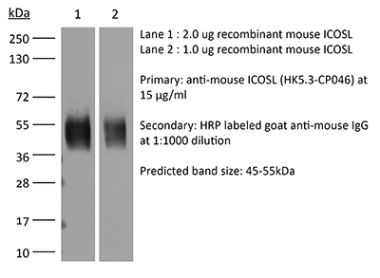
InVivoMAb anti-mouse ICOSL (CD275)
Product Details
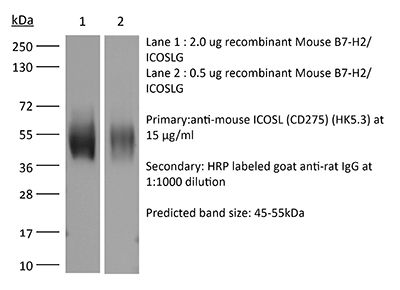
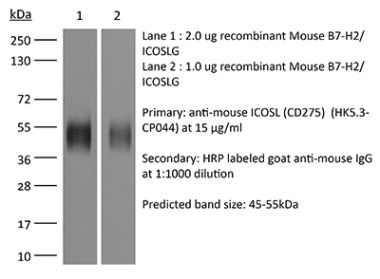
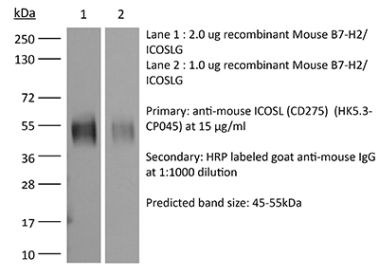
The HK5.3 monoclonal antibody reacts with mouse ICOSL (inducible T cell co-stimulator ligand) also known as CD275, B7RP-1, and B7-H2. ICOSL is a 40 kDa immune checkpoint protein belonging to the Ig receptor superfamily. Upon ICOSL binding, ICOS signaling co-stimulates T and B cell responses. The ligand Is expressed on antigen presenting cells including splenic B cells, dendritic cells, and macrophages. ICOS signaling is also thought to be important for maintaining regulatory T cell homeostasis. The HK5.3 antibody has been shown to block the binding of ICOSL to ICOS both in vitroand in vivo. HK5.3 treatment of mice has been reported to lead to a loss of regulatory T cells.Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse B7-RP1 transfected cell line |
| Reported Applications | in vivo ICOSL neutralization |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107566 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Additional Formats
Recommended Products
in vivo ICOSL neutralization
Stone, E. L., et al. (2015). "ICOS coreceptor signaling inactivates the transcription factor FOXO1 to promote Tfh cell differentiation" Immunity 42(2): 239-251. PubMed
T follicular helper (Tfh) cells are essential in the induction of high-affinity, class-switched antibodies. The differentiation of Tfh cells is a multi-step process that depends upon the co-receptor ICOS and the activation of phosphoinositide-3 kinase leading to the expression of key Tfh cell genes. We report that ICOS signaling inactivates the transcription factor FOXO1, and a Foxo1 genetic deletion allowed for generation of Tfh cells with reduced dependence on ICOS ligand. Conversely, enforced nuclear localization of FOXO1 inhibited Tfh cell development even though ICOS was overexpressed. FOXO1 regulated Tfh cell differentiation through a broad program of gene expression exemplified by its negative regulation of Bcl6. Final differentiation to germinal center Tfh cells (GC-Tfh) was instead FOXO1 dependent as the Foxo1(-/-) GC-Tfh cell population was substantially reduced. We propose that ICOS signaling transiently inactivates FOXO1 to initiate a Tfh cell contingency that is completed in a FOXO1-dependent manner.
in vivo ICOSL neutralization
Raynor, J., et al. (2015). "IL-6 and ICOS Antagonize Bim and Promote Regulatory T Cell Accrual with Age" J Immunol 195(3): 944-952. PubMed
Regulatory T cells (Tregs), a subset of CD4(+) T cells, dramatically accumulate with age in humans and mice and contribute to age-related immune suppression. Recently, we showed that a majority of accumulating Tregs in aged mice expressed low levels of CD25, and their accrual is associated with declining levels of IL-2 in aged mice. In this study, we further investigated the origin of CD25(lo) Tregs in aged mice. First, aged Tregs had high expression of neuropilin-1 and Helios, and had a broad Vbeta repertoire. Next, we analyzed the gene expression profile of Tregs, naive T cells, and memory T cells in aged mice. We found that the gene expression profile of aged CD25(lo) Tregs were more related to young CD25(lo) Tregs than to either naive or memory T cells. Further, the gene expression profile of aged Tregs was consistent with recently described “effector” Tregs (eTregs). Additional analysis revealed that nearly all Tregs in aged mice were of an effector phenotype (CD44(hi)CD62L(lo)) and could be further characterized by high levels of ICOS and CD69. ICOS contributed to Treg maintenance in aged mice, because in vivo Ab blockade of ICOSL led to a loss of eTregs, and this loss was rescued in Bim-deficient mice. Further, serum levels of IL-6 increased with age and contributed to elevated expression of ICOS on aged Tregs. Finally, Treg accrual was significantly blunted in aged IL-6-deficient mice. Together, our data show a role for IL-6 in promoting eTreg accrual with age likely through maintenance of ICOS expression.
in vivo ICOSL neutralization
Xin, L., et al. (2014). "Commensal microbes drive intestinal inflammation by IL-17-producing CD4+ T cells through ICOSL and OX40L costimulation in the absence of B7-1 and B7-2" Proc Natl Acad Sci U S A 111(29): 10672-10677. PubMed
The costimulatory B7-1 (CD80)/B7-2 (CD86) molecules, along with T-cell receptor stimulation, together facilitate T-cell activation. This explains why in vivo B7 costimulation neutralization efficiently silences a variety of human autoimmune disorders. Paradoxically, however, B7 blockade also potently moderates accumulation of immune-suppressive regulatory T cells (Tregs) essential for protection against multiorgan systemic autoimmunity. Here we show that B7 deprivation in mice overrides the necessity for Tregs in averting systemic autoimmunity and inflammation in extraintestinal tissues, whereas peripherally induced Tregs retained in the absence of B7 selectively mitigate intestinal inflammation caused by Th17 effector CD4(+) T cells. The need for additional immune suppression in the intestine reflects commensal microbe-driven T-cell activation through the accessory costimulation molecules ICOSL and OX40L. Eradication of commensal enteric bacteria mitigates intestinal inflammation and IL-17 production triggered by Treg depletion in B7-deficient mice, whereas re-establishing intestinal colonization with Candida albicans primes expansion of Th17 cells with commensal specificity. Thus, neutralizing B7 costimulation uncovers an essential role for Tregs in selectively averting intestinal inflammation by Th17 CD4(+) T cells with commensal microbe specificity.
in vivo ICOSL neutralization
Huang, W., et al. (2014). "IL-2-inducible T cell kinase tunes T regulatory cell development and is required for suppressive function" J Immunol 193(5): 2267-2272. PubMed
IL-2-inducible T cell kinase (ITK) is a key signaling mediator downstream of TCR, mediating T cell positive selection, as well as innate T cell and CD4(+) Th2/Th17 differentiation. In this article, we show that ITK also negatively tunes IL-2-induced expansion of conventional Foxp3-expressing regulatory T cells (Tregs). In vivo, Treg abundance is inversely correlated with ITK expression, and inducible Treg development is inversely dependent on ITK kinase activity. While Treg development normally requires both hematopoietic and thymic MHC class 2 (MHC2) expression, the absence of ITK allows Treg development with MHC2 expression in either compartment, with preference for selection by thymic MHC2, suggesting a gatekeeper role for ITK in ensuring that only Tregs selected by both thymic and hematopoietic MHC2 survive selection. Although ITK suppresses Treg development and is not required for maintenance of neuropilin-1-positive natural Tregs in the periphery, it is indispensable for Treg functional suppression of naive CD4(+) T cell-induced colitis in Rag(-/-) recipients. ITK thus regulates the development and function of Tregs.
in vivo ICOSL neutralization
Srivastava, S., et al. (2014). "Type I interferons directly inhibit regulatory T cells to allow optimal antiviral T cell responses during acute LCMV infection" J Exp Med 211(5): 961-974. PubMed
Regulatory T (T reg) cells play an essential role in preventing autoimmunity but can also impair clearance of foreign pathogens. Paradoxically, signals known to promote T reg cell function are abundant during infection and could inappropriately enhance T reg cell activity. How T reg cell function is restrained during infection to allow the generation of effective antiviral responses remains largely unclear. We demonstrate that the potent antiviral type I interferons (IFNs) directly inhibit co-stimulation-dependent T reg cell activation and proliferation, both in vitro and in vivo during acute infection with lymphocytic choriomeningitis virus (LCMV). Loss of the type I IFN receptor specifically in T reg cells results in functional impairment of virus-specific CD8(+) and CD4(+) T cells and inefficient viral clearance. Together, these data demonstrate that inhibition of T reg cells by IFNs is necessary for the generation of optimal antiviral T cell responses during acute LCMV infection.
in vivo ICOSL neutralization
Colino, J., et al. (2013). "Noncovalent association of protein and capsular polysaccharide on bacteria-sized latex beads as a model for polysaccharide-specific humoral immunity to intact gram-positive extracellular bacteria" J Immunol 191(6): 3254-3263. PubMed
Intact Streptococcus pneumoniae expressing type 14 capsular polysaccharide (PPS14) and type III S. agalactiae containing a PPS14 core capsule identical to PPS14 exhibit noncovalent associations of PPS14 and bacterial protein, in contrast to soluble covalent conjugates of these respective Ags. Both bacteria and conjugates induce murine PPS14-specific IgG responses dependent on CD4(+) T cells. Further, secondary immunization with conjugate and S. agalactiae, although not S. pneumoniae, results in a boosted response. However, in contrast to conjugate, PPS14-specific IgG responses to bacteria lack affinity maturation use the 44.1-idiotype and are dependent on marginal zone B cells. To better understand the mechanism underlying this dichotomy, we developed a minimal model of intact bacteria in which PPS14 and pneumococcal surface protein A (PspA) were stably attached to 1 mum (bacteria-sized) latex beads, but not directly linked to each other, in contrast to PPS14-PspA conjugate. Beads coated simultaneously with PPS14+, similar to conjugate, induced in mice boosted PPS14-specific IgG secondary responses, dependent on T cells and ICOS-dependent costimulation, and in which priming could be achieved with PspA alone. In contrast to conjugate, but similar to intact bacteria, the primary PPS14-specific IgG response to beads coated simultaneously with PPS14+ peaked rapidly, with the secondary response highly enriched for the 44.1-idiotype and lacking affinity maturation. These results demonstrate that noncovalent association in a particle, of polysaccharide and protein, recapitulates essential immunologic characteristics of intact bacteria that are distinct from soluble covalent conjugates of these respective Ags.
in vivo ICOSL neutralization
Baumjohann, D., et al. (2013). "Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype" Immunity 38(3): 596-605. PubMed
T follicular helper (Tfh) cells provide help to B cells and are crucial for establishment of germinal center (GC) reactions, including production of high-affinity antibodies and generation of memory B cells and long-lived plasma cells. Here we report that the magnitude of the Tfh cell response was dictated by the amount of antigen and directly correlated with the magnitude of the GC B cell response. In addition, maintenance of the Tfh cell phenotype required sustained antigenic stimulation by GC B cells. In lymphopenic conditions, a strong and prolonged Tfh cell response led to bystander B cell activation, hypergammaglobulinemia, and production of poly- and self-reactive antibodies. These data demonstrate that antigen dose determines the size and duration of the Tfh cell response and GC reaction, highlight the transient nature of the Tfh cell phenotype, and suggest a link between overstimulation of Tfh cells and the development of dysregulated humoral immune responses.
in vivo ICOSL neutralization
Arjunaraja, S., et al. (2012). "Structurally identical capsular polysaccharide expressed by intact group B streptococcus versus Streptococcus pneumoniae elicits distinct murine polysaccharide-specific IgG responses in vivo" J Immunol 188(11): 5238-5246. PubMed
We previously reported distinct differences in the murine in vivo Ig polysaccharide (PS)-specific responses to intact Streptococcus pneumoniae compared with responses to Neisseria meningitidis and that in each case, the bacterial subcapsular domain markedly influences the Ig response to the associated PS. In light of potentially unique contributions of biochemically distinct capsular PS and/or their characteristic attachments to the underlying bacterium, it remains unresolved whether different bacterial subcapsular domains can exert differential effects on PS-specific Ig responses to distinct bacterial pathogens. In this report, we used a mutant strain of group B Streptococcus (Streptococcus agalactiae) type III (GBS-III) that expresses desialylated capsular polysaccharide of GBS-III, biochemically identical to capsular pneumococcal polysaccharide type 14 (PPS14) of Streptococcus pneumoniae (intact inactivated Streptococcus pneumoniae, capsular type 14, Pn14), directly to compare the in vivo PPS14-specific IgG responses to two distinct gram-positive bacteria. Although both GBS-III and Pn14 elicited relatively rapid primary PPS14-specific IgG responses dependent on CD4(+) T cells, B7-dependent costimulation, and CD40-CD40L interactions, only GBS-III induced a highly boosted ICOS-dependent PPS14-specific IgG response after secondary immunization. Of note, priming with Pn14 and boosting with GBS-III, although not isolated PPS14, elicited a similar boosted PPS14-specific IgG response that was dependent on CD4(+) T cells during secondary immunization, indicating that Pn14 primes for memory but, unlike GBS-III, fails to elicit it. The inability of Pn14 to elicit a boosted PPS14-specific IgG response was overcome by coimmunization with unencapsulated GBS-III. Collectively, these data establish that structurally identical capsular PS expressed by two distinct gram-positive extracellular bacteria can indeed elicit distinct PS-specific IgG responses in vivo.
in vivo ICOSL neutralization
Arjunaraja, S., et al. (2012). "The nature of an in vivo anti-capsular polysaccharide response is markedly influenced by the composition and/or architecture of the bacterial subcapsular domain" J Immunol 188(2): 569-577. PubMed
In vivo anti-polysaccharide Ig responses to isolated polysaccharide (PS) are T cell independent, rapid, and fail to generate memory. However, little is known regarding PS-specific Ig responses to intact gram-positive and gram-negative extracellular bacteria. We previously demonstrated that intact heat-killed Streptococcus pneumoniae, a gram-positive bacterium, elicited a rapid primary pneumococcal capsular PS (PPS) response in mice that was dependent on CD4(+) T cells, B7-dependent costimulation, and CD40-CD40L interactions. However, this response was ICOS independent and failed to generate a boosted PPS-specific secondary IgG response. In the current study, we analyzed the murine meningococcal type C PS (MCPS)-specific Ig response to i.p.-injected intact, heat-killed Neisseria meningitidis, serogroup C (MenC), a gram-negative bacterium. In contrast to S. pneumoniae, the IgG anti-MCPS response to MenC exhibited delayed primary kinetics and was highly boosted after secondary immunization, whereas the IgG anti-MCPS response to isolated MCPS was rapid, without secondary boosting, and consisted of only IgG1 and IgG3, as opposed to all four IgG isotypes in response to intact MenC. The secondary, but not primary, IgG anti-MCPS response to MenC was dependent on CD4(+) T cells, CD40L, CD28, and ICOS. The primary and secondary IgG anti-MCPS responses were lower in TLR4-defective (C3H/HeJ) but not TLR2(-/-) or MyD88(-/-) mice, but secondary boosting was still observed. Of interest, coimmunization of S. pneumoniae and MenC resulted in a boosted secondary IgG anti-PPS response to S. pneumoniae. Our data demonstrate that the nature of the in vivo anti-PS response is markedly influenced by the composition and/or architecture of the bacterial subcapsular domain.
in vivo ICOSL neutralization
Choi, Y. S., et al. (2011). "ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6" Immunity 34(6): 932-946. PubMed
The nature of follicular helper CD4(+) T (Tfh) cell differentiation remains controversial, including the minimal signals required for Tfh cell differentiation and the time at which Tfh cell differentiation occurs. Here we determine that Tfh cell development initiates immediately during dendritic cell (DC) priming in vivo. We demonstrate that inducible costimulator (ICOS) provides a critical early signal to induce the transcription factor Bcl6, and Bcl6 then induces CXCR5, the canonical feature of Tfh cells. Strikingly, a bifurcation between Tfh and effector Th cells was measurable by the second cell division of CD4(+) T cells, at day 2 after an acute viral infection: IL2Ralpha(int) cells expressed Bcl6 and CXCR5 (Tfh cell program), whereas IL2Ralpha(hi) cells exhibited strong Blimp1 expression that repressed Bcl6 (effector Th cell program). Virtually complete polarization between Bcl6(+) Tfh cells and Blimp1(+) effector Th cell populations developed by 72 hr, even without B cells. Tfh cells were subsequently lost in the absence of B cells, demonstrating a B cell requirement for maintenance of Bcl6 and Tfh cell commitment via sequential ICOS signals.
- Immunology and Microbiology,
- Stem Cells and Developmental Biology,
- Mus musculus (House mouse)
Senescence-induced endothelial phenotypes underpin immune-mediated senescence surveillance.
In Genes and Development on 1 May 2022 by Yin, K., Patten, D. A., et al.
PubMed
Senescence is a stress-responsive tumor suppressor mechanism associated with expression of the senescence-associated secretory phenotype (SASP). Through the SASP, senescent cells trigger their own immune-mediated elimination, which if evaded leads to tumorigenesis. Senescent parenchymal cells are separated from circulating immunocytes by the endothelium, which is targeted by microenvironmental signaling. Here we show that SASP induces endothelial cell NF-κB activity and that SASP-induced endothelial expression of the canonical NF-κB component Rela underpins senescence surveillance. Using human liver sinusoidal endothelial cells (LSECs), we show that SASP-induced endothelial NF-κB activity regulates a conserved transcriptional program supporting immunocyte recruitment. Furthermore, oncogenic hepatocyte senescence drives murine LSEC NF-κB activity in vivo. Critically, we show two distinct endothelial pathways in senescence surveillance. First, endothelial-specific loss of Rela prevents development of Stat1-expressing CD4+ T lymphocytes. Second, the SASP up-regulates ICOSLG on LSECs, with the ICOS-ICOSLG axis contributing to senescence cell clearance. Our results show that the endothelium is a nonautonomous SASP target and an organizing center for immune-mediated senescence surveillance. © 2022 Yin et al.; Published by Cold Spring Harbor Laboratory Press.
- Immunology and Microbiology,
- Neuroscience,
- Mus musculus (House mouse)
Engagement of the costimulatory molecule ICOS in tissues promotes establishment of CD8+ tissue-resident memory T cells.
In Immunity on 11 January 2022 by Peng, C., Huggins, M. A., et al.
PubMed
Elevated gene expression of the costimulatory receptor Icos is a hallmark of CD8+ tissue-resident memory (Trm) T cells. Here, we examined the contribution of ICOS in Trm cell differentiation. Upon transfer into WT mice, Icos-/- CD8+ T cells exhibited defective Trm generation but produced recirculating memory populations normally. ICOS deficiency or ICOS-L blockade compromised establishment of CD8+ Trm cells but not their maintenance. ICOS ligation during CD8+ T cell priming did not determine Trm induction; rather, effector CD8+ T cells showed reduced Trm differentiation after seeding into Icosl-/- mice. IcosYF/YF CD8+ T cells were compromised in Trm generation, indicating a critical role for PI3K signaling. Modest transcriptional changes in the few Icos-/- Trm cells suggest that ICOS-PI3K signaling primarily enhances the efficiency of CD8+ T cell tissue residency. Thus, local ICOS signaling promotes production of Trm cells, providing insight into the contribution of costimulatory signals in the generation of tissue-resident populations. Copyright © 2021 Elsevier Inc. All rights reserved.
- Immunology and Microbiology
ICOS expression is required for maintenance but not the formation of germinal centers in the spleen in response to i>P. yoelii/i> infection
Preprint on BioRxiv : the Preprint Server for Biology on 25 August 2021 by O’Neal, K. A., Latham, L. E., et al.
PubMed
Inducible T cell co-stimulator (ICOS) plays a key role in the differentiation and maintenance of follicular helper T (Tfh) cells and thus germinal center (GC) formation. Previously, our lab showed in a Plasmodium chabaudi infection model that Icos -/- mice did not form GCs despite a persistent infection and thus a continued antigen (Ag) load. Here, we show that resolution of a primary infection with P. yoelii , was delayed in Icos -/- mice. This phenotype was associated with a reduction in the accumulation of Tfh-like and GC Tfh cells and an early deficiency in Ag-specific antibody (Ab) production. However, Icos -/- mice maintained their ability to form GCs, though they were less frequent in number than in wild-type (WT) mice. Furthermore, while Ab production in Icos -/- mice matched that of WT mice after the infection cleared, the Abs lacked signs of affinity maturation, suggesting functional defects associated with these GCs. Eventually, these GC structures dissipated more rapidly in Icos -/- mice than in WT mice. Moreover, the ability of Icos -/- mice to form these GC structures is not reliant on the high Ag load associated with P. yoelii infections, as GC formation was preserved in Icos -/- mice treated with early with atovaquone. Finally, mice were unable to form secondary GCs in the absence of ICOS after re-challenge. Overall, these data demonstrate the necessity of ICOS in the maintenance of Tfh cells, the formation and maintenance of sufficient numbers of functioning GCs, and the ability to generate new GC structures after re-infection with P. yoelii .
- Immunology and Microbiology
CTLA4-Ig-Based Bifunctional Costimulation Inhibitor Blocks CD28 and ICOS Signaling to Prevent T Cell Priming and Effector Function.
In The Journal of Immunology on 1 March 2021 by Goenka, R., Xu, Z., et al.
PubMed
CTLA4-Ig/abatacept dampens activation of naive T cells by blocking costimulation via CD28. It is an approved drug for rheumatoid arthritis but failed to deliver efficacy in a number of other autoimmune diseases. One explanation is that activated T cells rely less on CD28 signaling and use alternate coreceptors for effector function. ICOS is critical for activation of T-dependent humoral immune responses, which drives pathophysiology of IgG-mediated autoimmune diseases. In this study, we asked whether CD28 and ICOS play nonredundant roles for maintenance of T-dependent responses in mouse models. Using a hapten-protein immunization model, we show that during an ongoing germinal center response, combination treatment with CTLA4-Ig and ICOS ligand (ICOSL) blocking Ab completely dissolves ongoing germinal center responses, whereas single agents show only partial activity. Next, we took two approaches to engineer a therapeutic molecule that blocks both pathways. First, we engineered CTLA4-Ig to enhance binding to ICOSL while retaining affinity to CD80/CD86. Using a library approach, binding affinity of CTLA4-Ig to human ICOSL was increased significantly from undetectable to 15-42 nM; however, the affinity was still insufficient to completely block binding of ICOSL to ICOS. Second, we designed a bispecific costimulation inhibitor with high-affinity CTLA4 extracellular domains fused to anti-ICOSL Ab termed bifunctional costimulation inhibitor. With this bispecific approach, we achieved complete inhibition of CD80 and CD86 binding to CD28 as well as ICOS binding to ICOSL. Such bispecific molecules may provide greater therapeutic benefit in IgG-mediated inflammatory diseases compared with CTLA4-Ig alone. Copyright © 2021 by The American Association of Immunologists, Inc.
- In Vivo,
- Mus musculus (House mouse),
- Immunology and Microbiology
Cytomegalovirus restricts ICOSL expression on antigen-presenting cells disabling T cell co-stimulation and contributing to immune evasion.
In eLife on 18 January 2021 by Angulo, G., Zeleznjak, J., et al.
PubMed
Viral infections are controlled, and very often cleared, by activated T lymphocytes. The inducible co-stimulator (ICOS) mediates its functions by binding to its ligand ICOSL, enhancing T-cell activation and optimal germinal center (GC) formation. Here, we show that ICOSL is heavily downmodulated during infection of antigen-presenting cells by different herpesviruses. We found that, in murine cytomegalovirus (MCMV), the immunoevasin m138/fcr-1 physically interacts with ICOSL, impeding its maturation and promoting its lysosomal degradation. This viral protein counteracts T-cell responses, in an ICOS-dependent manner, and limits virus control during the acute MCMV infection. Additionally, we report that blockade of ICOSL in MCMV-infected mice critically regulates the production of MCMV-specific antibodies due to a reduction of T follicular helper and GC B cells. Altogether, these findings reveal a novel mechanism evolved by MCMV to counteract adaptive immune surveillance, and demonstrates a role of the ICOS:ICOSL axis in the host defense against herpesviruses. © 2021, Angulo et al.
- Mus musculus (House mouse),
- Immunology and Microbiology
Host immunology and rational immunotherapy for carbapenem-resistant Klebsiella pneumoniae infection.
In JCI Insight on 23 April 2020 by Iwanaga, N., Sandquist, I., et al.
PubMed
Infections due to carbapenem-resistant Klebsiella pneumoniae have emerged as a global threat due to its widespread antimicrobial resistance. Transplant recipients and patients with hematologic malignancies have high mortality rate, suggesting host factors in susceptibility. We developed a model of pulmonary infection using ST258 strain C4, KPC-2 clone, which are predominant K. pneumoniae carbapenemase-producing (KPC-producing) bacteria, and demonstrated that Rag2-/- Il2rg-/- mice - but not WT C57BL/6 or Rag2-/- mice - were susceptible to this opportunistic infection. Using single cell RNA sequencing in infected Rag2-/- mice, we identified distinct clusters of Ifng+ NK cells and Il17a+, Il22+, and inducible T cell costimulatory molecule-positive (ICOS+) group 3 innate lymphoid cells (ILCs) that were critical for host resistance. As solid organ transplantation is a risk factor, we generated a more clinically relevant model using FK506 in WT C57BL/6 mice. We further demonstrated that immunotherapy with recombinant IL-22 treatment ameliorated the ST258 pulmonary infection in both FK506-treated WT mice and Rag2-/- Il2rg-/- mice via hepatic IL-22ra1 signaling. These data support the development of host-directed immunotherapy as an adjunct treatment to new antibiotics.
- Block,
- Mus musculus (House mouse),
- Immunology and Microbiology
ICOS signaling promotes a secondary humoral response after re-challenge with Plasmodium chabaudi chabaudi AS.
In PLoS Pathogens on 1 April 2020 by Latham, L. E., Wikenheiser, D. J., et al.
PubMed
The co-stimulatory molecule ICOS is associated with the induction and regulation of T helper cell responses, including the differentiation of follicular helper T (Tfh) cells and the formation and maintenance of memory T cells. However, the role of ICOS signaling in secondary immune responses is largely unexplored. Here we show that memory T cell formation and maintenance are influenced by persistent infection with P. chabaudi chabaudi AS infection, as memory T cell numbers decline in wild-type and Icos-/- mice after drug-clearance. Following drug-clearance Icos-/- mice display a relapsing parasitemia that occurs more frequently and with higher peaks compared to wild-type mice after re-challenge. The secondary immune response in Icos-/- mice is characterized by significant impairment in the expansion of effector cells with a Tfh-like phenotype, which is associated with a diminished and delayed parasite-specific Ab response and the absence of germinal centers. Similarly, the administration of an anti-ICOSL antagonizing antibody to wild-type mice before and after reinfection with P. c. chabaudi AS leads to an early defect in Tfh cell expansion and parasite-specific antibody production, confirming a need for ICOS-ICOSL interactions to promote memory B cell responses. Furthermore, adoptive transfer of central memory T (TCM) cells from wild-type and Icos-/- mice into tcrb-/- mice to directly evaluate the ability of TCM cells to give rise to Tfh cells revealed that TCM cells from wild-type mice acquire a mixed Th1- and Tfh-like phenotype after P. c. chabaudi AS infection. While TCM cells from Icos-/- mice expand and display markers of activation to a similar degree as their WT counterparts, they displayed a reduced capacity to upregulate markers indicative of a Tfh cell phenotype, resulting in a diminished humoral response. Together these findings verify that ICOS signaling in memory T cells plays an integral role in promoting T cell effector responses during secondary infection with P. c. chabaudi AS.
- Neutralization,
- Mus musculus (House mouse),
- Immunology and Microbiology
Complement Signals Determine Opposite Effects of B Cells in Chemotherapy-Induced Immunity.
In Cell on 19 March 2020 by Lu, Y., Zhao, Q., et al.
PubMed
Understanding molecular mechanisms that dictate B cell diversity is important for targeting B cells as anti-cancer treatment. Through the single-cell dissection of B cell heterogeneity in longitudinal samples of patients with breast cancer before and after neoadjuvant chemotherapy, we revealed that an ICOSL+ B cell subset emerges after chemotherapy. Using three immunocompetent mouse models, we recapitulated the subset switch of human tumor-infiltrating B cells during chemotherapy. By employing B-cell-specific deletion mice, we showed that ICOSL in B cells boosts anti-tumor immunity by enhancing the effector to regulatory T cell ratio. The signature of ICOSL+ B cells is imprinted by complement-CR2 signaling, which is triggered by immunogenic cell death. Moreover, we identified that CD55, a complement inhibitory protein, determines the opposite roles of B cells in chemotherapy. Collectively, we demonstrated a critical role of the B cell subset switch in chemotherapy response, which has implications in designing novel anti-cancer therapies. VIDEO ABSTRACT.Copyright © 2020 Elsevier Inc. All rights reserved.
- Immunology and Microbiology
B cells are sufficient to prime the dominant CD4+ Tfh response to Plasmodium infection.
In The Journal of Experimental Medicine on 3 February 2020 by Arroyo, E. N. & Pepper, M.
PubMed
CD4+ T follicular helper (Tfh) cells dominate the acute response to a blood-stage Plasmodium infection and provide signals to direct B cell differentiation and protective antibody expression. We studied antigen-specific CD4+ Tfh cells responding to Plasmodium infection in order to understand the generation and maintenance of the Tfh response. We discovered that a dominant, phenotypically stable, CXCR5+ Tfh population emerges within the first 4 d of infection and results in a CXCR5+ CCR7+ Tfh/central memory T cell response that persists well after parasite clearance. We also found that CD4+ T cell priming by B cells was both necessary and sufficient to generate this Tfh-dominant response, whereas priming by conventional dendritic cells was dispensable. This study provides important insights into the development of CD4+ Tfh cells during Plasmodium infection and highlights the heterogeneity of antigen-presenting cells involved in CD4+ T cell priming. © 2019 Arroyo and Pepper.
- Genetics,
- Immunology and Microbiology
Histone methyltransferase Nsd2 is required for follicular helper T cell differentiation.
In The Journal of Experimental Medicine on 6 January 2020 by Long, X., Zhang, L., et al.
PubMed
Follicular helper T (Tfh) cells provide essential help for humoral immune response. Transcriptional factor Bcl6 is the master regulator for Tfh generation and is induced very early after T cell activation in a CD28-dependent manner, but how CD28 signal promotes Bcl6 early expression remains unknown. Here we found that CD28 signal quickly induces expression of the H3K36me2 methytransferase Nsd2, which is required for Bcl6 expression as early as the first cell division after T cell activation. Nsd2 deficiency in T cells leads to decreased Bcl6 expression, impaired Tfh generation, compromised germinal center response, and delayed virus clearance. Ectopic Bcl6 expression rescues the Tfh defect of Nsd2 KO cells. ICOS signal is dispensable for early Nsd2 induction but required for sustained Nsd2 expression, which is critical for Tfh maintenance. Overexpression of Nsd2 increases Bcl6 expression and enhances Tfh generation; 4-mo-old mice even develop spontaneous Tfh. Overall, our study reveals Nsd2 as a critical epigenetic regulator for Tfh differentiation. © 2019 Long et al.
- Neuroscience
Protection of bona fide T follicular memory cells during tissue isolation reveals their persistence, plasticity and functional impact
Preprint on BioRxiv : the Preprint Server for Biology on 23 June 2019 by Künzli, M., Schreiner, D., et al.
PubMed
h4>SUMMARY/h4> CD4 memory T cells play an important role in protective immunity and are a key target in vaccine development. Many studies have focused on T central memory (TCM) cells, while the existence and functional significance of T follicular helper (TFH) memory cells is controversial. Here we show that TFH memory cells are highly susceptible to NAD induced cell death (NICD) during isolation from tissues, leading to their under-representation in prior studies. NICD blockade reveals the persistence of abundant TFH memory cells, with high expression of hallmark TFH markers, that persist to at least 400 days after infection, by which time TCM cells are no longer found. Using single cell RNA-seq we demonstrate that TFH memory cells are transcriptionally distinct from TCM cells, maintain stemness and self-renewal gene expression, and, in contrast to TCM cells, are multipotent following recall. Surprisingly, TFH memory cells concurrently express a distinct glycolytic signature similar to trained immune cells, including elevated expression of mTOR, HIF-1 and cAMP regulated genes. Late disruption of glycolysis/ICOS signaling leads to TFH memory cell depletion concomitant with decreased splenic plasma cells and circulating antibody titers, demonstrating both unique homeostatic regulation of memory TFH and their sustained function during the memory phase of the immune response. These results highlight the metabolic heterogeneity underlying distinct memory T cell subsets and establish TFH memory cells as an attractive target for the induction of long-lived adaptive immunity. h4>HIGHLIGHTS/h4> Cell death during isolation from the tissue prevents the full recovery of TFH memory cells TFH memory cells are transcriptionally distinct from TCM cells and maintain a broader recall capacity TFH memory cells are maintained in the absence of antigen but require ICOS signaling and glycolysis TFH memory cells support late phase antibody production by splenic plasma cells
Food antigens drive spontaneous IgE elevation in the absence of commensal microbiota.
In Science Advances on 1 May 2019 by Hong, S. W., O, E., et al.
PubMed
Immunoglobulin E (IgE), a key mediator in allergic diseases, is spontaneously elevated in mice with disrupted commensal microbiota such as germ-free (GF) and antibiotics-treated mice. However, the underlying mechanisms for aberrant IgE elevation are still unclear. Here, we demonstrate that food antigens drive spontaneous IgE elevation in GF and antibiotics-treated mice by generating T helper 2 (TH2)-skewed T follicular helper (TFH) cells in gut-associated lymphoid tissues (GALTs). In these mice, depriving contact with food antigens results in defective IgE elevation as well as impaired generation of TFH cells and IgE-producing cells in GALT. Food antigen-driven TFH cells in GF mice are mostly generated in early life, especially during the weaning period. We also reveal that food antigen-driven TFH cells in GF mice are actively depleted by colonization with commensal microbiota. Thus, our findings provide a possible explanation for why the perturbation of commensal microbiota in early life increases the occurrence of allergic diseases.
- Cancer Research,
- Immunology and Microbiology
Acute Myeloid Leukemia Cells Express ICOS Ligand to Promote the Expansion of Regulatory T Cells.
In Frontiers in Immunology on 16 October 2018 by Han, Y., Dong, Y., et al.
PubMed
CD4+CD25+Foxp3+ regulatory T cells (Tregs) accumulate in bone marrow microenvironment in acute myeloid leukemia (AML). However, little is known about how the tumor environment including tumor cells themselves affects this process. Here we demonstrated that AML cells expressed inducible T-cell costimulator ligand (ICOSL) that can provide costimulation through ICOS for the conversion and expansion of Tregs sustaining high Foxp3 and CD25 expression as well as a suppressive function. TNF-a stimulation up-regulated the expression of ICOSL. Furthermore, both the conversion and expansion of CD4+CD25+Foxp3+ T cells and CD4+ICOS+Foxp3+ T cells were induced by co-culture with AML cells overexpressed ICOSL. CD4+CD25+ICOS+ T cells possessed stronger ability to secrete IL-10 than CD4+CD25+ICOS- T cells. The mechanism by which IL-10 promoted the proliferation of AML cells was dependent on the activation of the Akt, Erk1/2, p38, and Stat3 signaling pathways. Blockade of ICOS signaling using anti-ICOSL antibody impaired the generation of Tregs and retarded the progression of an AML mice model injected with C1498 cells. The expression of ICOSL of patient AML cells and ICOS+ Tregs were found to be predictors for overall survival and disease-free survival in patients with AML, with ICOS+ Treg cell subset being a stronger predictor than total Tregs. These results suggest that ICOSL expression by AML cells may directly drive Treg expansion as a mechanism of immune evasion and ICOS+ Treg cell frequency is a better prognostic predictor in patients with AML.
- Immunology and Microbiology,
- In Vivo,
- Mus musculus (House mouse)
Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis.
In Nature Communications on 15 March 2018 by Gaddis, D. E., Padgett, L. E., et al.
PubMed
Regulatory T (Treg) cells contribute to the anti-inflammatory response during atherogenesis. Here we show that during atherogenesis Treg cells lose Foxp3 expression and their immunosuppressive function, leading to the conversion of a fraction of these cells into T follicular helper (Tfh) cells. We show that Tfh cells are pro-atherogenic and that their depletion reduces atherosclerosis. Mechanistically, the conversion of Treg cells to Tfh cells correlates with reduced expression of IL-2Rα and pSTAT5 levels and increased expression of IL-6Rα. In vitro, incubation of naive T cells with oxLDL prevents their differentiation into Treg cells. Furthermore, injection of lipid-free Apolipoprotein AI (ApoAI) into ApoE-/- mice reduces intracellular cholesterol levels in Treg cells and prevents their conversion into Tfh cells. Together our results suggest that ApoAI, the main protein in high-density lipoprotein particles, modulates the cellular fate of Treg cells and thus influences the immune response during atherosclerosis.
Therapeutic ICOS blockade reduces T follicular helper cells and improves allergic airway disease
Preprint on BioRxiv : the Preprint Server for Biology on 18 January 2018 by Uwadiae, F. I., Pyle, C., et al.
PubMed
h4>ABSTRACT/h4> Allergic asthma is a disease of chronic airway inflammation and remodelling, characterised by a dysregulated type 2 response and allergen-specific IgE. T follicular helper cells (T FH ) are critical to antibody production and have recently been implicated in allergic airway disease (AAD) pathogenesis. The role of T FH in established disease and the therapeutic potential of targeting them are however not fully understood. Using two aeroallergen driven murine models of chronic AAD, T FH were first identified in the lung draining lymph nodes but with prolonged exposure were present in the lung itself. Sustained allergen exposure led to the accumulation of T FH , and concomitant development of germinal centre B cells. Blockade of Inducible T cell co-stimulator (ICOS) signalling during established AAD depleted T FH without adversely affecting the differentiation of other CD4 + T cell subsets. This resulted in impaired germinal centre responses, reduced allergen specific IgE and ameliorated inflammation and airway hyper-responsiveness, including reduced pulmonary IL-13. T FH did not however appear to produce IL-13 directly, suggesting they indirectly promote type-2 inflammation in the lungs. These data show that T FH play a pivotal role in the regulation of AAD and that targeting the ICOS-L pathway could represent a novel therapeutic approach in this disease.
- Immunology and Microbiology
Foxp1 Negatively Regulates T Follicular Helper Cell Differentiation and Germinal Center Responses by Controlling Cell Migration and CTLA-4.
In The Journal of Immunology on 15 January 2018 by Shi, B., Geng, J., et al.
PubMed
T follicular helper (Tfh) cells play an essential role in the formation of germinal centers (GC) and generation of high-affinity Abs. The homing of activated CD4+ T cells into B cell follicles and the involvement of key costimulatory and coinhibitory molecules are critical in controlling both the initiation and the magnitude of GC responses. Meanwhile, studies have shown that a high number of single clone B cells leads to intraclonal competition, which inhibits the generation of high-affinity Abs. Our previous work has shown that transcription factor Foxp1 is a critical negative regulator of Tfh cell differentiation. In this study, we report that the deletion of Foxp1 leads to a high proportion of activated CD4+ T cells homing into B cell follicles with faster kinetics, resulting in earlier GC formation. In addition, we show that Foxp1-deficient Tfh cells restore the generation of high-affinity Abs when cotransferred with high numbers of single clone B cells. We find that Foxp1 regulates the expression levels of cytotoxic T lymphocyte-associated Ag-4 (CTLA-4) in activated CD4+ T cells and that Ctla4 is a direct Foxp1 target. Finally, we demonstrate that CTLA-4 expression on conventional CD4+ T cells plays a cell-intrinsic role in Tfh cell differentiation in vivo, and CTLA-4 blockade helps abolish the intraclonal competition of B cells in generating high-affinity Abs. Copyright © 2018 by The American Association of Immunologists, Inc.
- Immunology and Microbiology
The strength of BCR signaling shapes terminal development of follicular helper T cells in mice.
In European Journal of Immunology on 1 August 2017 by Sacquin, A., Gador, M., et al.
PubMed
Antibody production is key for effective immune response and relies on follicular helper T (Tfh) cells. B cell-Tfh cell interactions result either in an extra-follicular low affinity B-cell response or in germinal center reactions producing high-affinity memory B cells and long-lived plasma cells. As Tfh cells influence B-cell commitment, it also became clear that B cells influence these interactions in ways that still remain unresolved. We observed that strong BCR signals decreased Tfh-cell differentiation in vitro, which correlated with decreased expression of ICOS-L at the surface of stimulated B cells. Further, we comprehensively demonstrated that ICOS-L expression correlated with the level of Tfh differentiation irrespective of antigen presentation at the surface of activated B cells. Our in vivo experiments could show that immunization with a high-affinity antigen for B cells resulted in much less Tfh development than immunization with low-affinity antigen. Furthermore, blocking ICOS-L in vivo inhibited Tfh development when using low-affinity antigen. Altogether, these results indicate that BCR affinity shapes Tfh-cell development in part through ICOS/ICOS-L interactions. Ultimately, we reveal new depths in the B cell-Tfh cell crosstalk that could eventually result in better vaccine protocols. © 2017 WILEY-VCH Verlag GmbH Co. KGaA, Weinheim.
- Immunology and Microbiology
Activation and Functional Specialization of Regulatory T Cells Lead to the Generation of Foxp3 Instability.
In The Journal of Immunology on 1 April 2017 by Zhang, Z., Zhang, W., et al.
PubMed
Accumulating evidence suggests that Foxp3+ cells can downregulate the expression of Foxp3, but whether thymically derived regulatory T cells (tTregs; especially committed tTregs) are capable of downregulating Foxp3 expression and being reprogrammed into other T effector cells remains controversial. Using a novel tTreg lineage-tracing mouse line, we were able to label epigenetically stable Foxp3+ cells derived from the thymus and demonstrate that mature tTregs are stable under homeostatic conditions. However, TCR engagement and sequential functional specialization of tTregs led to the generation of Foxp3 instability and reprogramming into the Th lineage. We further demonstrated that the signal switch from IL-2 to ICOS during Treg activation induced Treg instability and reprogramming. By using a dual lineage tracing model, we demonstrated that effector Tregs can revert to central Tregs, and this reversion is associated with increasing Foxp3 stability in vivo. Copyright © 2017 by The American Association of Immunologists, Inc.
- Cardiovascular biology,
- Immunology and Microbiology
IFNAR1-Signalling Obstructs ICOS-mediated Humoral Immunity during Non-lethal Blood-Stage Plasmodium Infection.
In PLoS Pathogens on 1 November 2016 by Sebina, I., James, K. R., et al.
PubMed
Parasite-specific antibodies protect against blood-stage Plasmodium infection. However, in malaria-endemic regions, it takes many months for naturally-exposed individuals to develop robust humoral immunity. Explanations for this have focused on antigenic variation by Plasmodium, but have considered less whether host production of parasite-specific antibody is sub-optimal. In particular, it is unclear whether host immune factors might limit antibody responses. Here, we explored the effect of Type I Interferon signalling via IFNAR1 on CD4+ T-cell and B-cell responses in two non-lethal murine models of malaria, P. chabaudi chabaudi AS (PcAS) and P. yoelii 17XNL (Py17XNL) infection. Firstly, we demonstrated that CD4+ T-cells and ICOS-signalling were crucial for generating germinal centre (GC) B-cells, plasmablasts and parasite-specific antibodies, and likewise that T follicular helper (Tfh) cell responses relied on B cells. Next, we found that IFNAR1-signalling impeded the resolution of non-lethal blood-stage infection, which was associated with impaired production of parasite-specific IgM and several IgG sub-classes. Consistent with this, GC B-cell formation, Ig-class switching, plasmablast and Tfh differentiation were all impaired by IFNAR1-signalling. IFNAR1-signalling proceeded via conventional dendritic cells, and acted early by limiting activation, proliferation and ICOS expression by CD4+ T-cells, by restricting the localization of activated CD4+ T-cells adjacent to and within B-cell areas of the spleen, and by simultaneously suppressing Th1 and Tfh responses. Finally, IFNAR1-deficiency accelerated humoral immune responses and parasite control by boosting ICOS-signalling. Thus, we provide evidence of a host innate cytokine response that impedes the onset of humoral immunity during experimental malaria.
- In Vivo,
- Mus musculus (House mouse),
- Immunology and Microbiology
The Costimulatory Molecule ICOS Regulates Host Th1 and Follicular Th Cell Differentiation in Response to Plasmodium chabaudi chabaudi AS Infection.
In The Journal of Immunology on 15 January 2016 by Wikenheiser, D. J., Ghosh, D., et al.
PubMed
Blood-stage Plasmodium chabaudi chabaudi AS infection requires cell- and Ab-mediated immunity to control acute and persistent infection, respectively. ICOS regulates CD4(+) T cell activation and promotes the induction of follicular Th (TFH) cells, CD4(+) T cells that support B cell affinity maturation within germinal centers (GCs), resulting in the production of high-affinity Abs. In this article, we demonstrate that, in response to P. c. chabaudi AS infection, the absence of ICOS resulted in an enhanced Th1 immune response that reduced peak parasitemia. Despite the absence of ICOS, CD4(+) T cells were capable of expressing PD-1, B cell lymphoma 6, and CXCR5 during early infection, indicating TFH development was not impaired. However, by day 21 postinfection, Icos(-/-) mice accumulated fewer splenic TFHs compared with Icos(+/+) mice, leading to substantially fewer GC B cells and a decrease in affinity, but not production, of parasite-specific isotype-switched Abs. Moreover, treatment of mice with anti-ICOS ligand Abs to modulate ICOS-ICOS ligand signaling revealed a requirement for ICOS in TFH differentiation only after day 6 postinfection. Ultimately, the quality and quantity of isotype-switched Abs produced in Icos(-/-) mice declined over time, resulting in impaired control of persistent parasitemia. Collectively, these data suggest ICOS is not required for TFH induction during P. c. chabaudi AS infection or production of isotype-switched Abs, but it is necessary for maintenance of a sustained high-affinity, protective Ab response. Copyright © 2016 by The American Association of Immunologists, Inc.