InVivoMAb anti-mouse IL-6
Product Details
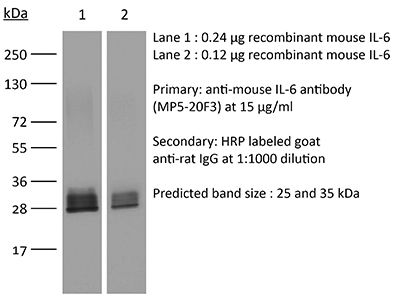
The MP5-20F3 monoclonal antibody reacts with mouse IL-6 (interleukin-6) a 21-28 kDa cytokine that is expressed by many cell types, including T lymphocytes, B lymphocytes, monocytes, fibroblasts, and endothelial cells. IL-6 signals through a cell-surface type I cytokine receptor complex consisting of the ligand-binding IL-6Rα chain (CD126), and the signal-transducing component gp130 (also called CD130). Upon receptor binding IL-6 influences antigen-specific immune responses, inflammatory responses, neuronal development, and is a major mediator of the acute phase reaction. The MP5-20F3 monoclonal antibody has been shown to neutralize the bioactivity of natural or recombinant IL-6.Specifications
| Isotype | Rat IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG1 isotype control, anti-horseradish peroxidase |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Recombinant mouse IL-6 |
| Reported Applications |
in vivo IL-6 neutralization in vitro IL-6 neutralization |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 μM filtered |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107709 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
in vivo IL-6 neutralization
Benevides, L., et al. (2015). "IL17 Promotes Mammary Tumor Progression by Changing the Behavior of Tumor Cells and Eliciting Tumorigenic Neutrophils Recruitment" Cancer Res 75(18): 3788-3799. PubMed
The aggressiveness of invasive ductal carcinoma (IDC) of the breast is associated with increased IL17 levels. Studying the role of IL17 in invasive breast tumor pathogenesis, we found that metastatic primary tumor-infiltrating T lymphocytes produced elevated levels of IL17, whereas IL17 neutralization inhibited tumor growth and prevented the migration of neutrophils and tumor cells to secondary disease sites. Tumorigenic neutrophils promote disease progression, producing CXCL1, MMP9, VEGF, and TNFalpha, and their depletion suppressed tumor growth. IL17A also induced IL6 and CCL20 production in metastatic tumor cells, favoring the recruitment and differentiation of Th17. In addition, IL17A changed the gene-expression profile and the behavior of nonmetastatic tumor cells, causing tumor growth in vivo, confirming the protumor role of IL17. Furthermore, high IL17 expression was associated with lower disease-free survival and worse prognosis in IDC patients. Thus, IL17 blockade represents an attractive approach for the control of invasive breast tumors. Cancer Res; 75(18); 3788-99. (c)2015 AACR.
in vivo IL-6 neutralization
Tsukamoto, H., et al. (2015). "IL-6-mediated environmental conditioning of defective Th1 differentiation dampens antitumour immune responses in old age" Nat Commun 6: 6702. PubMed
Decline in immune function and inflammation concomitantly develop with ageing. Here we focus on the impact of this inflammatory environment on T cells, and demonstrate that in contrast to successful tumour elimination in young mice, replenishment of tumour-specific CD4(+) T cells fails to induce tumour regression in aged hosts. The impaired antitumour effect of CD4(+) T cells with their defective Th1 differentiation in an aged environment is restored by interleukin (IL)-6 blockade or IL-6 deficiency. IL-6 blockade also restores the impaired ability of CD4(+) T cells to promote CD8(+) T-cell-dependent tumour elimination in aged mice, which requires IFN-gamma. Furthermore, IL-6-stimulated production of IL-4/IL-21 through c-Maf induction is responsible for impaired Th1 differentiation. IL-6 also contributes to IL-10 production from CD4(+) T cells in aged mice, causing attenuated responses of CD8(+) T cells. These findings suggest that IL-6 serves as an extrinsic factor counteracting CD4(+) T-cell-mediated immunity against tumour in old age.
in vivo IL-6 neutralization
Liang, Y., et al. (2015). "Innate lymphotoxin receptor mediated signaling promotes HSV-1 associated neuroinflammation and viral replication" Sci Rep 5: 10406. PubMed
Host anti-viral innate immunity plays important roles in the defense against HSV-1 infection. In this study, we find an unexpected role for innate LT/LIGHT signaling in promoting HSV-1 replication and virus induced inflammation in immunocompromised mice. Using a model of footpad HSV-1 infection in Rag1(-/-) mice, we observed that blocking LT/LIGHT signaling with LTbetaR-Ig could significantly delay disease progression and extend the survival of infected mice. LTbetaR-Ig treatment reduced late proinflammatory cytokine release in the serum and nervous tissue, and inhibited chemokine expression and inflammatory cells infiltration in the dorsal root ganglia (DRG). Intriguingly, LTbetaR-Ig treatment restricted HSV-1 replication in the DRG but not the footpad. These findings demonstrate a critical role for LT/LIGHT signaling in modulating innate inflammation and promoting HSV-1 replication in the nervous system, and suggest a new target for treatment of virus-induced adverse immune response and control of severe HSV-1 infection.
in vivo IL-6 neutralization
Hock, K., et al. (2014). "Donor CD4 T cells trigger costimulation blockade-resistant donor bone marrow rejection through bystander activation requiring IL-6" Am J Transplant 14(9): 2011-2022. PubMed
Bone marrow (BM) transplantation under costimulation blockade induces chimerism and tolerance. Cotransplantation of donor T cells (contained in substantial numbers in mobilized peripheral blood stem cells and donor lymphocyte infusions) together with donor BM paradoxically triggers rejection of donor BM through undefined mechanisms. Here, nonmyeloablatively irradiated C57BL/6 recipients simultaneously received donor BM (BALB/c) and donor T cells under costimulation blockade (anti-CD154 and CTLA4Ig). Donor CD4, but not CD8 cells, triggered natural killer-independent donor BM rejection which was associated with increased production of IL-6, interferon gamma (IFN-gamma) and IL-17A. BM rejection was prevented through neutralization of IL-6, but not of IFN-gamma or IL-17A. IL-6 counteracted the antiproliferative effect of anti-CD154 in vitro. Rapamycin and anti-lymphocyte function-associated antigen 1 negated this effect of IL-6 in vitro and prevented BM rejection in vivo. Simultaneous cotransplantation of (BALB/cxB6)F1, recipient or irradiated donor CD4 cells, or late transfer of donor CD4 cells did not lead to BM rejection, whereas cotransplantation of third party CD4 cells did. Transferred donor CD4 cells became activated, rapidly underwent apoptosis and triggered activation and proliferation of recipient T cells. Collectively, these results provide evidence that donor T cells recognizing the recipient as allogeneic lead to the release of IL-6, which abolishes the effect of anti-CD154, triggering donor BM rejection through bystander activation.
in vivo IL-6 neutralization
Khmaladze, I., et al. (2014). "Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice" Proc Natl Acad Sci U S A 111(35): E3669-3678. PubMed
Psoriasis (Ps) and psoriasis arthritis (PsA) are poorly understood common diseases, induced by unknown environmental factors, affecting skin and articular joints. A single i.p. exposure to mannan from Saccharomyces cerevisiae induced an acute inflammation in inbred mouse strains resembling human Ps and PsA-like disease, whereas multiple injections induced a relapsing disease. Exacerbation of disease severity was observed in mice deficient for generation of reactive oxygen species (ROS). Interestingly, restoration of ROS production, specifically in macrophages, ameliorated both skin and joint disease. Neutralization of IL-17A, mainly produced by gammadelta T cells, completely blocked disease symptoms. Furthermore, mice depleted of granulocytes were resistant to disease development. In contrast, certain acute inflammatory mediators (C5, Fcgamma receptor III, mast cells, and histamine) and adaptive immune players (alphabeta T and B cells) were redundant in disease induction. Hence, we propose that mannan-induced activation of macrophages leads to TNF-alpha secretion and stimulation of local gammadelta T cells secreting IL-17A. The combined action of activated macrophages and IL-17A produced in situ drives neutrophil infiltration in the epidermis and dermis of the skin, leading to disease manifestations. Thus, our finding suggests a new mechanism triggered by exposure to exogenous microbial components, such as mannan, that can induce and exacerbate Ps and PsA.
in vitro IL-6 neutralization
Jose, S., et al. (2014). "Mesenchymal stem cells exert anti-proliferative effect on lipopolysaccharide-stimulated BV2 microglia by reducing tumour necrosis factor-alpha levels" J Neuroinflammation 11: 149. PubMed
BACKGROUND: Progression of neurodegenerative diseases occurs when microglia, upon persistent activation, perpetuate a cycle of damage in the central nervous system. Use of mesenchymal stem cells (MSC) has been suggested as an approach to manage microglia activation based on their immunomodulatory functions. In the present study, we describe the mechanism through which bone marrow-derived MSC modulate the proliferative responses of lipopolysaccharide-stimulated BV2 microglia. METHODS: BV2 microglia were cultured with MSC and stimulated with 1 mug/ml lipopolysaccharide. Using an inducible nitric oxide synthase inhibitor, tritiated thymidine (3H-TdR) incorporation assay was performed to determine the role of nitric oxide in the anti-proliferative effect of MSC. We also studied apoptosis and the cell cycle of both cell types using flow cytometry and explored their cytokine profile using protein and cytometric arrays. Moreover, the role of IL-6 and TNF-alpha in immunomodulation was deduced using specific blocking antibodies and recombinant proteins. RESULTS: MSC reduces microglia proliferation upon lipopolysaccharide stimulation by 21 to 28% and modulates the levels of nitric oxide, IL-6 and TNF-alpha. The role of nitric oxide in conferring the anti-proliferative effect of MSC was ruled out. Furthermore, we found that MSC exert their anti-proliferative effect by restoring the percentage of BV2 cells at S and G2/M phase to levels similar to unstimulated cells. MSC undergo a G0/G1 arrest while exerting this effect. We have also identified that MSC-mediated modulation of microglia is independent of IL-6, whilst reduction of TNF-alpha in co-culture is critical for inhibition of microglia proliferation. CONCLUSIONS: Our study demonstrates that MSC inhibit microglia proliferation independent of nitric oxide and IL-6, although reduction of TNF-alpha is critical for this effect. The inhibition of proliferation is through cell cycle modulation. These findings shed light on the mechanisms of microglial immunomodulation by MSC.
in vivo IL-6 neutralization
Barber, D. L., et al. (2014). "Role of IL-6 in Mycobacterium avium–associated immune reconstitution inflammatory syndrome" J Immunol 192(2): 676-682. PubMed
Immune reconstitution inflammatory syndrome (IRIS) is a major adverse event of antiretroviral therapy in HIV infection, and paradoxically occurs as HIV viremia is suppressed and CD4 T cell numbers recover. IRIS reflects pathogenic immune responses against opportunistic infections acquired during the period of immunodeficiency, but little is understood about the mechanisms of inflammatory pathology. In this study, we show that IL-6 and C-reactive protein levels transiently rise at the time of the IRIS event in HIV-infected patients, unmasking Mycobacterium avium complex infection after starting antiretroviral therapy. To directly test the role of IL-6 in IRIS pathology, we used a model of experimentally inducible IRIS in which M. avium-infected T cell-deficient mice undergo a fatal inflammatory disease after reconstitution with CD4 T cells. We find that IL-6 neutralization reduces C-reactive protein levels, alleviates wasting disease, and extends host survival during experimental IRIS. Moreover, we show that combined blockade of IL-6 and IFN-gamma further reduces IRIS pathology, even after the onset of wasting disease. The combination of these clinical and experimental-model data show that the IL-6 pathway is not only a biomarker of mycobacterial IRIS but also a major mediator of pathology distinct from IFN-gamma and may be a useful target for therapeutic intervention.
in vivo IL-6 neutralization
Kugler, D. G., et al. (2013). "CD4+ T cells are trigger and target of the glucocorticoid response that prevents lethal immunopathology in toxoplasma infection" J Exp Med 210(10): 1919-1927. PubMed
Synthetic glucocorticoids (GCs) are commonly used in the treatment of inflammatory diseases, but the role of endogenous GCs in the regulation of host-protective immune responses is poorly understood. Here we show that GCs are induced during acute Toxoplasma gondii infection and directly control the T cell response to the parasite. When infected with toxoplasma, mice that selectively lack GC receptor (GR) expression in T cells (GR(lck-Cre)) rapidly succumb to infection despite displaying parasite burdens indistinguishable from control animals and unaltered levels of the innate cytokines IL-12 and IL-27. Mortality in the GR(lck-Cre) mice was associated with immunopathology and hyperactive Th1 cell function as revealed by enhanced IFN-gamma and TNF production in vivo. Unexpectedly, these CD4(+) T lymphocytes also overexpressed IL-10. Importantly, CD4(+) T cell depletion in wild-type or GR(lck-Cre) mice led to ablation of the GC response to infection. Moreover, in toxoplasma-infected RAG(-/-) animals, adoptive transfer of CD4(+) T lymphocytes was required for GC induction. These findings establish a novel IL-10-independent immunomodulatory circuit in which CD4(+) T cells trigger a GC response that in turn dampens their own effector function. In the case of T. gondii infection, this self-regulatory pathway is critical for preventing collateral tissue damage and promoting host survival.
in vivo IL-6 neutralization
Berger, H., et al. (2013). "SOCS3 transactivation by PPARgamma prevents IL-17-driven cancer growth" Cancer Res 73(12): 3578-3590. PubMed
Activation of the transcription factor PPARgamma by the n-3 fatty acid docosahexaenoic acid (DHA) is implicated in controlling proinflammatory cytokine secretion, but the intracellular signaling pathways engaged by PPARgamma are incompletely characterized. Here, we identify the adapter-encoding gene SOCS3 as a critical transcriptional target of PPARgamma. SOCS3 promoter binding and gene transactivation by PPARgamma was associated with a repression in differentiation of proinflammatory T-helper (TH)17 cells. Accordingly, TH17 cells induced in vitro displayed increased SOCS3 expression and diminished capacity to produce interleukin (IL)-17 following activation of PPARgamma by DHA. Furthermore, naive CD4 T cells derived from mice fed a DHA-enriched diet displayed less capability to differentiate into TH17 cells. In two different mouse models of cancer, DHA prevented tumor outgrowth and angiogenesis in an IL-17-dependent manner. Altogether, our results uncover a novel molecular pathway by which PPARgamma-induced SOCS3 expression prevents IL-17-mediated cancer growth.
in vivo IL-6 neutralization
Debock, I., et al. (2012). "Th17 alloimmunity prevents neonatal establishment of lymphoid chimerism in IL-4-deprived mice" Am J Transplant 12(1): 81-89. PubMed
Immune responses in newborn mice are known to be biased toward the helper type 2 phenotype. This may account for their propensity to develop tolerance. Herein, we evaluated the effects of IL-4 deprivation on CD4(+) T-cell activities elicited by neonatal exposure to allogeneic spleen cells. We showed that chimerism, Th2-type polarization and pathology, as well as skin allograft acceptance were inhibited in BALB/c mice immunized at birth with (A/J x BALB/c) F(1) spleen cells upon in vivo IL-4 neutralization. While IL-4 neutralization inhibited the development of Th2 cells in this model, it led to the accumulation of IL-17A, IL-17F, IL-22, IL-6 and RORgammat mRNA in the spleen or graft tissues. Moreover, IL-4 deprivation led to the differentiation of donor-specific Th17 cells with a concomitant Th1 response characterized by IFN-gamma production. The Th17-type response emerging in IL-4-deprived mice was found to mediate both intragraft neutrophil infiltration and the abrogation of B-cell chimerism. Neutralization of this Th17 response failed however to restore functional skin graft acceptance. Collectively, our observations indicate that the neonatal Th2 response opposes the development of Th17 cells, and that Th17 cells are responsible for controlling lymphoid chimerism in mice neonatally injected with semiallogeneic cells.
in vitro IL-6 neutralization
Molinero, L. L., et al. (2011). "High TCR stimuli prevent induced regulatory T cell differentiation in a NF-kappaB-dependent manner" J Immunol 186(8): 4609-4617. PubMed
The concentration of Ag or mitogenic stimuli is known to play an important role in controlling the differentiation of naive CD4(+) T cells into different effector phenotypes. In particular, whereas TCR engagement at low Ag doses in the presence of TGF-beta and IL-2 can promote differentiation of Foxp3-expressing induced regulatory T cells (iTregs), high levels of Ag have been shown in vitro and in vivo to prevent Foxp3 upregulation. This tight control of iTreg differentiation dictated by Ag dose most likely determines the quality and duration of an immune response. However, the molecular mechanism by which this high-dose inhibition of Foxp3 induction occurs is not well understood. In this study, we demonstrate that when cells are in the presence of CD28 costimulation, TCR-dependent NF-kappaB signaling is essential for Foxp3 inhibition at high doses of TCR engagement in mouse T cells. Prevention of Foxp3 induction depends on the production of NF-kappaB-dependent cytokines by the T cells themselves. Moreover, T cells that fail to upregulate Foxp3 under iTreg-differentiating conditions and high TCR stimulation acquire the capacity to make TNF and IFN-gamma, as well as IL-17 and IL-9. Thus, NF-kappaB helps T cells control their differentiation fate in a cell-intrinsic manner and prevents peripheral iTreg development under conditions of high Ag load that may require more vigorous effector T cell responses.
in vivo IL-6 neutralization
Prabhakara, R., et al. (2011). "Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus" Infect Immun 79(12): 5010-5018. PubMed
Staphylococcus aureus is a common cause of prosthetic implant infections, which can become chronic due to the ability of S. aureus to grow as a biofilm. Little is known about adaptive immune responses to these infections in vivo. We hypothesized that S. aureus elicits inflammatory Th1/Th17 responses, associated with biofilm formation, instead of protective Th2/Treg responses. We used an adapted mouse model of biofilm-mediated prosthetic implant infection to determine chronic infection rates, Treg cell frequencies, and local cytokine levels in Th1-biased C57BL/6 and Th2-biased BALB/c mice. All C57BL/6 mice developed chronic S. aureus implant infection at all time points tested. However, over 75% of BALB/c mice spontaneously cleared the infection without adjunctive therapy and demonstrated higher levels of Th2 cytokines and anti-inflammatory Treg cells. When chronic infection rates in mice deficient in the Th2 cytokine interleukin-4 (IL-4) via STAT6 mutation in a BALB/c background were assessed, the mice were unable to clear the S. aureus implant infection. Additionally, BALB/c mice depleted of Treg cells via an anti-CD25 monoclonal antibody (MAb) were also unable to clear the infection. In contrast, the C57BL/6 mice that were susceptible to infection were able to eliminate S. aureus biofilm populations on infected intramedullary pins once the Th1 and Th17 responses were diminished by MAb treatment with anti-IL-12 p40. Together, these results indicate that Th2/Treg responses are mechanisms of protection against chronic S. aureus implant infection, as opposed to Th1/Th17 responses, which may play a role in the development of chronic infection.
- Mus musculus (House mouse),
- Immunology and Microbiology
Inhibition of IL-25/IL-17RA improves immune-related adverse events of checkpoint inhibitors and reveals antitumor activity.
In Journal for Immunotherapy of Cancer on 21 March 2024 by Hu, X., Bukhari, S. M., et al.
PubMed
Immune checkpoint inhibitors (ICIs) have improved outcomes and extended patient survival in several tumor types. However, ICIs often induce immune-related adverse events (irAEs) that warrant therapy cessation, thereby limiting the overall effectiveness of this class of therapeutic agents. Currently, available therapies used to treat irAEs might also blunt the antitumor activity of the ICI themselves. Therefore, there is an urgent need to identify treatments that have the potential to be administered alongside ICI to optimize their use. Using a translationally relevant murine model of anti-PD-1 and anti-CTLA-4 antibodies-induced irAEs, we compared the safety and efficacy of prednisolone, anti-IL-6, anti-TNFɑ, anti-IL-25 (IL-17E), and anti-IL-17RA (the receptor for IL-25) administration to prevent irAEs and to reduce tumor size. While all interventions were adequate to inhibit the onset of irAEs pneumonitis and hepatitis, treatment with anti-IL-25 or anti-IL-17RA antibodies also exerted additional antitumor activity. Mechanistically, IL-25/IL-17RA blockade reduced the number of organ-infiltrating lymphocytes. These findings suggest that IL-25/IL-17RA may serve as an additional target when treating ICI-responsive tumors, allowing for better tumor control while suppressing immune-related toxicities. © Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
- Cancer Research,
- Immunology and Microbiology,
- Cell Biology,
- Biochemistry and Molecular biology
Tumor cell senescence-induced macrophage CD73 expression is a critical metabolic immune checkpoint in the aging tumor microenvironment.
In Theranostics on 7 February 2024 by Deng, Y., Chen, Q., et al.
PubMed
Background: The role of senescent cells in the tumor microenvironment (TME) is usually bilateral, and diverse therapeutic approaches, such as radiotherapy and chemotherapy, can induce cellular senescence. Cellular interactions are widespread in the TME, and tumor cells reprogram immune cells metabolically by producing metabolites. However, how senescent cells remodel the metabolism of TME remains unclear. This study aimed to explore precise targets to enhance senescent cells-induced anti-tumor immunity from a metabolic perspective. Methods: The in vivo senescence model was induced by 8 Gy×3 radiotherapy or cisplatin chemotherapy, and the in vitro model was induced by 10 Gy-irradiation or cisplatin treatment. Metabonomic analysis and ELISA assay on tumor interstitial fluid were performed for metabolites screening. Marker expression and immune cell infiltration in the TME were analyzed by flow cytometry. Cell co-culture system and senescence-conditioned medium were used for crosstalk validation in vitro. RNA sequencing and rescue experiments were conducted for mechanism excavation. Immunofluorescence staining and single-cell transcriptome profiling analysis were performed for clinical validation. Results: We innovatively reveal the metabolic landscape of the senescent TME, characterized with the elevation of adenosine. It is attributed to the senescent tumor cell-induced CD73 upregulation of tumor-associated macrophages (TAMs). CD73 expression in TAMs is evoked by SASP-related pro-inflammatory cytokines, especially IL-6, and regulated by JAK/STAT3 pathway. Consistently, a positive correlation between tumor cells senescence and TAMs CD73 expression is identified in lung cancer clinical specimens and databases. Lastly, blocking CD73 in a senescent background suppresses tumors and activates CD8+ T cell-mediated antitumor immunity. Conclusions: TAMs expressed CD73 contributes significantly to the adenosine accumulation in the senescent TME, suggesting targeting CD73 is a novel synergistic anti-tumor strategy in the aging microenvironment. © The author(s).
- Mus musculus (House mouse),
- Immunology and Microbiology,
- Cell Biology
Resveratrol-βcd inhibited premature ovarian insufficiency progression by regulating granulosa cell autophagy.
In Journal of Ovarian Research on 15 January 2024 by Hu, B., Zheng, X., et al.
PubMed
The ovarian environment of premature ovarian insufficiency (POI) patients exhibits immune dysregulation, which leads to excessive secretion of numerous proinflammatory cytokines that affect ovarian function. An abnormal level of macrophage polarization directly or indirectly inhibits the differentiation of ovarian granulosa cells and steroid hormone production, ultimately leading to POI. Resveratrol, as a health supplement, has been widely recognized for its safety. There is a substantial amount of evidence indicating that resveratrol and its analogs possess significant immune-regulatory functions. It has also been reported that resveratrol can effectively inhibit the progression of POI. However, the underlying immunological and molecular mechanisms through which resveratrol inhibits the progression of POI are still unclear. Our preliminary reports have shown that resveratrol-βcd, the beta-cyclodextrin complex of resveratrol, significantly enhances the stability of resveratrol. Resveratrol-βcd could regulate the dysfunctional immune status of macrophages and T cells in the tumor microenvironment. In this study, we treated busulfan and cyclophosphamide (B/C)-treated mice, which were used as a POI model, with resveratrol-βcd. After resveratrol-βcd treatment, the levels of IL-6 in the ovaries were significantly increased, and the progression of POI was suppressed. IL-6 activated granulosa cells (GCs) through soluble IL-6R (sIL-6R), promoting autophagy in GCs. Resveratrol-βcd and IL-6 had a synergistic effect on enhancing autophagy in GCs and promoting E2 secretion. We partially elucidated the immune mechanism by which resveratrol inhibits the progression of POI and the autophagy-regulating function of GCs. This provides a theoretical basis for using resveratrol to prevent POI in future studies and clinical guidance. © 2024. The Author(s).
- Mus musculus (House mouse),
- Immunology and Microbiology
Elimination of Chlamydia muridarum from the female reproductive tract is IL-12p40 dependent, but independent of Th1 and Th2 cells.
In PLoS Pathogens on 1 January 2024 by Rixon, J. A., Fong, K. D., et al.
PubMed
Chlamydia vaccine approaches aspire to induce Th1 cells for optimal protection, despite the fact that there is no direct evidence demonstrating Th1-mediated Chlamydia clearance from the female reproductive tract (FRT). We recently reported that T-bet-deficient mice can resolve primary Chlamydia infection normally, undermining the potentially protective role of Th1 cells in Chlamydia immunity. Here, we show that T-bet-deficient mice develop robust Th17 responses and that mice deficient in Th17 cells exhibit delayed bacterial clearance, demonstrating that Chlamydia-specific Th17 cells represent an underappreciated protective population. Additionally, Th2-deficient mice competently clear cervicovaginal infection. Furthermore, we show that sensing of IFN-γ by non-hematopoietic cells is essential for Chlamydia immunity, yet bacterial clearance in the FRT does not require IFN-γ secretion by CD4 T cells. Despite the fact that Th1 cells are not necessary for Chlamydia clearance, protective immunity to Chlamydia is still dependent on MHC class-II-restricted CD4 T cells and IL-12p40. Together, these data point to IL-12p40-dependent CD4 effector maturation as essential for Chlamydia immunity, and Th17 cells to a lesser extent, yet neither Th1 nor Th2 cell development is critical. Future Chlamydia vaccination efforts will be more effective if they focus on induction of this protective CD4 T cell population. Copyright: © 2024 Rixon et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
- Mus musculus (House mouse),
- Genetics,
- Cancer Research
Adenosine A2A receptor is a tumor suppressor of NASH-associated hepatocellular carcinoma.
In Cell Reports Medicine on 19 September 2023 by Allard, B., Jacoberger-Foissac, C., et al.
PubMed
Inhibition of adenosine A2A receptor (A2AR) is a promising approach for cancer immunotherapy currently evaluated in several clinical trials. We here report that anti-obesogenic and anti-inflammatory functions of A2AR, however, significantly restrain hepatocellular carcinoma (HCC) development. Adora2a deletion in mice triggers obesity, non-alcoholic steatohepatitis (NASH), and systemic inflammation, leading to spontaneous HCC and promoting dimethylbenzyl-anthracene (DMBA)- or diethylnitrosamine (DEN)-induced HCC. Conditional Adora2a deletion reveals critical roles of myeloid and hepatocyte-derived A2AR signaling in restraining HCC by limiting hepatic inflammation and steatosis. Remarkably, the impact of A2AR pharmacological blockade on HCC development is dependent on pre-existing NASH. In support of our animal studies, low ADORA2A gene expression in human HCC is associated with cirrhosis, hepatic inflammation, and poor survival. Together, our study uncovers a previously unappreciated tumor-suppressive function for A2AR in the liver and suggests caution in the use of A2AR antagonists in patients with NASH and NASH-associated HCC. Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
- Cancer Research,
- Immunology and Microbiology
OX40L-Armed Oncolytic Virus Boosts T-cell Response and Remodels Tumor Microenvironment for Pancreatic Cancer Treatment.
In Theranostics on 9 August 2023 by Liu, S., Li, F., et al.
PubMed
Rationale: The resistance of pancreatic ductal adenocarcinoma (PDAC) to immunotherapies is caused by the immunosuppressive tumor microenvironment (TME) and dense extracellular matrix. Currently, the efficacy of an isolated strategy targeting stromal desmoplasia or immune cells has been met with limited success in the treatment of pancreatic cancer. Oncolytic virus (OV) therapy can remodel the TME and damage tumor cells either by directly killing them or by enhancing the anti-tumor immune response, which holds promise for the treatment of PDAC. This study aimed to investigate the therapeutic effect of OX40L-armed OV on PDAC and to elucidate the underlying mechanisms. Methods: Murine OX40L was inserted into herpes simplex virus-1 (HSV-1) to construct OV-mOX40L. Its expression and function were assessed using reporter cells, cytopathic effect, and immunogenic cell death assays. The efficacy of OV-mOX40L was then evaluated in a KPC syngeneic mouse model. Tumor-infiltrating immune and stromal cells were analyzed using flow cytometry and single-cell RNA sequencing to gain insight into the mechanisms of oncolytic virotherapy. Results: OV-mOX40L treatment delayed tumor growth in KPC tumor-bearing C57BL/6 mice. It also boosted the tumor-infiltrating CD4+ T cell response, mitigated cytotoxic T lymphocyte (CTL) exhaustion, and reduced the number of regulatory T cells. The treatment of OV-mOX40L reprogrammed macrophages and neutrophils to a more pro-inflammatory anti-tumor state. In addition, the number of myofibroblastic cancer-associated fibroblasts (CAF) was reduced after treatment. Based on single-cell sequencing analysis, OV-mOX40L, in combination with anti-IL6 and anti-PD-1, significantly extended the lifespan of PDAC mice. Conclusion: OV-mOX40L converted the immunosuppressive tumor immune microenvironment to a more activated state, remodeled the stromal matrix, and enhanced T cell response. OV-mOX40L significantly prolonged the survival of PDAC mice, either as a monotherapy or in combination with synergistic antibodies. Thus, this study provides a multimodal therapeutic strategy for pancreatic cancer treatment. © The author(s).
Hyaluronic acid-bilirubin nanomedicine-based combination chemoimmunotherapy.
In Nature Communications on 8 August 2023 by Lee, Y., Shinn, J., et al.
PubMed
Despite significant advances in immune checkpoint blockade (ICB), immunosuppression mediated by tumor-associated myeloid cells (TAMCs) poses a major barrier to cancer immunotherapy. In addition, while immunogenic cell death (ICD) provides a viable approach to inducing anti-tumor immune response, it remains unknown how to effectively trigger ICD while addressing immunosuppressive TAMCs. Here, we show that SC144, a gp130 inhibitor that blocks the IL-6/gp130/STAT3 pathway, induces ICD of tumor cells and polarizes macrophages to M1-phenotype in vitro. However, as SC144 also induces killing of CD8+ T-cells, we sought to deliver SC144 selectively to tumor cells and TAMCs. Toward this goal, we have developed hyaluronic acid-bilirubin nanoparticles (HABN) that accumulate in CD44hi tumor cells and TAMCs. Systemic administration of SC144 loaded in HABN (SC144@HABN) induces apoptosis and ICD of tumor cells, increases the ratio of M1-like to M2-like macrophages, and decreases the frequency of myeloid-derived suppressor cells and CD4+ regulatory T-cells, while promoting anti-tumor CD8+ T-cells. Moreover, SC144@HABN combined with anti-PD-L1 ICB efficiently eliminates MC38 tumors and ICB-resistant 4T1 tumors. Overall, our work demonstrates a therapeutic strategy based on coordinated ICD induction and TAMC modulation and highlights the potential of combination chemoimmunotherapy. © 2023. Springer Nature Limited.
- Immunology and Microbiology,
- Mus musculus (House mouse)
Early Infiltration of Innate Immune Cells to the Liver Depletes HNF4α and Promotes Extrahepatic Carcinogenesis.
In Cancer Discovery on 7 July 2023 by Goldman, O., Adler, L. N., et al.
PubMed
Multiple studies have identified metabolic changes within the tumor and its microenvironment during carcinogenesis. Yet, the mechanisms by which tumors affect the host metabolism are unclear. We find that systemic inflammation induced by cancer leads to liver infiltration of myeloid cells at early extrahepatic carcinogenesis. The infiltrating immune cells via IL6-pSTAT3 immune-hepatocyte cross-talk cause the depletion of a master metabolic regulator, HNF4α, consequently leading to systemic metabolic changes that promote breast and pancreatic cancer proliferation and a worse outcome. Preserving HNF4α levels maintains liver metabolism and restricts carcinogenesis. Standard liver biochemical tests can identify early metabolic changes and predict patients' outcomes and weight loss. Thus, the tumor induces early metabolic changes in its macroenvironment with diagnostic and potentially therapeutic implications for the host. Cancer growth requires a permanent nutrient supply starting from early disease stages. We find that the tumor extends its effect to the host's liver to obtain nutrients and rewires the systemic and tissue-specific metabolism early during carcinogenesis. Preserving liver metabolism restricts tumor growth and improves cancer outcomes. This article is highlighted in the In This Issue feature, p. 1501. ©2023 The Authors; Published by the American Association for Cancer Research.
- Mus musculus (House mouse),
- Cancer Research,
- Endocrinology and Physiology,
- Neuroscience
Schwann cell insulin-like growth factor receptor type-1 mediates metastatic bone cancer pain in mice.
In Brain, Behavior, and Immunity on 1 May 2023 by Landini, L., Marini, M., et al.
PubMed
Insulin growth factor-1 (IGF-1), an osteoclast-dependent osteolysis biomarker, contributes to metastatic bone cancer pain (MBCP), but the underlying mechanism is poorly understood. In mice, the femur metastasis caused by intramammary inoculation of breast cancer cells resulted in IGF-1 increase in femur and sciatic nerve, and IGF-1-dependent stimulus/non-stimulus-evoked pain-like behaviors. Adeno-associated virus-based shRNA selective silencing of IGF-1 receptor (IGF-1R) in Schwann cells, but not in dorsal root ganglion (DRG) neurons, attenuated pain-like behaviors. Intraplantar IGF-1 evoked acute nociception and mechanical/cold allodynia, which were reduced by selective IGF-1R silencing in DRG neurons and Schwann cells, respectively. Schwann cell IGF-1R signaling promoted an endothelial nitric oxide synthase-mediated transient receptor potential ankyrin 1 (TRPA1) activation and release of reactive oxygen species that, via macrophage-colony stimulating factor-dependent endoneurial macrophage expansion, sustained pain-like behaviors. Osteoclast derived IGF-1 initiates a Schwann cell-dependent neuroinflammatory response that sustains a proalgesic pathway that provides new options for MBCP treatment. Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
- Cancer Research,
- Immunology and Microbiology
Semi-supervised analysis of myeloid and T cell behavior in ex vivo ovarian tumor slices reveals changes in cell motility after treatments.
In IScience on 21 April 2023 by Lafôrets, F., Kotantaki, P., et al.
PubMed
Studies of the high-grade serous ovarian cancer (HGSOC) tumor microenvironment, the most lethal gynecological cancer, aim to enhance the efficiency of established therapies. Cell motility is an important process of anti-tumor response. Using ex vivo human and mouse HGSOC tumor slices combined with time-lapse imaging, we assessed the motility of CD8+ T and myeloid cells. We developed a semi-supervised analysis of cell movements, identifying four cell behaviors: migrating, long migrating, static, and wobbling. Tumor slices were maintained 24h ex vivo, retaining viability and cell movements. Ex vivo treatments with lipopolysaccharide altered CD8+ T and myeloid cell behavior. In vivo chemotherapy reduced ex vivo cell movements in human and mouse tumors and differentially affected CD8+ T and myeloid cells in chemo-sensitive and chemo-resistant mouse models. Ex vivo tumor slices can extend in vivo mouse studies to human, providing a stepping stone to translate mouse cancer studies to clinical trials. © 2023 The Authors.
IL-1/MyD88-Dependent G-CSF and IL-6 Secretion Mediates Postburn Anemia.
In The Journal of Immunology on 1 April 2023 by Noel, J. G., Ramser, S. W., et al.
PubMed
The anemia of critical illness (ACI) is a nearly universal pathophysiological consequence of burn injury and a primary reason burn patients require massive quantities of transfused blood. Inflammatory processes are expected to drive postburn ACI and prevent meaningful erythropoietic stimulation through iron or erythropoietin supplementation, but to this day no specific inflammatory pathways have been identified as a critical mechanism. In this study, we examined whether secretion of G-CSF and IL-6 mediates distinct features of postburn ACI and interrogated inflammatory mechanisms that could be responsible for their secretion. Our analysis of mouse and human skin samples identified the burn wound as a primary source of G-CSF and IL-6 secretion. We show that G-CSF and IL-6 are secreted independently through an IL-1/MyD88-dependent mechanism, and we ruled out TLR2 and TLR4 as critical receptors. Our results indicate that IL-1/MyD88-dependent G-CSF secretion plays a key role in impairing medullary erythropoiesis and IL-6 secretion plays a key role in limiting the access of erythroid cells to iron. Importantly, we found that IL-1α/β neutralizing Abs broadly attenuated features of postburn ACI that could be attributed to G-CSF or IL-6 secretion and rescued deficits of circulating RBC counts, hemoglobin, and hematocrit caused by burn injury. We conclude that wound-based IL-1/MyD88 signaling mediates postburn ACI through induction of G-CSF and IL-6 secretion. Copyright © 2023 by The American Association of Immunologists, Inc.
- Mus musculus (House mouse),
- Immunology and Microbiology
CD169+ macrophage intrinsic IL-10 production regulates immune homeostasis during sepsis.
In Cell Reports on 28 March 2023 by Yeung, S. T., Ovando, L. J., et al.
PubMed
Macrophages facilitate critical functions in regulating pathogen clearance and immune homeostasis in tissues. The remarkable functional diversity exhibited by macrophage subsets is dependent on tissue environment and the nature of the pathological insult. Our current knowledge of the mechanisms that regulate the multifaceted counter-inflammatory responses mediated by macrophages remains incomplete. Here, we report that CD169+ macrophage subsets are necessary for protection under excessive inflammatory conditions. We show that in the absence of these macrophages, even under mild septic conditions, mice fail to survive and exhibit increased production of inflammatory cytokines. Mechanistically, CD169+ macrophages control inflammatory responses via interleukin-10 (IL-10), as CD169+ macrophage-specific deletion of IL-10 was lethal during septic conditions, and recombinant IL-10 treatment reduced lipopolysaccharide (LPS)-induced lethality in mice lacking CD169+ macrophages. Collectively, our findings show a pivotal homeostatic role for CD169+ macrophages and suggest they may serve as an important target for therapy under damaging inflammatory conditions. Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
- Cancer Research,
- Immunology and Microbiology,
- Pharmacology
Pexidartinib synergize PD-1 antibody through inhibiting treg infiltration by reducing TAM-derived CCL22 in lung adenocarcinoma.
In Frontiers in Pharmacology on 28 March 2023 by Zhang, W., Jiang, X., et al.
PubMed
There is a crosstalk between Tumor-associated macrophages (TAM) and tumor-infiltrating T cells in tumor environment. TAM could inhibit the activity of cytotoxic T cells; TAM could also regulate the composition of T cells in tumor immune environment. The combination therapy for TAM and tumor infiltrated T cells has been widely noticed, but the crosstalk between TAM and tumor infiltrated T cells remains unclear in the process of combination therapy. We treated lung adenocarcinoma tumor models with pexidartinib, which targets macrophage colony stimulating factor receptor (M-CSFR) and c-kit tyrosine kinase, to inhibited TAM. Pexidartinib inhibited the ratio of macrophages in the tumor and also altered macrophage polarization. In addition to reprogram TAM, pexidartinib also changed the composition of tumor-invasive T cells. After pexidartinib treatment, the total number of T cells, CD8+ T cells and Treg cells were all decreased, the ratio of CD8+T/Treg increased significantly. According to the analysis of cytokines and chemokines during the treatment of pexidartinib, CCL22, as a chemokine for Treg recruitment, significantly decreased after the treatment of pexidartinib. Base on the above observation, the combination of pexidartinib and PD-1 antibody were used in the treatment of lung adenocarcinoma subcutaneous tumor model, the combination therapy has significantly improved the efficacy of tumor treatment compared with the monotherapy. Meanwhile, compared with pexidartinib monotherapy, the combination treatment further switches the polarization status of tumor-associated macrophages. In summary, our results showed that the combination of pexidartinib and PD-1 antibody showed a synergy and significantly improved the anti-tumor efficacy, through pexidartinib increasing CD8T/Treg ratio by reducing TAM-derived CCL22. Copyright © 2023 Zhang, Jiang, Zou, Yuan and Wang.
- Cancer Research,
- Neuroscience
Area postrema neurons mediate interleukin-6 function in cancer-associated cachexia
Preprint on BioRxiv : the Preprint Server for Biology on 13 January 2023 by Sun, Q., van de Lisdonk, D., et al.
PubMed
Interleukin-6 (IL-6) has been long considered a key player in cancer-associated cachexia 1-15 . It is believed that sustained elevation of IL-6 production during cancer progression causes brain dysfunctions, which ultimately result in cachexia 16-20 . However, how peripheral IL-6 influences the brain remains poorly understood. Here we show that neurons in the area postrema (AP), a circumventricular structure in the hindbrain, mediate the function of IL-6 in cancer-associated cachexia in mice. We found that circulating IL-6 can rapidly enter the AP and activate AP neurons. Peripheral tumor, known to increase circulating IL-6 1-5,15,18,21-23 , leads to elevated IL-6 and neuronal hyperactivity in the AP, and causes potentiated excitatory synaptic transmission onto AP neurons. Remarkably, neutralization of IL-6 in the brain of tumor-bearing mice with an IL-6 antibody prevents cachexia, reduces the hyperactivity in an AP network, and markedly prolongs lifespan. Furthermore, suppression of Il6ra , the gene encoding IL-6 receptor, specifically in AP neurons with CRISPR/dCas9 interference achieves similar effects. Silencing of Gfral-expressing AP neurons also ameliorates the cancer-associated cachectic phenotypes and AP network hyperactivity. Our study identifies a central mechanism underlying the function of peripheral IL-6, which may serve as a target for treating cancer-associated cachexia.
- Cancer Research,
- Immunology and Microbiology,
- Mus musculus (House mouse)
Blockade of IL-6 inhibits tumor immune evasion and improves anti-PD-1 immunotherapy.
In Cytokine on 1 October 2022 by Li, W., Wu, Z., et al.
PubMed
Long-standing inflammatory bowel disease predisposes to the development of colorectal cancer (CRC). Interleukin (IL) -6, a pivotal link between chronic inflammation and tumor progression, has recently been recognized as a potential therapeutic target. The effect of IL-6 on proliferation and metastasis of CRC by activating the STAT3 pathway has been widely demonstrated in recent years, but few on mediating tumor immune evasion. In this study, we found that IL-6 was remarkably overexpressed in CRC and its elevation was associated with a poor prognosis. We studied CRC tumorigenesis in vivo by inoculating MC38 tumors and induced-CRC model via AOM/DSS (azoxymethane/dextransulfate sodium) in IL-6 deficient (IL-6-/-) and wild-type (WT) mice and found that IL-6-/- mice were less susceptible to develop tumors, compared to WT mice. We detected CD8+ T cells via immunofluorescence and found they exhibit high expression in tumor of IL-6-/- mice. High level of IL-6 was found in colitis model, with down-regulation of MHC-I molecules. In in vitro experiments, we found that IL-6 may act as a negative regulator in IFNγ-STAT1-MHC-I signaling. In addition, vivo trials also confirmed that MHC-I mRNA level was negatively related to the existence of IL-6. Furthermore, the blockade of IL-6 also activated CD8+T-cell accumulation and led to the high PD-L1 expression in CRC, which can sensitize animals to anti-PD-1 therapy. Our study provides a research basis for the significant role of IL-6 in tumor evasion and highlights a novel target to improve the efficacy of immunotherapy. Copyright © 2022 Elsevier Ltd. All rights reserved.
- Mus musculus (House mouse),
- Stem Cells and Developmental Biology
The trophectoderm acts as a niche for the inner cell mass through C/EBPα-regulated IL-6 signaling.
In Stem Cell Reports on 13 September 2022 by Plana-Carmona, M., Stik, G., et al.
PubMed
IL-6 has been shown to be required for somatic cell reprogramming into induced pluripotent stem cells (iPSCs). However, how Il6 expression is regulated and whether it plays a role during embryo development remains unknown. Here, we describe that IL-6 is necessary for C/EBPα-enhanced reprogramming of B cells into iPSCs but not for B cell to macrophage transdifferentiation. C/EBPα overexpression activates both Il6 and Il6ra genes in B cells and in PSCs. In embryo development, Cebpa is enriched in the trophectoderm of blastocysts together with Il6, while Il6ra is mostly expressed in the inner cell mass (ICM). In addition, Il6 expression in blastocysts requires Cebpa. Blastocysts secrete IL-6 and neutralization of the cytokine delays the morula to blastocyst transition. The observed requirement of C/EBPα-regulated IL-6 signaling for pluripotency during somatic cell reprogramming thus recapitulates a physiologic mechanism in which the trophectoderm acts as niche for the ICM through the secretion of IL-6. Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
- WB,
- Mus musculus (House mouse),
- Cancer Research
Bone marrow-confined IL-6 signaling mediates the progression of myelodysplastic syndromes to acute myeloid leukemia.
In The Journal of Clinical Investigation on 1 September 2022 by Mei, Y., Ren, K., et al.
PubMed
Myelodysplastic syndromes (MDS) are age-related myeloid neoplasms with increased risk of progression to acute myeloid leukemia (AML). The mechanisms of transformation of MDS to AML are poorly understood, especially in relation to the aging microenvironment. We previously established an mDia1/miR-146a double knockout (DKO) mouse model phenocopying MDS. These mice develop age-related pancytopenia with oversecretion of proinflammatory cytokines. Here, we found that most of the DKO mice underwent leukemic transformation at 12-14 months of age. These mice showed myeloblast replacement of fibrotic bone marrow and widespread leukemic infiltration. Strikingly, depletion of IL-6 in these mice largely rescued the leukemic transformation and markedly extended survival. Single-cell RNA sequencing analyses revealed that DKO leukemic mice had increased monocytic blasts that were reduced with IL-6 knockout. We further revealed that the levels of surface and soluble IL-6 receptor (IL-6R) in the bone marrow were significantly increased in high-risk MDS patients. Similarly, IL-6R was also highly expressed in older DKO mice. Blocking of IL-6 signaling significantly ameliorated AML progression in the DKO model and clonogenicity of CD34-positive cells from MDS patients. Our study establishes a mouse model of progression of age-related MDS to AML and indicates the clinical significance of targeting IL-6 signaling in treating high-risk MDS.
- Cancer Research
Nucleolin Therapeutic Targeting Decreases Pancreatic Cancer Immunosuppression.
In Cancers on 31 August 2022 by Ponzo, M., Debesset, A., et al.
PubMed
The pancreatic ductal adenocarcinoma (PDAC) microenvironment is highly fibrotic and hypoxic, with poor immune cell infiltration. Recently, we showed that nucleolin (NCL) inhibition normalizes tumour vessels and impairs PDAC growth. Immunocompetent mouse models of PDAC were treated by the pseudopeptide N6L,amp;nbsp;which selectively inhibits NCL. Tumour-infiltrating immune cells and changes in the tumour microenvironment were analysed. N6L reduced the proportion of regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) and increased tumour-infiltrated T lymphocytes (TILs) with an activated phenotype. Low-dose anti-VEGFR2 treatment normalized PDAC vessels but did not modulate the immune suppressive microenvironment. RNAseq analysis of N6L-treated PDAC tumours revealed a reduction of cancer-associated fibroblast (CAF) expansion in vivo and in vitro. Notably, N6L treatment decreased IL-6 levels both in tumour tissues and in serum. Treating mPDAC by an antibody blocking IL-6 reduced the proportion of Tregs and MDSCs and increased the amount of TILs, thus mimicking the effects of N6L. These results demonstrate that NCL inhibition blocks the amplification of lymphoid and myeloid immunosuppressive cells and promotes T cell activation in PDAC through a new mechanism of action dependent on the direct inhibition of the tumoral stroma.
- Cancer Research,
- Immunology and Microbiology
Selective suppression of melanoma lacking IFN-γ pathway by JAK inhibition depends on T cells and host TNF signaling.
In Nature Communications on 25 August 2022 by Shen, H., Huang, F., et al.
PubMed
Therapeutic resistance to immune checkpoint blockers (ICBs) in melanoma patients is a pressing issue, of which tumor loss of IFN-γ signaling genes is a major underlying mechanism. However, strategies of overcoming this resistance mechanism have been largely elusive. Moreover, given the indispensable role of tumor-infiltrating T cells (TILs) in ICBs, little is known about how tumor-intrinsic loss of IFN-γ signaling (IFNγR1KO) impacts TILs. Here, we report that IFNγR1KO melanomas have reduced infiltration and function of TILs. IFNγR1KO melanomas harbor a network of constitutively active protein tyrosine kinases centered on activated JAK1/2. Mechanistically, JAK1/2 activation is mediated by augmented mTOR. Importantly, JAK1/2 inhibition with Ruxolitinib selectively suppresses the growth of IFNγR1KO but not scrambled control melanomas, depending on T cells and host TNF. Together, our results reveal an important role of tumor-intrinsic IFN-γ signaling in shaping TILs and manifest a targeted therapy to bypass ICB resistance of melanomas defective of IFN-γ signaling. © 2022. The Author(s).
- Biochemistry and Molecular biology,
- Cell Biology,
- Immunology and Microbiology
CD24-Siglec axis is an innate immune checkpoint against metaflammation and metabolic disorder.
In Cell Metabolism on 2 August 2022 by Wang, X., Liu, M., et al.
PubMed
The molecular interactions that regulate chronic inflammation underlying metabolic disease remain largely unknown. Since the CD24-Siglec interaction regulates inflammatory response to danger-associated molecular patterns (DAMPs), we have generated multiple mouse strains with single or combined mutations of Cd24 or Siglec genes to explore the role of the CD24-Siglec interaction in metaflammation and metabolic disorder. Here, we report that the CD24-Siglec-E axis, but not other Siglecs, is a key suppressor of obesity-related metabolic dysfunction. Inactivation of the CD24-Siglec-E pathway exacerbates, while CD24Fc treatment alleviates, diet-induced metabolic disorders, including obesity, dyslipidemia, insulin resistance, and nonalcoholic steatohepatitis (NASH). Mechanistically, sialylation-dependent recognition of CD24 by Siglec-E induces SHP-1 recruitment and represses metaflammation to protect against metabolic syndrome. A first-in-human study of CD24Fc (NCT02650895) supports the significance of this pathway in human lipid metabolism and inflammation. These findings identify the CD24-Siglec-E axis as an innate immune checkpoint against metaflammation and metabolic disorder and suggest a promising therapeutic target for metabolic disease. Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.