumor cell cytotoxicity, resident T cell proliferation and peripheral blood T cell recruitment into tumor. By blocking the IFNγ-CXCL10 axis, the recruitment of peripheral T cells was abrogated, partially affecting the efficacy of CD20-TCB treatment which rely only on resident T cell proliferation. Altogether these data reveal that CD20-TCB’s anti-tumor activity relies on a triple effect: i) fast formation of stable T cell-tumor cell synapses which induce tumor cytotoxicity and cytokine production, ii) resident T cell proliferation and iii) recruitment of fresh peripheral T cells to the tumor core to allow a positive enhancement of the anti-tumor effect.
in vitro IFNγ neutralization
Mitson-Salazar, A., et al. (2015). "Hematopoietic prostaglandin D synthase defines a proeosinophilic pathogenic effector human T2 cell subpopulation with enhanced function" J Allergy Clin Immunol. pii : S0091-6749(15)01186-0. PubMed
BACKGROUND: IL-5+ pathogenic effector TH2 (peTH2) cells are a TH2 cell subpopulation with enhanced proinflammatory function that has largely been characterized in murine models of allergic inflammation. OBJECTIVE: We sought to identify phenotype markers for human peTH2 cells and characterize their function in patients with allergic eosinophilic inflammatory diseases. METHODS: Patients with eosinophilic gastrointestinal disease (EGID), patients with atopic dermatitis (AD), and nonatopic healthy control (NA) subjects were enrolled. peTH2 and conventional TH2 (cTH2) cell phenotype, function, and cytokine production were analyzed by using flow cytometry. Confirmatory gene expression was measured by using quantitative RT-PCR. Prostaglandin D2 levels were measured with ELISA. Gut TH2 cells were obtained by means of esophagogastroduodenoscopy. RESULTS: peTH2 cells were identified as chemoattractant receptor-homologous molecule expressed on TH2 cells-positive (CRTH2+), hematopoietic prostaglandin D synthase-positive CD161hi CD4 T cells. peTH2 cells expressed significantly greater IL-5 and IL-13 than did hematopoietic prostaglandin D synthase-negative and CD161- cTH2 cells. peTH2 cells were highly correlated with blood eosinophilia (r = 0.78-0.98) and were present in 30- to 40-fold greater numbers in subjects with EGID and those with AD versus NA subjects. Relative to cTH2 cells, peTH2 cells preferentially expressed receptors for thymic stromal lymphopoietin, IL-25, and IL-33 and demonstrated greater responsiveness to these innate pro-TH2 cytokines. peTH2 but not cTH2 cells produced prostaglandin D2. In patients with EGID and those with AD, peTH2 cells expressed gut- and skin-homing receptors, respectively. There were significantly greater numbers of peTH2 cells in gut tissue from patients with EGID versus NA subjects. CONCLUSION: peTH2 cells are the primary functional proinflammatory human TH2 cell subpopulation underlying allergic eosinophilic inflammation. The unambiguous phenotypic identification of human peTH2 cells provides a powerful tool to track these cells in future pathogenesis studies and clinical trials.
- Immunology and Microbiology,
CD8 T cell response and its released cytokine IFN-γ are necessary for lung alveolar epithelial repair during bacterial pneumonia.
In Frontiers in Immunology on 13 November 2023 by Zhang, X., Ali, M., et al.
PubMed
Alveolar epithelial regeneration depends on the activity of resident quiescent progenitor cells. Alveolar epithelial type II (AT2) cells are known as the alveolar epithelial progenitor cells. They exit quiescent state, proliferate rapidly in response to injury and differentiate into alveolar epithelial type I (AT1) cells to regenerate the damaged alveolar epithelium. Although AT2 cell plasticity has been a very intense field of research, the role of CD8 T cell response and their released cytokine IFN-γ, in regulating AT2 cell plasticity and alveolar epithelial repair and regeneration after injury remains largely unknown. We used flow cytometry to quantify the amount of CD8 T cells in mouse lungs after bacterial pneumonia caused by Streptococcus pneumoniae. To determine whether CD8 T cells and their released cytokine IFN-γ are necessary for AT2 cell activity during alveolar epithelial regeneration, we performed loss of function studies using anti-CD8 or anti-IFN-γ monoclonal antibody (mAb) treatment in vivo. We assessed the effects of CD8 T cells and cytokine IFN-γ on AT2 cell differentiation capacity using the AT2- CD8 T cell co-culture system in vitro. We detected a transient wave of accumulation of CD8 T cells in mouse lungs, which coincided with the burst of AT2 cell proliferation during alveolar epithelial repair and regeneration in mice following bacterial pneumonia caused by Streptococcus pneumoniae. Depletion of CD8 T cells or neutralization of cytokine IFN-γ using anti-CD8 or anti-IFN-γ monoclonal antibody significantly reduced AT2 cell proliferation and differentiation into AT1 cells in mice after bacterial pneumonia. Furthermore, co-culture of CD8 T cells or cytokine IFN-γ with AT2 cells promoted AT2-to-AT1 cell differentiation in both murine and human systems. Conversely, blockade of IFN-γ signaling abrogated the increase in AT2-to-AT1 cell differentiation in the AT2- CD8 T cell co-culture system. Our data demonstrate that CD8 T-cell response and cytokine IFN-γ are necessary for promoting AT2 cell activity during alveolar epithelial repair and regeneration after acute lung injury caused by bacterial pneumonia. Copyright © 2023 Zhang, Ali, Pantuck, Yang, Lin, Bahmed, Kosmider and Tian.
- Biochemistry and Molecular biology,
- Cancer Research,
- Cell Biology,
- Stem Cells and Developmental Biology
Metabolic Reprogramming via ACOD1 depletion enhances function of human induced pluripotent stem cell-derived CAR-macrophages in solid tumors.
In Nature Communications on 18 September 2023 by Wang, X., Su, S., et al.
PubMed
The pro-inflammatory state of macrophages, underpinned by their metabolic condition, is essentially affecting their capacity of combating tumor cells. Here we find, via a pooled metabolic gene knockout CRISPR screen that KEAP1 and ACOD1 are strong regulators of the pro-inflammatory state in macrophages. We show that ACOD1 knockout macrophages, generated in our induced pluripotent stem cell-derived CAR-macrophage (CAR-iMAC) platform, are strongly and persistently polarized toward the pro-inflammatory state, which manifests in increased reactive oxygen species (ROS) production, more potent phagocytosis and enhanced cytotoxic functions against cancer cells in vitro. In ovarian or pancreatic cancer mouse models, ACOD1-depleted CAR-iMACs exhibit enhanced capacity in repressing tumors, leading to increased survival. In addition, combining ACOD1-depleted CAR-iMACs with immune checkpoint inhibitors (ICI), such as anti-CD47 or anti-PD1 antibodies, result in even stronger tumor suppressing effect. Mechanistically, the depletion of ACOD1 reduces levels of the immuno-metabolite itaconate, allowing KEAP1 to prevent NRF2 from entering the nucleus to activate an anti-inflammatory program. This study thus lays down the proof of principle for targeting ACOD1 in myeloid cells for cancer immunotherapy and introduces metabolically engineered human iPSC-derived CAR-iMACs cells with enhanced polarization and anti-tumor functions in adoptive cell transfer therapies. © 2023. Springer Nature Limited.
- COVID-19
An immunostimulatory glycolipid that blocks SARS-CoV-2, RSV, and influenza infections in vivo.
In Nature Communications on 5 July 2023 by Tsuji, M., Nair, M. S., et al.
PubMed
Prophylactic vaccines for SARS-CoV-2 have lowered the incidence of severe COVID-19, but emergence of viral variants that are antigenically distinct from the vaccine strains are of concern and additional, broadly acting preventive approaches are desirable. Here, we report on a glycolipid termed 7DW8-5 that exploits the host innate immune system to enable rapid control of viral infections in vivo. This glycolipid binds to CD1d on antigen-presenting cells and thereby stimulates NKT cells to release a cascade of cytokines and chemokines. The intranasal administration of 7DW8-5 prior to virus exposure significantly blocked infection by three different authentic variants of SARS-CoV-2, as well as by respiratory syncytial virus and influenza virus, in mice or hamsters. We also found that this protective antiviral effect is both host-directed and mechanism-specific, requiring both the CD1d molecule and interferon-[Formula: see text]. A chemical compound like 7DW8-5 that is easy to administer and cheap to manufacture may be useful not only in slowing the spread of COVID-19 but also in responding to future pandemics long before vaccines or drugs are developed. © 2023. The Author(s).
- In Vitro,
- Mus musculus (House mouse),
- Stem Cells and Developmental Biology
Dysregulated lung stroma drives emphysema exacerbation by potentiating resident lymphocytes to suppress an epithelial stem cell reservoir.
In Immunity on 14 March 2023 by Wang, C., Hyams, B., et al.
PubMed
Aberrant tissue-immune interactions are the hallmark of diverse chronic lung diseases. Here, we sought to define these interactions in emphysema, a progressive disease characterized by infectious exacerbations and loss of alveolar epithelium. Single-cell analysis of human emphysema lungs revealed the expansion of tissue-resident lymphocytes (TRLs). Murine studies identified a stromal niche for TRLs that expresses Hhip, a disease-variant gene downregulated in emphysema. Stromal-specific deletion of Hhip induced the topographic expansion of TRLs in the lung that was mediated by a hyperactive hedgehog-IL-7 axis. 3D immune-stem cell organoids and animal models of viral exacerbations demonstrated that expanded TRLs suppressed alveolar stem cell growth through interferon gamma (IFNγ). Finally, we uncovered an IFNγ-sensitive subset of human alveolar stem cells that was preferentially lost in emphysema. Thus, we delineate a stromal-lymphocyte-epithelial stem cell axis in the lung that is modified by a disease-variant gene and confers host susceptibility to emphysema. Published by Elsevier Inc.
- Cancer Research,
- Immunology and Microbiology
Induction of tumor cell autosis by myxoma virus-infected CAR-T and TCR-T cells to overcome primary and acquired resistance.
In Cancer Cell on 12 September 2022 by Zheng, N., Fang, J., et al.
PubMed
Cytotoxicity of tumor-specific T cells requires tumor cell-to-T cell contact-dependent induction of classic tumor cell apoptosis and pyroptosis. However, this may not trigger sufficient primary responses of solid tumors to adoptive cell therapy or prevent tumor antigen escape-mediated acquired resistance. Here we test myxoma virus (MYXV)-infected tumor-specific T (TMYXV) cells expressing chimeric antigen receptor (CAR) or T cell receptor (TCR), which systemically deliver MYXV into solid tumors to overcome primary resistance. In addition to T cell-induced apoptosis and pyroptosis, tumor eradication by CAR/TCR-TMYXV cells is also attributed to tumor cell autosis induction, a special type of cell death. Mechanistically, T cell-derived interferon γ (IFNγ)-protein kinase B (AKT) signaling synergizes with MYXV-induced M-T5-SKP-1-VPS34 signaling to trigger robust tumor cell autosis. CAR/TCR-TMYXV-elicited autosis functions as a type of potent bystander killing to restrain antigen escape. We uncover an unexpected synergy between T cells and MYXV to bolster solid tumor cell autosis that reinforces tumor clearance. Copyright © 2022 Elsevier Inc. All rights reserved.
- In Vitro,
- Homo sapiens (Human),
- Endocrinology and Physiology,
- Immunology and Microbiology
PD-1 mediates decidual γδ T cells cytotoxicity during recurrent pregnancy loss.
In American Journal of Reproductive Immunology (New York, N.Y. : 1989) on 1 September 2022 by Guo, R., Jiang, S., et al.
PubMed
Recurrent pregnancy loss (RPL) is one of the big challenges of normal pregnancy. Immune dysregulation has been proposed for the key underline mechanisms of RPL. However, the essential roles of T cells, especially γδ T cells, have not been defined. Decidua were obtained from normal pregnancy women or recurrent pregnancy loss patients and the surface molecules of γδ T cells in decidua were evaluated via flow cytometric analysis. The expression of PD-1 in clinical samples was analyzed by immunohistochemistry assay. The intracellular cytokines of decidual PD-1+ and PD-1- γδ T cells were evaluated by flow cytometric analysis. The cytotoxicity of PD-1- γδ T cells were confirmed via an in vitro co-culture experiment. The specific inhibitors for Erk, p38 and JNK against the MAPK pathway were added to the co-culture media to evaluate the functions of the Erk, p38 and JNK. We demonstrated that PD-1 was significantly decreased on decidual tissue γδ T cells of patients with RPL, resulting in the enhanced cytotoxicity of γδ T cells against trophoblasts. We further elucidated an Erk-dependent TNF-α production mediates the γδ T cell cytotoxicity against the trophoblast cells. Finally, the reduced expression of PD-L1 in the villi tissues of patients with RPL might be the cause of the reduction of PD-1 on the tissue γδ T cells. Our study uncovers an important role of PD-1 expression on decidual γδ T cells in maintaining the normal pregnancy, and may provide a new strategy for immune therapy against RPL. © 2022 John Wiley Sons A/S. Published by John Wiley Sons Ltd.
- FC/FACS,
- Homo sapiens (Human),
- Immunology and Microbiology,
- Neuroscience
Contact-Dependent Granzyme B-Mediated Cytotoxicity of Th17-Polarized Cells Toward Human Oligodendrocytes.
In Frontiers in Immunology on 29 April 2022 by Jamann, H., Cui, Q. L., et al.
PubMed
Multiple sclerosis (MS) is characterized by the loss of myelin and of myelin-producing oligodendrocytes (OLs) in the central nervous system (CNS). Pro-inflammatory CD4+ Th17 cells are considered pathogenic in MS and are harmful to OLs. We investigated the mechanisms driving human CD4+ T cell-mediated OL cell death. Using fluorescent and brightfield in vitro live imaging, we found that compared to Th2-polarized cells, Th17-polarized cells show greater interactions with primary human OLs and human oligodendrocytic cell line MO3.13, displaying longer duration of contact, lower mean speed, and higher rate of vesicle-like structure formation at the sites of contact. Using single-cell RNA sequencing, we assessed the transcriptomic profile of primary human OLs and Th17-polarized cells in direct contact or separated by an insert. We showed that upon close interaction, OLs upregulate the expression of mRNA coding for chemokines and antioxidant/anti-apoptotic molecules, while Th17-polarized cells upregulate the expression of mRNA coding for chemokines and pro-inflammatory cytokines such as IL-17A, IFN-γ, and granzyme B. We found that secretion of CCL3, CXCL10, IFN-γ, TNFα, and granzyme B is induced upon direct contact in cocultures of human Th17-polarized cells with human OLs. In addition, we validated by flow cytometry and immunofluorescence that granzyme B levels are upregulated in Th17-polarized compared to Th2-polarized cells and are even higher in Th17-polarized cells upon direct contact with OLs or MO3.13 cells compared to Th17-polarized cells separated from OLs by an insert. Moreover, granzyme B is detected in OLs and MO3.13 cells following direct contact with Th17-polarized cells, suggesting the release of granzyme B from Th17-polarized cells into OLs/MO3.13 cells. To confirm granzyme B-mediated cytotoxicity toward OLs, we showed that recombinant human granzyme B can induce OLs and MO3.13 cell death. Furthermore, pretreatment of Th17-polarized cells with a reversible granzyme B blocker (Ac-IEPD-CHO) or a natural granzyme B blocker (serpina3N) improved survival of MO3.13 cells upon coculture with Th17 cells. In conclusion, we showed that human Th17-polarized cells form biologically significant contacts with human OLs and exert direct toxicity by releasing granzyme B. Copyright © 2022 Jamann, Cui, Desu, Pernin, Tastet, Halaweh, Farzam-kia, Mamane, Ouédraogo, Cleret-Buhot, Daigneault, Balthazard, Klement, Lemaître, Arbour, Antel, Stratton and Larochelle.
- FC/FACS
IL-9/STAT3/fatty acid oxidation-mediated lipid peroxidation contributes to Tc9 cell longevity and enhanced antitumor activity.
In The Journal of Clinical Investigation on 1 April 2022 by Xiao, L., Ma, X., et al.
PubMed
CD8+ T cell longevity regulated by metabolic activity plays important roles in cancer immunotherapy. Although in vitro-polarized, transferred IL-9-secreting CD8+ Tc9 (cytotoxic T lymphocyte subset 9) cells exert greater persistence and antitumor efficacy than Tc1 cells, the underlying mechanism remains unclear. Here, we show that tumor-infiltrating Tc9 cells display significantly lower lipid peroxidation than Tc1 cells in several mouse models, which is strongly correlated with their persistence. Using RNA-sequence and functional validation, we found that Tc9 cells exhibited unique lipid metabolic programs. Tc9 cell-derived IL-9 activated STAT3, upregulated fatty acid oxidation and mitochondrial activity, and rendered Tc9 cells with reduced lipid peroxidation and resistance to tumor- or ROS-induced ferroptosis in the tumor microenvironment. IL-9 signaling deficiency, inhibiting STAT3, or fatty acid oxidation increased lipid peroxidation and ferroptosis of Tc9 cells, resulting in impaired longevity and antitumor ability. Similarly, human Tc9 cells also exhibited lower lipid peroxidation than Tc1 cells and tumor-infiltrating CD8+ T cells expressed lower IL9 and higher lipid peroxidation- and ferroptosis-related genes than circulating CD8+ T cells in patients with melanoma. This study indicates that lipid peroxidation regulates Tc9 cell longevity and antitumor effects via the IL-9/STAT3/fatty acid oxidation pathway and regulating T cell lipid peroxidation can be used to enhance T cell-based immunotherapy in human cancer.
- In Vivo,
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
Cross-linking of T cell to B cell lymphoma by the T cell bispecific antibody CD20-TCB induces IFNγ/CXCL10-dependent peripheral T cell recruitment in humanized murine model.
In PLoS ONE on 7 January 2021 by Cremasco, F., Menietti, E., et al.
PubMed
Diffuse large B cell lymphomas (DLBCL) are a highly heterogeneous subtype of Non Hodgkin Lymphoma (NHL), accounting for about 25% of NHL. Despite an increased progression-free survival upon therapy, 40-50% of patients develop relapse/refractory disease, therefore there remains an important medical need. T cell recruiting therapies, such as the CD20xCD3 T cell bi-specific antibody CD20-TCB (RG6026 or glofitamab), represent a novel approach to target all stages of DLBCL, especially those that fail to respond to multiple lines of treatment. We aimed for a better understanding of the molecular features related to the mode of action (MoA) of CD20-TCB in inducing Target/T cell synapse formation and human T cell recruitment to the tumor. To directly evaluate the correlation between synapse, cytokine production and anti-tumor efficacy using CD20-TCB, we developed an innovative preclinical human DLBCL in vivo model that allowed tracking in vivo human T cell dynamics by multiphoton intravital microscopy (MP-IVM). By ex vivo and in vivo approaches, we revealed that CD20-TCB is inducing strong and stable synapses between human T cell and tumor cells, which are dependent on the dose of CD20-TCB and on LFA-1 activity but not on FAS-L. Moreover, despite CD20-TCB being a large molecule (194.342 kDa), we observed that intra-tumor CD20-TCB-mediated human T cell-tumor cell synapses occur within 1 hour upon CD20-TCB administration. These tight interactions, observed for at least 72 hours post TCB administration, result in tumor cell cytotoxicity, resident T cell proliferation and peripheral blood T cell recruitment into tumor. By blocking the IFNγ-CXCL10 axis, the recruitment of peripheral T cells was abrogated, partially affecting the efficacy of CD20-TCB treatment which rely only on resident T cell proliferation. Altogether these data reveal that CD20-TCB's anti-tumor activity relies on a triple effect: i) fast formation of stable T cell-tumor cell synapses which induce tumor cytotoxicity and cytokine production, ii) resident T cell proliferation and iii) recruitment of fresh peripheral T cells to the tumor core to allow a positive enhancement of the anti-tumor effect.
- Cancer Research,
- Immunology and Microbiology
Cross-Linking of T cell to B cell lymphoma by the T cell bispecific antibody CD20-TCB induces IFNγ/CXCL10-dependent peripheral T cell recruitment in humanized murine model
Preprint on BioRxiv : the Preprint Server for Biology on 9 October 2020 by Cremasco, F., Menietti, E., et al.
PubMed
Diffuse large B cell lymphomas (DLBCL) are a highly heterogeneous subtype of Non Hodgkin Lymphoma (NHL), accounting for about 25% of NHL [1]. Despite an increased progression-free survival upon therapy, 40-50% of patients develop relapse/refractory disease, therefore there remains an important medical need [2]. T cell recruiting therapies, such as the CD20xCD3 T cell bi-specific antibody CD20-TCB (RG6026 or glofitamab), represent a novel approach to target all stages of DLBCL, especially those that fail to respond to multiple lines of treatment [3, 4]. We aimed for a better understanding of the molecular features related to the mode of action (MoA) of CD20-TCB in inducing Target/T cell synapse formation and human T cell recruitment to the tumor. To directly evaluate the correlation between synapse, cytokine production and anti-tumor efficacy using CD20-TCB, we developed an innovative preclinical human DLBCL in vivo model that allowed tracking in vivo human T cell dynamics by multiphoton intravital microscopy (MP-IVM). By ex vivo and in vivo approaches, we revealed that CD20-TCB is inducing strong and stable synapses between human T cell and tumor cells, which are dependent on the dose of CD20-TCB and on LFA-1 activity but not on FAS-L. Moreover, despite CD20-TCB being a large molecule (194.342 kDa), we observed that intra-tumor CD20-TCB - mediated human T cell-tumor cell synapses occur within 1 hour upon CD20-TCB administration. These tight interactions, observed for at least 72 hours post TCB administration, result in tumor cell cytotoxicity, resident T cell proliferation and peripheral blood T cell recruitment into tumor. By blocking the IFNγ-CXCL10 axis, the recruitment of peripheral T cells was abrogated, partially affecting the efficacy of CD20-TCB treatment which rely only on resident T cell proliferation. Altogether these data reveal that CD20-TCB’s anti-tumor activity relies on a triple effect: i) fast formation of stable T cell-tumor cell synapses which induce tumor cytotoxicity and cytokine production, ii) resident T cell proliferation and iii) recruitment of fresh peripheral T cells to the tumor core to allow a positive enhancement of the anti-tumor effect.
- Neutralization,
- Homo sapiens (Human),
- Biochemistry and Molecular biology,
- Cancer Research,
- Cell Biology,
- Immunology and Microbiology
TLR8-Mediated Metabolic Control of Human Treg Function: A Mechanistic Target for Cancer Immunotherapy.
In Cell Metabolism on 8 January 2019 by Li, L., Liu, X., et al.
PubMed
Regulatory T (Treg) cells induce an immunosuppressive microenvironment that is a major obstacle for successful tumor immunotherapy. Dissecting the regulatory mechanisms between energy metabolism and functionality in Treg cells will provide insight toward developing novel immunotherapies against cancer. Here we report that human naturally occurring and tumor-associated Treg cells exhibit distinct metabolic profiles with selectivity for glucose metabolism compared with effector T cells. Treg-mediated accelerated glucose consumption induces cellular senescence and suppression of responder T cells through cross-talk. TLR8 signaling selectively inhibits glucose uptake and glycolysis in human Treg cells, resulting in reversal of Treg suppression. Importantly, TLR8 signaling-mediated reprogramming of glucose metabolism and function in human Treg cells can enhance anti-tumor immunity in vivo in a melanoma adoptive transfer T cell therapy model. Our studies identify mechanistic links between innate signaling and metabolic regulation of human Treg suppression, which may be used as a strategy to advance tumor immunotherapy.Copyright © 2018 Elsevier Inc. All rights reserved.