InVivoMAb anti-mouse TIM-4
Product Details
The RMT4-53 monoclonal antibody reacts with mouse T cell immunoglobulin and mucin domain 4 (TIM-4), a phosphatidylserine-binding receptor and member of the Ig superfamily. TIM-4 is preferentially expressed on antigen-presenting cells. TIM-4 is thought to enhance the engulfment of apoptotic cells and play a role in regulating T cell proliferation. The RMT4-53 antibody has been shown to block TIM-4 in vitro and in vivo.Specifications
| Isotype | Rat IgG2b, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2b isotype control, anti-keyhole limpet hemocyanin |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse TIM-4-Ig fusion protein |
| Reported Applications |
in vivo TIM-4 blockade in vitro TIM-4 blockade Immunofluorescence |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687695 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
in vivo TIM-4 blockade
Ji, H., et al. (2014). "T-cell immunoglobulin and mucin domain 4 (TIM-4) signaling in innate immune-mediated liver ischemia-reperfusion injury" Hepatology 60(6): 2052-2064. PubMed
Hepatic ischemia-reperfusion injury (IRI), an innate immunity-driven inflammation response, occurs in multiple clinical settings including liver resection, transplantation, trauma, and shock. T-cell immunoglobulin and mucin (TIM)-4, the only TIM protein not expressed on T cells, is found on macrophages and dendritic cells. The regulatory function of macrophage TIM-4 in the engulfment of apoptotic/necrotic bodies in innate immunity-mediated disease states remains unknown. This study focuses on the putative role of TIM-4 signaling in a model of liver warm ischemia (90 minutes) and reperfusion. The ischemia insult triggered TIM-4 expression by stressed hepatocellular phosphatidylserine (PS) presentation, peaking at 6 hours of reperfusion, and coinciding with the maximal hepatocellular damage. TIM-4-deficient or wild-type WT mice treated with antagonistic TIM-4 monoclonal antibody (mAb) were resistant against liver IRI, evidenced by diminished serum alanine aminotransferase (sALT) levels and well-preserved hepatic architecture. Liver hepatoprotection rendered by TIM-4 deficiency was accompanied by diminished macrophage infiltration/chemoattraction, phagocytosis, and activation of Toll-like receptor (TLR)2/4/9-dependent signaling. Correlating with in vivo kinetics, the peak of TIM-4 induction in lipopolysaccharide (LPS)-activated bone marrow derived-macrophages (BMM) was detected in 6-hour cultures. To mimic liver IRI, we employed hydrogen peroxide-necrotic hepatocytes, which readily present PS. Indeed, necrotic hepatocytes were efficiently captured/engulfed by WT (TIM-4+) but not by TIM-4-deficient BMM. Finally, in a newly established model of liver IRI, adoptive transfer of WT but not TIM-4-deficient BMM readily recreated local inflammation response/hepatocellular damage in the CD11b-DTR mouse system. CONCLUSION: These findings document the importance of macrophage-specific TIM-4 activation in the mechanism of hepatic IRI. Macrophage TIM-4 may represent a therapeutic target to minimize innate inflammatory responses in IR-stressed organs.
in vivo TIM-4 blockade, in vitro TIM-4 blockade
Yeung, M. Y., et al. (2013). "Interruption of dendritic cell-mediated TIM-4 signaling induces regulatory T cells and promotes skin allograft survival" J Immunol 191(8): 4447-4455. PubMed
Dendritic cells (DCs) are the central architects of the immune response, inducing inflammatory or tolerogenic immunity, dependent on their activation status. As such, DCs are highly attractive therapeutic targets and may hold the potential to control detrimental immune responses. TIM-4, expressed on APCs, has complex functions in vivo, acting both as a costimulatory molecule and a phosphatidylserine receptor. The effect of TIM-4 costimulation on T cell activation remains unclear. In this study, we demonstrate that Ab blockade of DC-expressed TIM-4 leads to increased induction of induced regulatory T cells (iTregs) from naive CD4(+) T cells, both in vitro and in vivo. iTreg induction occurs through suppression of IL-4/STAT6/Gata3-induced Th2 differentiation. In addition, blockade of TIM-4 on previously activated DCs still leads to increased iTreg induction. iTregs induced under TIM-4 blockade have equivalent potency to control and, upon adoptive transfer, significantly prolong skin allograft survival in vivo. In RAG(-/-) recipients of skin allografts adoptively transferred with CD4(+) T cells, we show that TIM-4 blockade in vivo is associated with a 3-fold prolongation in allograft survival. Furthermore, in this mouse model of skin transplantation, increased induction of allospecific iTregs and a reduction in T effector responses were observed, with decreased Th1 and Th2 responses. This enhanced allograft survival and protolerogenic skewing of the alloresponse is critically dependent on conversion of naive CD4(+) to Tregs in vivo. Collectively, these studies identify blockade of DC-expressed TIM-4 as a novel strategy that holds the capacity to induce regulatory immunity in vivo.
Immunofluorescence
Zhang, Y., et al. (2013). "Targeting TIM-1 on CD4 T cells depresses macrophage activation and overcomes ischemia-reperfusion injury in mouse orthotopic liver transplantation" Am J Transplant 13(1): 56-66. PubMed
Hepatic injury due to cold storage followed by reperfusion remains a major cause of morbidity and mortality after orthotopic liver transplantation (OLT). CD4 T cell TIM-1 signaling costimulates a variety of immune responses in allograft recipients. This study analyzes mechanisms by which TIM-1 affects liver ischemia-reperfusion injury (IRI) in a murine model of prolonged cold storage followed by OLT. Livers from C57BL/6 mice, preserved at 4 degrees C in the UW solution for 20 h, were transplanted to syngeneic recipients. There was an early (1 h) increased accumulation of TIM-1+ activated CD4 T cells in the ischemic OLTs. Disruption of TIM-1 signaling with a blocking mAb (RMT1-10) ameliorated liver damage, evidenced by reduced sALT levels and well-preserved architecture. Unlike in controls, TIM-1 blockade diminished OLT expression of Tbet/IFN-gamma, but amplified IL-4/IL-10/IL-22; abolished neutrophil and macrophage infiltration/activation and inhibited NF-kappaB while enhancing Bcl-2/Bcl-xl. Although adoptive transfer of CD4 T cells triggered liver damage in otherwise IR-resistant RAG(-/-) mice, adjunctive TIM-1 blockade reduced Tbet transcription and abolished macrophage activation, restoring homeostasis in IR-stressed livers. Further, transfer of TIM-1(Hi) CD4+, but not TIM-1(Lo) CD4+ T cells, recreated liver IRI in RAG(-/-) mice. Thus, TIM-1 expressing CD4 T cells are required in the mechanism of innate immune-mediated hepatic IRI in OLTs.
- In Vivo,
- Mus musculus (House mouse),
- Immunology and Microbiology
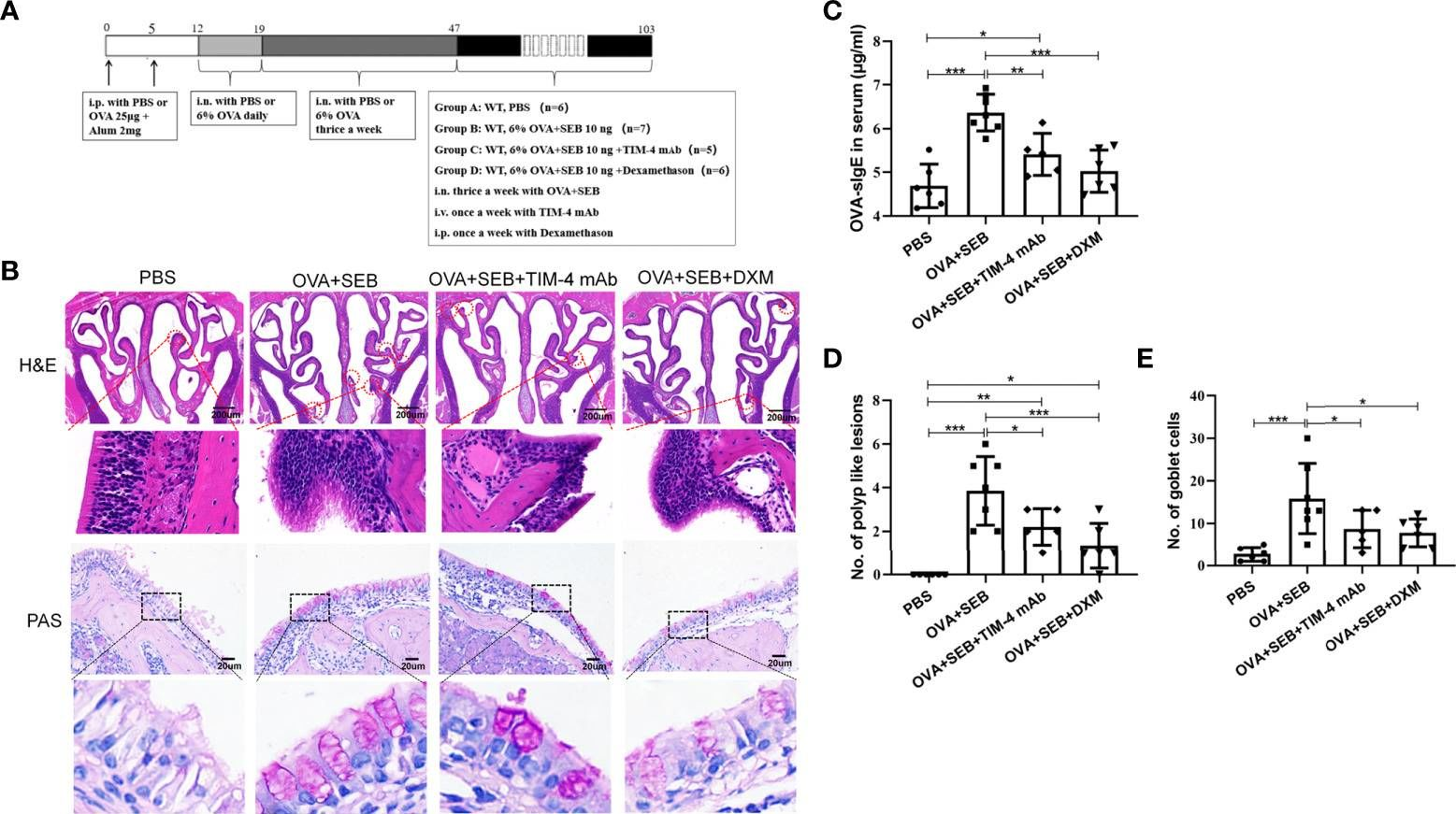
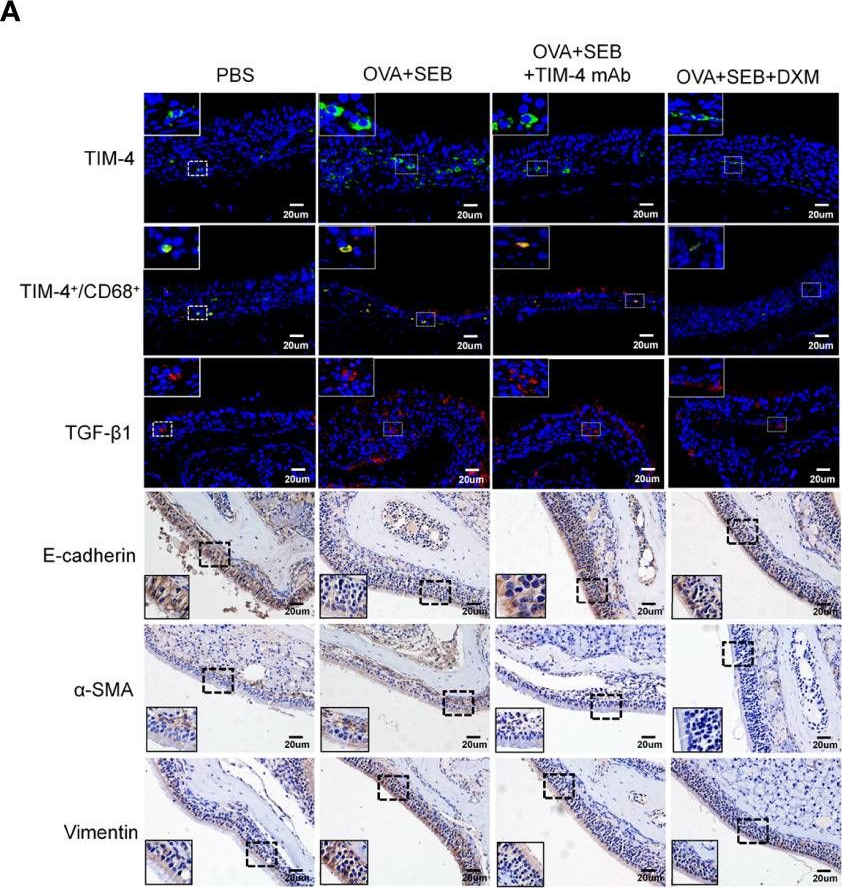
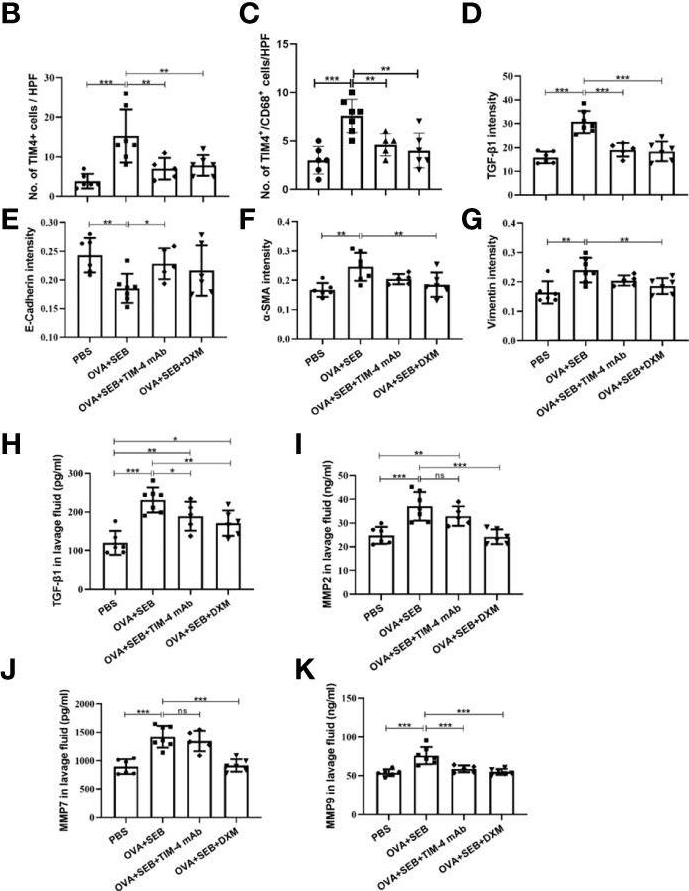
TIM-4 in macrophages contributes to nasal polyp formation through the TGF-β1-mediated epithelial to mesenchymal transition in nasal epithelial cells.
In Frontiers in Immunology on 23 August 2022 by Qin, D., Liu, P., et al.
PubMed
Chronic rhinosinusitis with nasal polyps (CRSwNP) is caused by prolonged inflammation of the paranasal sinus mucosa. The epithelial to mesenchymal transition (EMT) is involved in the occurrence and development of CRSwNP. The T-cell immunoglobulin domain and the mucin domain 4 (TIM-4) is closely related to chronic inflammation, but its mechanism in CRSwNP is poorly understood. In our study, we found that TIM-4 was increased in the sinonasal mucosa of CRSwNP patients and, especially, in macrophages. TIM-4 was positively correlated with α-SMA but negatively correlated with E-cadherin in CRS. Moreover, we confirmed that TIM-4 was positively correlated with the clinical parameters of the Lund-Mackay and Lund-Kennedy scores. In the NP mouse model, administration of TIM-4 neutralizing antibody significantly reduced the polypoid lesions and inhibited the EMT process. TIM-4 activation by stimulating with tissue extracts of CRSwNP led to a significant increase of TGF-β1 expression in macrophages in vitro. Furthermore, coculture of macrophages and human nasal epithelial cells (hNECs) results suggested that the overexpression of TIM-4 in macrophages made a contribution to the EMT process in hNECs. Mechanistically, TIM-4 upregulated TGF-β1 expression in macrophages via the ROS/p38 MAPK/Egr-1 pathway. In conclusion, TIM-4 contributes to the EMT process and aggravates the development of CRSwNP by facilitating the production of TGF-β1 in macrophages. Inhibition of TIM-4 expression suppresses nasal polyp formation, which might provide a new therapeutic approach for CRSwNP. Copyright © 2022 Qin, Liu, Zhou, Jin, Gong, Liu, Chen, Huang, Fan, Tao and Xu.
- In Vivo,
- Mus musculus (House mouse)
Role of Tim4 in the regulation of ABCA1+ adipose tissue macrophages and post-prandial cholesterol levels.
In Nature Communications on 21 July 2021 by MAGALHAES, M. S., Smith, P., et al.
PubMed
Dyslipidemia is a main driver of cardiovascular diseases. The ability of macrophages to scavenge excess lipids implicate them as mediators in this process and understanding the mechanisms underlying macrophage lipid metabolism is key to the development of new treatments. Here, we investigated how adipose tissue macrophages regulate post-prandial cholesterol transport. Single-cell RNA sequencing and protected bone marrow chimeras demonstrated that ingestion of lipids led to specific transcriptional activation of a population of resident macrophages expressing Lyve1, Tim4, and ABCA1. Blocking the phosphatidylserine receptor Tim4 inhibited lysosomal activation and the release of post-prandial high density lipoprotein cholesterol following a high fat meal. Both effects were recapitulated by chloroquine, an inhibitor of lysosomal function. Moreover, clodronate-mediated cell-depletion implicated Tim4+ resident adipose tissue macrophages in this process. Thus, these data indicate that Tim4 is a key regulator of post-prandial cholesterol transport and adipose tissue macrophage function and may represent a novel pathway to treat dyslipidemia. © 2021. The Author(s).
- In Vivo,
- Mus musculus (House mouse)
Hepatic Ischemic Preconditioning Alleviates Ischemia-Reperfusion Injury by Decreasing TIM4 Expression.
In International Journal of Biological Sciences on 21 August 2018 by Zhang, Y., Shen, Q., et al.
PubMed
Ischemia-reperfusion injury (IRI) of the liver is a primary cause of post-liver-surgery complications and ischemic preconditioning (IPC) has been verified to protect against ischemia-reperfusion injury. TIM-4 activation plays an important role in macrophage mediated hepatic IRI. This study aimed to determine whether IPC protects against hepatic IRI through inhibiting TIM-4 activation. In this study, a model of warm liver ischemia (90 min) and reperfusion for 6 h was used. Mice were subjected to ischemia-reperfusion injury with or without ischemic preconditioning and TIM4 blocking antibody. Western blot was determined to detect the expression of TIM4 protein and mitochondrial apoptosis-related protein expression. Liver function was evaluated using the level of alanine transaminase (ALT) and aspartate transaminase (AST), cell apoptosis and pathological examination. We found that compared with the control group, ischemic preconditioning reduced IRI by decreasing hepatocyte apoptosis, ALT, AST, CD68 and CD3 positive cells, tissue myeloperoxidase activity(MPO), and downregulating TIM-4 expression. TIM4 blocking could reduce CD68 and CD3 positive cells in liver. Furthermore, activated monocytes transfusion significantly abolished the protect effect of IPC with increased hepatocyte apoptosis, ALT, AST, CD68 and CD3 positive cells while TIM-4 knockdown monocytes lost this effect. These results suggested that IPC protects against hepatic IRI by downregulating TIM-4 and indicated TIM-4 would be a novel therapeutic target to minimize IRI.
- In Vivo,
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
TIM-3 Regulates CD103+ Dendritic Cell Function and Response to Chemotherapy in Breast Cancer.
In Cancer Cell on 8 January 2018 by de Mingo Pulido, A., Gardner, A., et al.
PubMed
Intratumoral CD103+ dendritic cells (DCs) are necessary for anti-tumor immunity. Here we evaluated the expression of immune regulators by CD103+ DCs in a murine model of breast cancer and identified expression of TIM-3 as a target for therapy. Anti-TIM-3 antibody improved response to paclitaxel chemotherapy in models of triple-negative and luminal B disease, with no evidence of toxicity. Combined efficacy was CD8+ T cell dependent and associated with increased granzyme B expression; however, TIM-3 expression was predominantly localized to myeloid cells in both human and murine tumors. Gene expression analysis identified upregulation of Cxcl9 within intratumoral DCs during combination therapy, and therapeutic efficacy was ablated by CXCR3 blockade, Batf3 deficiency, or Irf8 deficiency. Copyright © 2017 Elsevier Inc. All rights reserved.
- In Vivo,
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
TIM-4 Identifies IFN-γ-Expressing Proinflammatory B Effector 1 Cells That Promote Tumor and Allograft Rejection.
In The Journal of Immunology on 1 October 2017 by Ding, Q., Mohib, K., et al.
PubMed
B cells give rise to polarized subsets, including B effector 1 (Be1) cells and regulatory B cells, which can promote or inhibit immune responses through expression of IFN-γ and IL-10, respectively. Such subsets likely explain why B cell depletion can either ameliorate or exacerbate inflammatory diseases; however, these cells remain poorly understood because of the absence of specific markers. Although T cell Ig and mucin domain-containing molecule (TIM)-1 broadly identifies IL-10+ regulatory B cells, no similar markers for Be1 cells have been described. We now show that TIM-4 is expressed by a subset of B cells distinct from those expressing TIM-1. Although TIM-1+ B cells are enriched for IL-10, TIM-4+ B cells are enriched for IFN-γ. TIM-1+ B cells enhanced the growth of B16-F10 melanoma. In contrast, TIM-4+ B cells decreased B16-F10 metastasis and s.c. tumor growth, and this was IFN-γ dependent. TIM-1+ B cells prolonged islet allograft survival in B-deficient mice, whereas TIM-4+ B cells accelerated rejection in an IFN-γ-dependent manner. Moreover, TIM-4+ B cells promoted proinflammatory Th differentiation in vivo, increasing IFN-γ while decreasing IL-4, IL-10, and Foxp3 expression by CD4+ T cells-effects that are opposite from those of TIM-1+ B cells. Importantly, a monoclonal anti-TIM-4 Ab promoted allograft tolerance, and this was dependent on B cell expression of TIM-4. Anti-TIM-4 downregulated T-bet and IFN-γ expression by TIM-4+ B cells and indirectly increased IL-10 expression by TIM-1+ B cells. Thus, TIM-4+ B cells are enriched for IFN-γ-producing proinflammatory Be1 cells that enhance immune responsiveness and can be specifically targeted with anti-TIM-4. Copyright © 2017 by The American Association of Immunologists, Inc.
- In Vivo,
- Mus musculus (House mouse),
- Immunology and Microbiology
T-cell immunoglobulin and mucin domain 4 (TIM-4) signaling in innate immune-mediated liver ischemia-reperfusion injury.
In Hepatology on 1 December 2014 by Ji, H., Liu, Y., et al.
PubMed
Hepatic ischemia-reperfusion injury (IRI), an innate immunity-driven inflammation response, occurs in multiple clinical settings including liver resection, transplantation, trauma, and shock. T-cell immunoglobulin and mucin (TIM)-4, the only TIM protein not expressed on T cells, is found on macrophages and dendritic cells. The regulatory function of macrophage TIM-4 in the engulfment of apoptotic/necrotic bodies in innate immunity-mediated disease states remains unknown. This study focuses on the putative role of TIM-4 signaling in a model of liver warm ischemia (90 minutes) and reperfusion. The ischemia insult triggered TIM-4 expression by stressed hepatocellular phosphatidylserine (PS) presentation, peaking at 6 hours of reperfusion, and coinciding with the maximal hepatocellular damage. TIM-4-deficient or wild-type WT mice treated with antagonistic TIM-4 monoclonal antibody (mAb) were resistant against liver IRI, evidenced by diminished serum alanine aminotransferase (sALT) levels and well-preserved hepatic architecture. Liver hepatoprotection rendered by TIM-4 deficiency was accompanied by diminished macrophage infiltration/chemoattraction, phagocytosis, and activation of Toll-like receptor (TLR)2/4/9-dependent signaling. Correlating with in vivo kinetics, the peak of TIM-4 induction in lipopolysaccharide (LPS)-activated bone marrow derived-macrophages (BMM) was detected in 6-hour cultures. To mimic liver IRI, we employed hydrogen peroxide-necrotic hepatocytes, which readily present PS. Indeed, necrotic hepatocytes were efficiently captured/engulfed by WT (TIM-4+) but not by TIM-4-deficient BMM. Finally, in a newly established model of liver IRI, adoptive transfer of WT but not TIM-4-deficient BMM readily recreated local inflammation response/hepatocellular damage in the CD11b-DTR mouse system. These findings document the importance of macrophage-specific TIM-4 activation in the mechanism of hepatic IRI. Macrophage TIM-4 may represent a therapeutic target to minimize innate inflammatory responses in IR-stressed organs. © 2014 by the American Association for the Study of Liver Diseases.
- In Vivo,
- Mus musculus (House mouse)
TIM4 Regulates the Anti-Islet Th2 Alloimmune Response.
In Cell Transplantation on 13 March 2014 by Vergani, A., Gatti, F., et al.
PubMed
The role of the novel costimulatory molecule TIM4 in anti-islet response is unknown. We explored TIM4 expression and targeting in Th1 (BALB/c islets into C57BL/6 mice) and Th2 (BALB/c islets into Tbet(-/-) C57BL/6 mice) models of anti-islet alloimmune response and in a model of anti-islet autoimmune response (diabetes onset in NOD mice). The targeting of TIM4, using the monoclonal antibody RMT4-53, promotes islet graft survival in a Th1 model, with 30% of the graft surviving in the long term; islet graft protection appears to be mediated by a Th1 to Th2 skewing of the immune response. Differently, in the Th2 model, TIM4 targeting precipitates graft rejection by further enhancing the Th2 response. The effect of anti-TIM4 treatment in preventing autoimmune diabetes was marginal with only minor Th1 to Th2 skewing. B-Cell depletion abolished the effect of TIM4 targeting. TIM4 is expressed on human B-cells and is upregulated in diabetic and islet-transplanted patients. Our data suggest a model in which TIM4 targeting promotes Th2 response over Th1 via B-cells. The targeting of TIM4 could become a component of an immunoregulatory protocol in clinical islet transplantation, aiming at redirecting the immune system toward a Th2 response.