InVivoMAb anti-mouse IL-4
Product Details
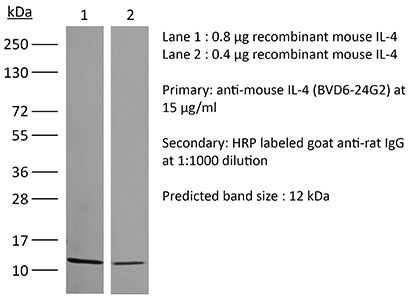
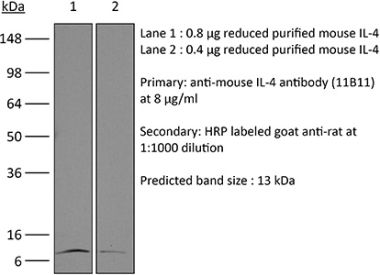
The BVD6-24G2 monoclonal antibody reacts with mouse IL-4 (interleukin-4) a multifunctional 14 kDa cytokine. IL-4 is expressed primarily by activated Th2 cells and NK cells, and at lower levels by mast cells, and basophils. IL-4 signals through the IL-4Rα. Upon receptor binding IL-4 stimulates activated B and T lymphocyte proliferation, and the differentiation of B cells into plasma cells. It also induces B cell class switching to IgE, and up-regulates MHC class II production while decreasing the production of Th1 cells, macrophages, IFNγ, and dendritic cell IL-12. Like other Th2 associated cytokines, IL-4 is involved in the airway inflammation observed in the lungs of patients with allergic asthma. The BVD6-24G2 antibody cannot neutralize the bioactivity of natural or recombinant IL-4.Specifications
| Isotype | Rat IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG1 isotype control, anti-horseradish peroxidase |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Recombinant mouse IL-4 |
| Reported Applications |
ELISA ELISPOT Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_10949184 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
Flow Cytometry
Chennupati, V., et al. (2016). "Notch Signaling Regulates the Homeostasis of Tissue-Restricted Innate-like T Cells" J Immunol 197(3): 771-782. PubMed
Although Notch signaling plays important roles in lineage commitment and differentiation of multiple cell types including conventional T cells, nothing is currently known concerning Notch function in innate-like T cells. We have found that the homeostasis of several well-characterized populations of innate-like T cells including invariant NKT cells (iNKT), CD8alphaalphaTCRalphabeta small intestinal intraepithelial lymphocytes, and innate memory phenotype CD8 T cells is controlled by Notch. Notch selectively regulates hepatic iNKT cell survival via tissue-restricted control of B cell lymphoma 2 and IL-7Ralpha expression. More generally, Notch regulation of innate-like T cell homeostasis involves both cell-intrinsic and -extrinsic mechanisms and relies upon context-dependent interactions with Notch ligand-expressing fibroblastic stromal cells. Collectively, using conditional ablation of Notch receptors on peripheral T cells or Notch ligands on putative fibroblastic stromal cells, we show that Notch signaling is indispensable for the homeostasis of three tissue-restricted populations of innate-like T cells: hepatic iNKT, CD8alphaalphaTCRalphabeta small intestinal intraepithelial lymphocytes, and innate memory phenotype CD8 T cells, thus supporting a generalized role for Notch in innate T cell homeostasis.
Flow Cytometry
Deligne, C., et al. (2015). "Anti-CD20 therapy induces a memory Th1 response through the IFN-gamma/IL-12 axis and prevents protumor regulatory T-cell expansion in mice" Leukemia 29(4): 947-957. PubMed
The long-lasting clinical response by lymphoma patients to anti-CD20 therapy has been attributed to the induction of an anti-tumor adaptive immunity. We previously demonstrated that a CD4-dependent mechanism is responsible for the long-term protection of CD20(+) tumor-bearing mice by anti-CD20 treatment. Here, we compare tumor immunity in tumor-bearing animals that did or did not receive anti-CD20 treatment. Splenic CD4(+)FoxP3(+) regulatory T cells (Tregs) expanded substantially in untreated mice that exhibited then a reduced survival, whereas Tregs depletion led to long-term survival of the animals, suggesting the establishment of a Treg-dependent immunosuppressive environment after tumor injection. Strikingly, anti-CD20 therapy reversed the initial expansion of Tregs, and was accompanied by a marked increase in the number of Th1 cells, with no detectable change in Th2 and Th17 cell numbers. Interleukin-12 serum level was also increased by the anti-CD20 treatment, and activated myeloid dendritic cells producing interleukin-12 could be detected in lymph nodes of treated animals, while interferon-gamma blockade strongly reduced survival. Also, CD4(+) effector memory T cells were evidenced in surviving animals, and the transfer of CD4(+) T cells induced long-term protection. Thus, anti-CD20 therapy promotes strong anti-tumor adaptive immunity, opposes Treg expansion and inhibits tumor cells from maintaining an immunosuppressive environment.
ELISA
Oeser, K., et al. (2015). "Conditional IL-4/IL-13-deficient mice reveal a critical role of innate immune cells for protective immunity against gastrointestinal helminths" Mucosal Immunol 8(3): 672-682. PubMed
Approximately one-third of the world population is infected with gastrointestinal helminths. Studies in mouse models have demonstrated that the cytokines interleukin (IL)-4 and IL-13 are essential for worm expulsion, but the critical cellular source of these cytokines is poorly defined. Here, we compared the immune response to Nippostrongylus brasiliensis in wild-type, T cell-specific IL-4/IL-13-deficient and general IL-4/IL-13-deficient mice. We show that T cell-derived IL-4/IL-13 promoted T helper 2 (Th2) polarization in a paracrine manner, differentiation of alternatively activated macrophages, and tissue recruitment of innate effector cells. However, innate IL-4/IL-13 played the critical role for induction of goblet cell hyperplasia and secretion of effector molecules like Mucin5ac and RELMbeta in the small intestine. Surprisingly, T cell-specific IL-4/IL-13-deficient and wild-type mice cleared the parasite with comparable efficiency, whereas IL-4/IL-13-deficient mice showed impaired expulsion. These findings demonstrate that IL-4/IL-13 produced by cells of the innate immune system is required and sufficient to initiate effective type 2 immune responses resulting in protective immunity against N. brasiliensis.
ELISA
Tanaka, S., et al. (2014). "CCAAT/enhancer-binding protein alpha negatively regulates IFN-gamma expression in T cells" J Immunol 193(12): 6152-6160. PubMed
Humoral immunity, including Ab switching and somatic hypermutation, is critically regulated by CD4(+) T cells. T follicular helper (Tfh) cells have been recently shown to be a distinct T cell subset important in germinal center reactions. The transcriptional regulation of Tfh cell development and function has not been well understood. In this study, we report that C/EBPalpha, a basic region/leucine zipper transcription factor, is highly expressed in Tfh cells. Cebpa-deficient CD4(+) T cells exhibit enhanced IFN-gamma expression in vitro and in vivo. T cell-specific Cebpa knockout mice, although not defective in Tfh cell generation, produce significantly increased levels of IgG2a/b and IgG3 following immunization with a protein Ag. Moreover, C/EBPalpha binds to the Ifng gene and inhibits T-bet-driven Ifng transcription in a DNA binding-dependent manner. Our study thus demonstrates that C/EBPalpha restricts IFN-gamma expression in T cells to allow proper class switching by B cells.
ELISPOT
Booth, A. J., et al. (2011). "IL-6 promotes cardiac graft rejection mediated by CD4+ cells" J Immunol 187(11): 5764-5771. PubMed
IL-6 mediates numerous immunologic effects relevant to transplant rejection; however, its specific contributions to these processes are not fully understood. To this end, we neutralized IL-6 in settings of acute cardiac allograft rejection associated with either CD8(+) or CD4(+) cell-dominant responses. In a setting of CD8(+) cell-dominant graft rejection, IL-6 neutralization delayed the onset of acute rejection while decreasing graft infiltrate and inverting anti-graft Th1/Th2 priming dominance in recipients. IL-6 neutralization markedly prolonged graft survival in the setting of CD4(+) cell-mediated acute rejection and was associated with decreased graft infiltrate, altered Th1 responses, and reduced serum alloantibody. Furthermore, in CD4(+) cell-dominated rejection, IL-6 neutralization was effective when anti-IL-6 administration was delayed by as many as 6 d posttransplant. Finally, IL-6-deficient graft recipients were protected from CD4(+) cell-dominant responses, suggesting that IL-6 production by graft recipients, rather than grafts, is necessary for this type of rejection. Collectively, these observations define IL-6 as a critical promoter of graft infiltration and a shaper of T cell lineage development in cardiac graft rejection. In light of these findings, the utility of therapeutics targeting IL-6 should be considered for preventing cardiac allograft rejection.
- Mus musculus (House mouse),
- Biochemistry and Molecular biology,
- Cell Biology,
- Immunology and Microbiology
Mitochondrial Integrity Regulated by Lipid Metabolism Is a Cell-Intrinsic Checkpoint for Treg Suppressive Function.
In Cell Metabolism on 4 February 2020 by Field, C. S., Baixauli, F., et al.
PubMed
Regulatory T cells (Tregs) subdue immune responses. Central to Treg activation are changes in lipid metabolism that support their survival and function. Fatty acid binding proteins (FABPs) are a family of lipid chaperones required to facilitate uptake and intracellular lipid trafficking. One family member, FABP5, is expressed in T cells, but its function remains unclear. We show that in Tregs, genetic or pharmacologic inhibition of FABP5 function causes mitochondrial changes underscored by decreased OXPHOS, impaired lipid metabolism, and loss of cristae structure. FABP5 inhibition in Tregs triggers mtDNA release and consequent cGAS-STING-dependent type I IFN signaling, which induces heightened production of the regulatory cytokine IL-10 and promotes Treg suppressive activity. We find evidence of this pathway, along with correlative mitochondrial changes in tumor infiltrating Tregs, which may underlie enhanced immunosuppression in the tumor microenvironment. Together, our data reveal that FABP5 is a gatekeeper of mitochondrial integrity that modulates Treg function. Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
- Mus musculus (House mouse),
- Biochemistry and Molecular biology,
- Immunology and Microbiology
Signal transducer and activator of transcription 5 (STAT5) paralog dose governs T cell effector and regulatory functions.
In eLife on 21 March 2016 by Villarino, A. V., Laurence, A., et al.
PubMed
The transcription factor STAT5 is fundamental to the mammalian immune system. However, the relationship between its two paralogs, STAT5A and STAT5B, and the extent to which they are functionally distinct, remain uncertain. Using mouse models of paralog deficiency, we demonstrate that they are not equivalent for CD4(+) 'helper' T cells, the principal orchestrators of adaptive immunity. Instead, we find that STAT5B is dominant for both effector and regulatory (Treg) responses and, therefore, uniquely necessary for immunological tolerance. Comparative analysis of genomic distribution and transcriptomic output confirm that STAT5B has fargreater impact but, surprisingly, the data point towards asymmetric expression (i.e. paralog dose), rather than distinct functional properties, as the key distinguishing feature. Thus, we propose a quantitative model of STAT5 paralog activity whereby relative abundance imposes functional specificity (or dominance) in the face of widespread structural homology.