InVivoMAb anti-mouse CD276 (B7-H3)
Product Details
The MJ18 monoclonal antibody reacts with mouse CD276 also known as B7-H3. CD276 is a type I transmembrane protein and a member of the B7 family of co-stimulatory proteins. CD276 is expressed weakly on activated lymphocytes, macrophages, dendritic cells, nasal and airway epithelial cells, osteoblasts, and some tumor cell lines. A soluble form of CD276 is also secreted by monocytes, dendritic cells, and activated T cells. The biological role of CD276 is still under investigation however, recent studies suggest a negative regulatory role for CD276 in T cell responses. The MJ18 antibody has been shown to block CD276 when administered in vivo.Specifications
| Isotype | Rat IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG1 isotype control, anti-horseradish peroxidase |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse B7-H3 IgG2a fusion protein |
| Reported Applications |
in vivo B7-H3 blockade Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_10950149 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
Flow Cytometry
Kamachi, F., et al. (2015). "ICOS promotes group 2 innate lymphoid cell activation in lungs" Biochem Biophys Res Commun 463(4): 739-745. PubMed
Group 2 innate lymphoid cells (ILC2s) are newly identified, potent producers of type 2 cytokines, such as IL-5 and IL-13, and contribute to the development of allergic lung inflammation induced by cysteine proteases. Although it has been shown that inducible costimulator (ICOS), a costimulatory molecule, is expressed on ILC2s, the role of ICOS in ILC2 responses is largely unknown. In the present study, we investigated whether the interaction of ICOS with its ligand B7-related protein-1 (B7RP-1) can promote ILC2 activation. Cytokine production in ILC2s purified from mouse lungs was significantly increased by coculture with B7RP-1-transfected cells, and increased cytokine production was inhibited by monoclonal antibody-mediated blocking of the ICOS/B7RP-1 interaction. ILC2 expansion and eosinophil influx induced by papain, a cysteine protease antigen, in mouse lungs were significantly abrogated by blocking the ICOS/B7RP-1 interaction. Dendritic cells (DCs) in the lungs expressed B7RP-1 and the number of DCs markedly increased with papain administration. B7RP-1 expression on lung DCs was reduced after papain administration. This downregulation of B7RP-1 expression may be an indication of ICOS/B7RP-1 binding. These results indicate that ILC2s might interact with B7RP-1-expressing DCs in allergic inflammatory lung, and ICOS signaling can positively regulate the protease allergen-induced ILC2 activation followed by eosinophil infiltration into the lungs.
in vivo B7-H3 blockade
Yamato, I., et al. (2009). "Clinical importance of B7-H3 expression in human pancreatic cancer" Br J Cancer 101(10): 1709-1716. PubMed
BACKGROUND: B7-H3 is a new member of the B7 ligand family and regulates T-cell responses in various conditions. However, the role of B7-H3 in tumour immunity is largely unknown. The purpose of this study was to evaluate the clinical significance of B7-H3 expression in human pancreatic cancer and the therapeutic potential for cancer immunotherapy. METHODS: We investigated B7-H3 expression in 59 patients with pancreatic cancer by immunohistochemistry and real-time PCR. Furthermore, we examined the anti-tumour effect of B7-H3-blocking monoclonal antibody in vivo in a murine pancreatic cancer model. RESULTS: Tumour-related B7-H3 expression was abundant in most human pancreatic cancer tissues and was significantly higher compared with that in non-cancer tissue or normal pancreas. Moreover, its expression was significantly more intense in cases with lymph node metastasis and advanced pathological stage. B7-H3 blockade promoted CD8(+) T-cell infiltration into the tumour and induced a substantial anti-tumour effect on murine pancreatic cancer. In addition, the combination of gemcitabine with B7-H3 blockade showed a synergistic anti-tumour effect without overt toxicity. CONCLUSION: Our data show for the first time that B7-H3 may have a critical role in pancreatic cancer and provide the rationale for developing a novel cancer immunotherapy against this fatal disease.
Flow Cytometry
Nagashima, O., et al. (2008). "B7-H3 contributes to the development of pathogenic Th2 cells in a murine model of asthma" J Immunol 181(6): 4062-4071. PubMed
B7-H3 is a new member of the B7 family. The receptor for B7-H3 has not been identified, but it seems to be expressed on activated T cells. Initial studies have shown that B7-H3 provides a stimulatory signal to T cells. However, recent studies suggest a negative regulatory role for B7-H3 in T cell responses. Thus, the immunological function of B7-H3 is controversial and unclear. In this study, we investigated the effects of neutralizing anti-B7-H3 mAb in a mouse model of allergic asthma to determine whether B7-H3 contributes to the development of pathogenic Th2 cells and pulmonary inflammation. Administration of anti-B7-H3 mAb significantly reduced airway hyperreactivity with a concomitant decrease in eosinophils in the lung as compared with control IgG-treated mice. Treatment with anti-B7-H3 mAb also resulted in decreased production of Th2 cytokines (IL-4, IL-5, and IL-13) in the draining lymph node cells. Although blockade of B7-H3 during the induction phase abrogated the development of asthmatic responses, B7-H3 blockade during the effector phase did not inhibit asthmatic responses. These results indicated an important role for B7-H3 in the development of pathogenic Th2 cells during the induction phase in a murine model of asthma.
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
CD276-dependent efferocytosis by tumor-associated macrophages promotes immune evasion in bladder cancer.
In Nature Communications on 1 April 2024 by Cheng, M., Chen, S., et al.
PubMed
Interplay between innate and adaptive immune cells is important for the antitumor immune response. However, the tumor microenvironment may turn immune suppressive, and tumor associated macrophages are playing a role in this transition. Here, we show that CD276, expressed on tumor-associated macrophages (TAM), play a role in diminishing the immune response against tumors. Using a model of tumors induced by N-butyl-N-(4-hydroxybutyl) nitrosamine in BLCA male mice we show that genetic ablation of CD276 in TAMs blocks efferocytosis and enhances the expression of the major histocompatibility complex class II (MHCII) of TAMs. This in turn increases CD4 + and cytotoxic CD8 + T cell infiltration of the tumor. Combined single cell RNA sequencing and functional experiments reveal that CD276 activates the lysosomal signaling pathway and the transcription factor JUN to regulate the expression of AXL and MerTK, resulting in enhanced efferocytosis in TAMs. Proving the principle, we show that simultaneous blockade of CD276 and PD-1 restrain tumor growth better than any of the components as a single intervention. Taken together, our study supports a role for CD276 in efferocytosis by TAMs, which is potentially targetable for combination immune therapy. © 2024. The Author(s).
- Mus musculus (House mouse),
- Cancer Research
The anti-B7-H3 blocking antibody MJ18 does not recognize B7-H3 in murine tumor models
Preprint on BioRxiv : the Preprint Server for Biology on 17 November 2023 by Nammor, T., Frizzell, J., et al.
PubMed
The immune checkpoint molecule B7-H3 is regarded as one of the most promising therapeutic targets for the treatment of human cancers. B7-H3 is highly expressed in many cancers and its expression has been associated to impaired antitumor immunity and poor patient prognosis. In immunocompetent mouse tumor models, genetic deletion of B7-H3 in tumor cells enhances antitumor immune response leading to tumor shrinkage. The underlying mechanisms of B7-H3 inhibitory function remain largely uncharacterized and the identity of potential cognate(s) receptor(s) of B7-H3 is still to be defined. To better understand B7-H3 function in vivo , several studies have employed MJ18, a monoclonal antibody reported to bind murine B7-H3 and blocks its immune-inhibitory function. In this brief research report, we show that 1) MJ18 does not bind B7-H3, 2) MJ18 binds the Fc receptor FcγRIIB on surface of murine splenocytes, and 3) MJ18 does not induce tumor regression in a mouse model responsive to B7-H3 knockout. Given the high profile of B7-H3 as therapeutic target for human cancers, our work emphasizes that murine B7-H3 studies using the MJ18 antibody should be interpreted with caution. Finally, we hope that our study will motivate the scientific community to establish much-needed validated research tools to study B7-H3 biology in mouse models.
- Cancer Research,
- Immunology and Microbiology
Immune checkpoint B7-H3 is a therapeutic vulnerability in prostate cancer harboring PTEN and TP53 deficiencies.
In Science Translational Medicine on 10 May 2023 by Shi, W., Wang, Y., et al.
PubMed
Checkpoint immunotherapy has yielded meaningful responses across many cancers but has shown modest efficacy in advanced prostate cancer. B7 homolog 3 protein (B7-H3/CD276) is an immune checkpoint molecule and has emerged as a promising therapeutic target. However, much remains to be understood regarding B7-H3's role in cancer progression, predictive biomarkers for B7-H3-targeted therapy, and combinatorial strategies. Our multi-omics analyses identified B7-H3 as one of the most abundant immune checkpoints in prostate tumors containing PTEN and TP53 genetic inactivation. Here, we sought in vivo genetic evidence for, and mechanistic understanding of, the role of B7-H3 in PTEN/TP53-deficient prostate cancer. We found that loss of PTEN and TP53 induced B7-H3 expression by activating transcriptional factor Sp1. Prostate-specific deletion of Cd276 resulted in delayed tumor progression and reversed the suppression of tumor-infiltrating T cells and NK cells in Pten/Trp53 genetically engineered mouse models. Furthermore, we tested the efficacy of the B7-H3 inhibitor in preclinical models of castration-resistant prostate cancer (CRPC). We demonstrated that enriched regulatory T cells and elevated programmed cell death ligand 1 (PD-L1) in myeloid cells hinder the therapeutic efficacy of B7-H3 inhibition in prostate tumors. Last, we showed that B7-H3 inhibition combined with blockade of PD-L1 or cytotoxic T lymphocyte-associated protein 4 (CTLA-4) achieved durable antitumor effects and had curative potential in a PTEN/TP53-deficient CRPC model. Given that B7-H3-targeted therapies have been evaluated in early clinical trials, our studies provide insights into the potential of biomarker-driven combinatorial immunotherapy targeting B7-H3 in prostate cancer, among other malignancies.
- Cancer Research,
- Immunology and Microbiology
mTORC1 upregulates B7-H3/CD276 to inhibit antitumor T cells and drive tumor immune evasion.
In Nature Communications on 3 March 2023 by Liu, H. J., Du, H., et al.
PubMed
Identifying the mechanisms underlying the regulation of immune checkpoint molecules and the therapeutic impact of targeting them in cancer is critical. Here we show that high expression of the immune checkpoint B7-H3 (CD276) and high mTORC1 activity correlate with immunosuppressive phenotypes and worse clinical outcomes in 11,060 TCGA human tumors. We find that mTORC1 upregulates B7-H3 expression via direct phosphorylation of the transcription factor YY2 by p70 S6 kinase. Inhibition of B7-H3 suppresses mTORC1-hyperactive tumor growth via an immune-mediated mechanism involving increased T-cell activity and IFN-γ responses coupled with increased tumor cell expression of MHC-II. CITE-seq reveals strikingly increased cytotoxic CD38+CD39+CD4+ T cells in B7-H3-deficient tumors. In pan-human cancers, a high cytotoxic CD38+CD39+CD4+ T-cell gene signature correlates with better clinical prognosis. These results show that mTORC1-hyperactivity, present in many human tumors including tuberous sclerosis complex (TSC) and lymphangioleiomyomatosis (LAM), drives B7-H3 expression leading to suppression of cytotoxic CD4+ T cells. © 2023. The Author(s).
- In Vivo,
- Mus musculus (House mouse),
- Cardiovascular biology,
- Immunology and Microbiology
B7H3-dependent myeloid-derived suppressor cell recruitment and activation in pulmonary fibrosis.
In Frontiers in Immunology on 2 September 2022 by Liu, T., Gonzalez De Los Santos, F., et al.
PubMed
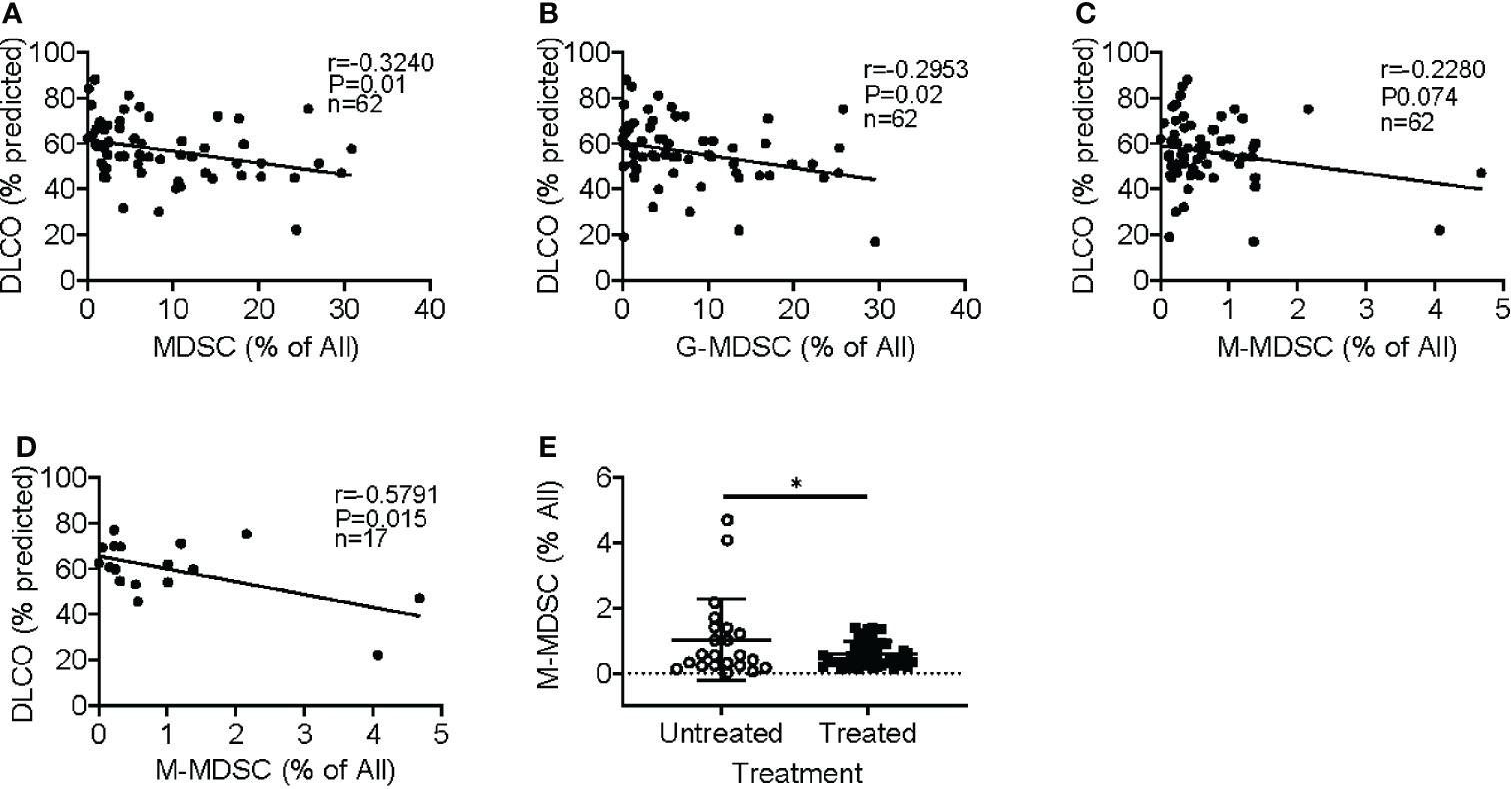
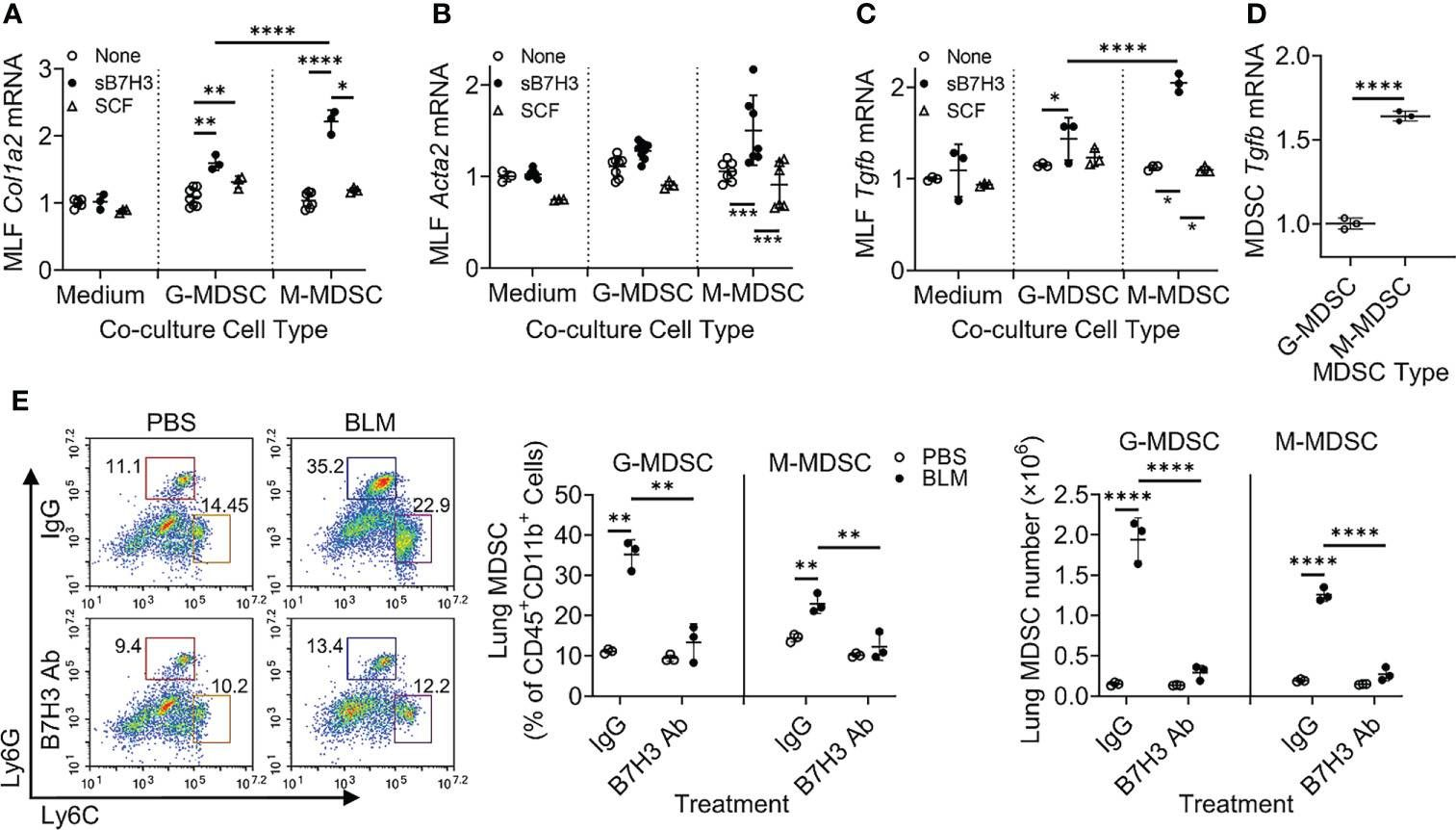
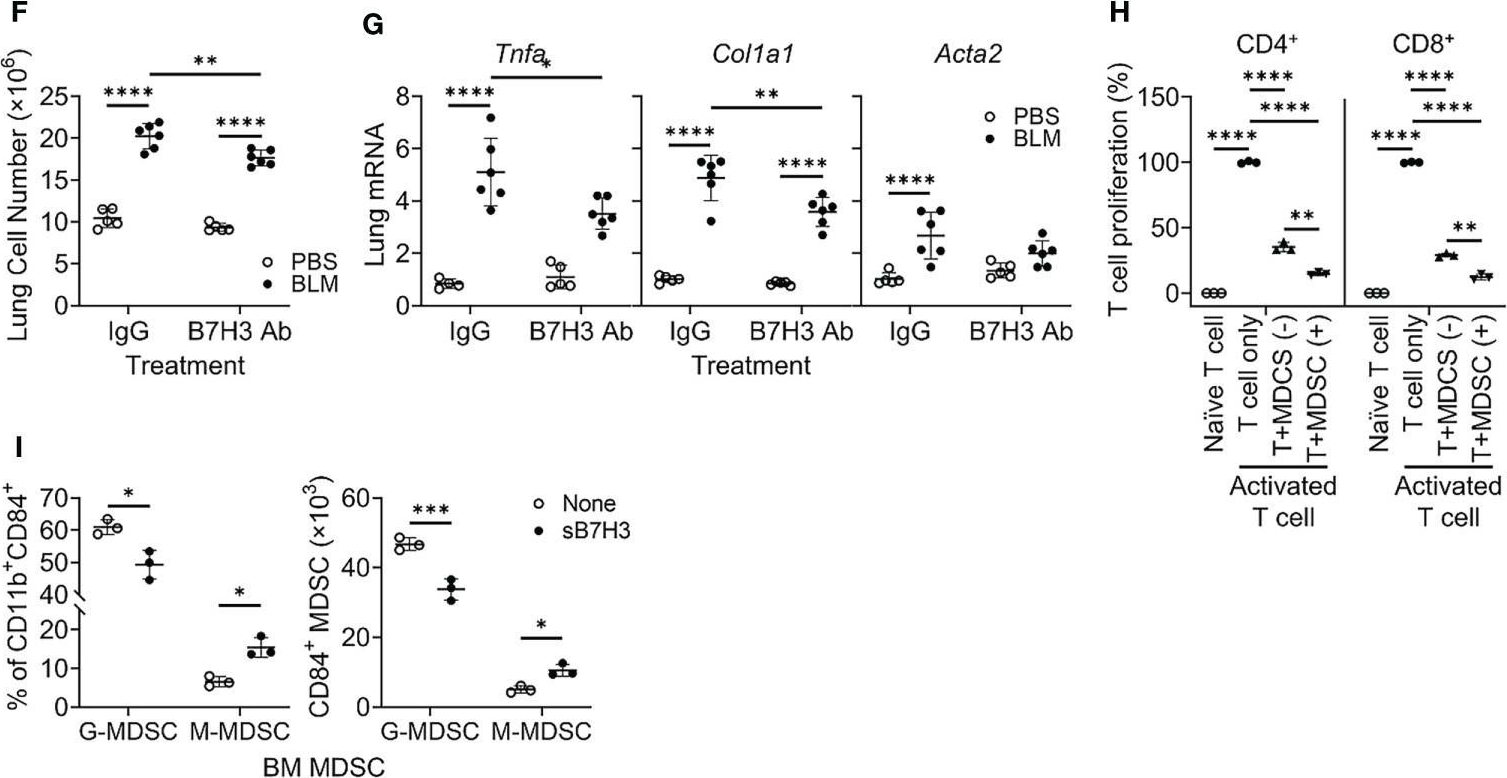
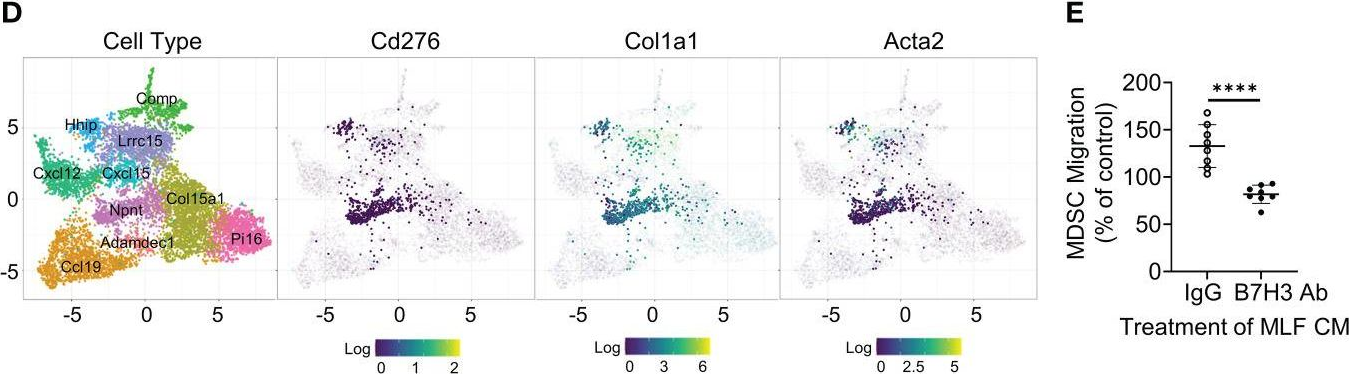
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic lung disease without effective curative therapy. Recent evidence shows increased circulating myeloid-derived suppressor cells (MDSCs) in cancer, inflammation, and fibrosis, with some of these cells expressing B7H3. We sought to investigate the role of MDSCs in IPF and its potential mediation via B7H3. Here we prospectively collected peripheral blood samples from IPF patients to analyze for circulating MDSCs and B7H3 expression to assess their clinical significance and potential impact on co-cultured lung fibroblasts and T-cell activation. In parallel, we assess MDSC recruitment and potential B7H3 dependence in a mouse model of pulmonary fibrosis. Expansion of MDSCs in IPF patients correlated with disease severity. Co-culture of soluble B7H3 (sB7H3)-treated mouse monocytic MDSCs (M-MDSCs), but not granulocytic MDSCs (G-MDSCs), activated lung fibroblasts and myofibroblast differentiation. Additionally, sB7H3 significantly enhanced MDSC suppression of T-cell proliferation. Activated M-MDSCs displayed elevated TGFβ and Arg1 expression relative to that in G-MDSCs. Treatment with anti-B7H3 antibodies inhibited bone marrow-derived MDSC recruitment into the bleomycin-injured lung, accompanied by reduced expression of inflammation and fibrosis markers. Selective telomerase reverse transcriptase (TERT) deficiency in myeloid cells also diminished MDSC recruitment associated with the reduced plasma level of sB7H3, lung recruitment of c-Kit+ hematopoietic progenitors, myofibroblast differentiation, and fibrosis. Lung single-cell RNA sequencing (scRNA-seq) revealed fibroblasts as a predominant potential source of sB7H3, and indeed the conditioned medium from activated mouse lung fibroblasts had a chemotactic effect on bone marrow (BM)-MDSC, which was abolished by B7H3 blocking antibody. Thus, in addition to their immunosuppressive activity, TERT and B7H3-dependent MDSC expansion/recruitment from BM could play a paracrine role to activate myofibroblast differentiation during pulmonary fibrosis with potential significance for disease progression mediated by sB7H3. Copyright © 2022 Liu, Gonzalez De Los Santos, Rinke, Fang, Flaherty and Phan.
- FC/FACS,
- Mus musculus (House mouse),
- Cancer Research
Elimination of acquired resistance to PD-1 blockade via the concurrent depletion of tumour cells and immunosuppressive cells.
In Nature Biomedical Engineering on 1 November 2021 by Xue, G., Wang, Z., et al.
PubMed
Antigen release resulting from the death of tumour cells induced by chemotherapies and targeted therapies can augment the antitumour responses induced by immune checkpoint blockade (ICB). However, tumours responding to ICB therapies often become resistant to them. Here we show that the specific targeting of tumour cells promotes the growth of tumour-cell variants that are resistant to ICB, and that the acquired resistance can be overcome via the concurrent depletion of tumour cells and of major types of immunosuppressive cell via a monoclonal antibody binding the enzyme CD73, which we identified as highly expressed on tumour cells and on regulatory T cells, myeloid-derived suppressor cells and tumour-associated macrophages, but not on cytolytic T lymphocytes, natural killer cells and dendritic cells. In mice with murine tumours, the systemic administration of anti-PD1 antibodies and anti-CD73 antibodies conjugated to a near-infrared dye prevented near-infrared-irradiated tumours from acquiring resistance to ICB and resulted in the eradication of advanced tumours. The elimination of immunosuppressive cells may overcome acquired resistance to ICB across a range of tumour types and combination therapies. © 2021. The Author(s), under exclusive licence to Springer Nature Limited.
- Cancer Research,
- Immunology and Microbiology,
- Stem Cells and Developmental Biology,
- In Vivo,
- Mus musculus (House mouse)
CD276 expression enables squamous cell carcinoma stem cells to evade immune surveillance.
In Cell Stem Cell on 2 September 2021 by Wang, C., Li, Y., et al.
PubMed
Immunosurveillance is a critical mechanism guarding against tumor development and progression. Checkpoint inhibitors have shown significant success in cancer treatment, but expression of key factors such as PD-L1 in putative cancer stem cell (CSC) populations in squamous cell carcinoma has been inconclusive, suggesting that CSCs may have developed other mechanisms to escape immune surveillance. Here we show that CSCs upregulate the immune checkpoint molecule CD276 (B7-H3) to evade host immune responses. CD276 is highly expressed by CSCs in mouse and human head and neck squamous cell carcinoma (HNSCC) and can be used to prospectively isolate tumorigenic CSCs. Anti-CD276 antibodies eliminate CSCs in a CD8+ T cell-dependent manner, inhibiting tumor growth and lymph node metastases in a mouse HNSCC model. Single-cell RNA sequencing (RNA-seq) showed that CD276 blockade remodels SCC heterogeneity and reduces epithelial-mesenchymal transition. These results show that CSCs utilize CD276 for immune escape and suggest that targeting CD276 may reduce CSCs in HNSCC. Copyright © 2021 Elsevier Inc. All rights reserved.