InVivoMAb anti-human CD47
Product Details
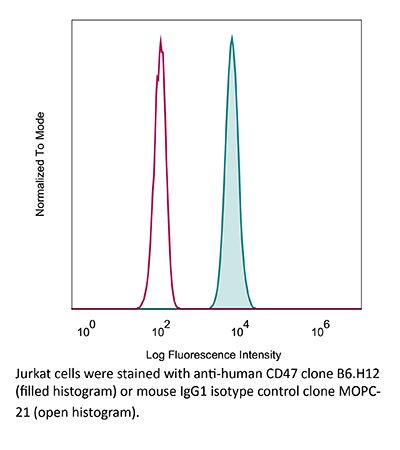
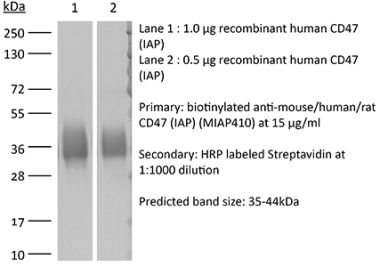
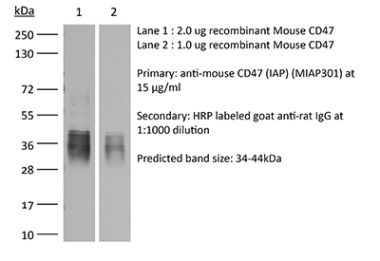
The B6H12 monoclonal antibody reacts with human CD47 otherwise known as integrin-associated protein (IAP), and neurophilin. CD47 is an approximately 50 kDa glycosylated five transmembrane protein that is ubiquitously expressed by both hematopoietic cells such as T and B lymphocytes, monocytes, platelets and erythrocytes and non-hematopoietic cells. CD47 is involved in a range of cellular processes, including apoptosis, proliferation, adhesion, and migration. Furthermore, it plays a key role in immune and angiogenic responses. CD47 is a receptor for thrombospondin-1 (TSP-1), a secreted glycoprotein that plays a role in vascular development and angiogenesis. CD47 Is has been found to be overexpressed in many different tumor cells. Because of this, anti-CD47 monoclonal antibodies have been proposed and studied as a therapeutic treatment for human cancers. The B6H12 antibody has been shown to neutralize CD47 and inhibit the growth of hepatocellular carcinoma cells in vitro.Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Intact CD47 purified from placenta |
| Reported Applications |
in vitro CD47 neutralization in vivo CD47 neutralization in human tumor xenograft models or humanized mice Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107655 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
in vivo CD47 neutralization in human tumor xenograft model
Gordon, S. R., et al. (2017). "PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity" Nature 545(7655): 495-499. PubMed
Programmed cell death protein 1 (PD-1) is an immune checkpoint receptor that is upregulated on activated T cells for the induction of immune tolerance. Tumour cells frequently overexpress the ligand for PD-1, programmed cell death ligand 1 (PD-L1), facilitating their escape from the immune system. Monoclonal antibodies that block the interaction between PD-1 and PD-L1, by binding to either the ligand or receptor, have shown notable clinical efficacy in patients with a variety of cancers, including melanoma, colorectal cancer, non-small-cell lung cancer and Hodgkin’s lymphoma. Although it is well established that PD-1-PD-L1 blockade activates T cells, little is known about the role that this pathway may have in tumour-associated macrophages (TAMs). Here we show that both mouse and human TAMs express PD-1. TAM PD-1 expression increases over time in mouse models of cancer and with increasing disease stage in primary human cancers. TAM PD-1 expression correlates negatively with phagocytic potency against tumour cells, and blockade of PD-1-PD-L1 in vivo increases macrophage phagocytosis, reduces tumour growth and lengthens the survival of mice in mouse models of cancer in a macrophage-dependent fashion. This suggests that PD-1-PD-L1 therapies may also function through a direct effect on macrophages, with substantial implications for the treatment of cancer with these agents.
in vivo CD47 neutralization in humanized mice, in vitro CD47 neutralization
Xiao, Z., et al. (2015). "Antibody mediated therapy targeting CD47 inhibits tumor progression of hepatocellular carcinoma" Cancer Lett 360(2): 302-309. PubMed
Human hepatocellular carcinoma (HCC) has a high rate of tumor recurrence and metastasis, resulting in shortened survival times. The efficacy of current systemic therapies for HCC is limited. In this study, we used xenograft tumor models to investigate the use of antibodies that block CD47 and inhibit HCC tumor growth. Immunostaining of tumor tissue and HCC cell lines demonstrated CD47 over-expression in HCC as compared to normal hepatocytes. Macrophage phagocytosis of HCC cells was increased after treatment with CD47 antibodies (CD47mAbs) that block CD47 binding to SIRPalpha. Further, CD47 blockade inhibited tumor growth in both heterotopic and orthotopic models of HCC, and promoted the migration of macrophages into the tumor mass. Our results demonstrate that targeting CD47 by specific antibodies has potential immunotherapeutic efficacy in human HCC.
in vivo CD47 neutralization in humanized mice
Lo, J., et al. (2015). "Anti-CD47 antibody suppresses tumour growth and augments the effect of chemotherapy treatment in hepatocellular carcinoma" Liver Int. doi : 10.1111/liv.12963. PubMed
BACKGROUND & AIMS: Hepatocellular carcinoma (HCC) is often associated with metastasis and recurrence leading to a poor prognosis. Therefore, development of novel treatment regimens is urgently needed to improve the survival of HCC patients. In this study, we aimed to investigate the in vitro and in vivo effects of anti-CD47 antibody alone and in combination with chemotherapy in HCC. METHODS: In this study, we examined the functional effects of anti-CD47 antibody (B6H12) on cell proliferation, sphere formation, migration and invasion, chemosensitivity, macrophage-mediated phagocytosis and tumourigenicity both in vitro and in vivo. The therapeutic efficacy of anti-CD47 antibody alone or in combination with doxorubicin was examined in patient-derived HCC xenograft. RESULTS: Blocking CD47 with anti-CD47 monoclonal antibody (B6H12) at 10 mug/ml could suppress self-renewal, tumourigenicity and migration and invasion abilities of MHCC-97L and Huh-7 cells. Interestingly, anti-CD47 antibody synergized the effect of HCC cells to chemotherapeutic drugs including doxorubicin and cisplatin. Blockade of CD47 by anti-CD47 antibody induced macrophage-mediated phagocytosis. Using a patient-derived HCC xenograft mouse model, we found that anti-CD47 antibody (400 mug/mouse) in combination with doxorubicin (2 mg/kg) exerted maximal effects on tumour suppression, as compared with doxorubicin and anti-CD47 antibody alone. CONCLUSIONS: Anti-CD47 antibody treatment could complement chemotherapy which may be a promising therapeutic strategy for the treatment of HCC patients.
in vitro CD47 neutralization
Lo, J., et al. (2015). "Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice" Hepatology 62(2): 534-545. PubMed
Sorafenib is a new standard treatment for patients with advanced hepatocellular carcinoma (HCC). However, the survival benefit of this treatment is modest, partly owing to drug resistance. Recent evidence has demonstrated the existence of tumor-initiating cells (T-ICs) as the culprit for treatment resistance. To examine whether sorafenib resistance was a result of the presence of liver T-ICs, we developed sorafenib-resistant HCC cells both in vitro and in vivo through continuous exposure to sorafenib. Using these models, we found that sorafenib-resistant clones demonstrated enhanced T-IC properties, including tumorigenicity, self-renewal, and invasiveness. In addition, several T-IC markers were found to be up-regulated, among which CD47 was found to be most significant. Using chromatin immunoprecipitation assays and expression analyses, CD47 expression was found to be regulated by nuclear factor kappa B (NF-kappaB) through a specific response element in the promoter of CD47, and the site occupancy and expression were increased and decreased upon stimulation and inhibition of NF-kappaB, respectively. Consistently, NF-kappaB was activated in sorafenib-resistant HCC cells, and this finding was confirmed in clinical HCC samples, which showed a positive correlation between NF-kappaB and CD47 expression. Functional characterization of CD47 in sorafenib-resistant HCC cells was evaluated using a lentivirus-based knockdown approach and showed increased sensitization to sorafenib upon CD47 knockdown. Furthermore, blockade of CD47 using anti-CD47 antibody (Ab) showed a similar effect. Using a patient-derived HCC xenograft mouse model, we found that anti-CD47 Ab (500 mug/mouse) in combination with sorafenib (100 mg/kg, orally) exerted synergistic effects on tumor suppression, as compared with sorafenib and anti-CD47 Ab alone. CONCLUSIONS: NF-kappaB-mediated CD47 up-regulation promotes sorafenib resistance, and targeting CD47 in combination with sorafenib is an attractive therapeutic regimen for the treatment of HCC patients.
in vivo CD47 neutralization in humanized mice
Lee, T. K., et al. (2014). "Blockade of CD47-mediated cathepsin S/protease-activated receptor 2 signaling provides a therapeutic target for hepatocellular carcinoma" Hepatology 60(1): 179-191. PubMed
Identification of therapeutic targets against tumor-initiating cells (TICs) is a priority in the development of new therapeutic paradigms against cancer. We enriched a TIC population capable of tumor initiation and self-renewal by serial passages of hepatospheres with chemotherapeutic agents. In chemoresistant hepatospheres, CD47 was found to be up-regulated, when compared with differentiated progenies. CD47 is preferentially expressed in liver TICs, which contributed to tumor initiation, self-renewal, and metastasis and significantly affected patients’ clinical outcome. Knockdown of CD47 suppressed stem/progenitor cell characteristics. CD47(+) hepatocellular carcinoma (HCC) cells preferentially secreted cathepsin S (CTSS), which regulates liver TICs through the CTSS/protease-activated receptor 2 (PAR2) loop. Suppression of CD47 by morpholino approach suppressed growth of HCC in vivo and exerted a chemosensitization effect through blockade of CTSS/PAR2 signaling. CONCLUSION: These data suggest that CD47 may be an attractive therapeutic target for HCC therapy.
in vitro CD47 neutralization, Flow Cytometry
Tseng, D., et al. (2013). "Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response" Proc Natl Acad Sci U S A 110(27): 11103-11108. PubMed
Mobilization of the T-cell response against cancer has the potential to achieve long-lasting cures. However, it is not known how to harness antigen-presenting cells optimally to achieve an effective antitumor T-cell response. In this study, we show that anti-CD47 antibody-mediated phagocytosis of cancer by macrophages can initiate an antitumor T-cell immune response. Using the ovalbumin model antigen system, anti-CD47 antibody-mediated phagocytosis of cancer cells by macrophages resulted in increased priming of OT-I T cells [cluster of differentiation 8-positive (CD8(+))] but decreased priming of OT-II T cells (CD4(+)). The CD4(+) T-cell response was characterized by a reduction in forkhead box P3-positive (Foxp3(+)) regulatory T cells. Macrophages following anti-CD47-mediated phagocytosis primed CD8(+) T cells to exhibit cytotoxic function in vivo. This response protected animals from tumor challenge. We conclude that anti-CD47 antibody treatment not only enables macrophage phagocytosis of cancer but also can initiate an antitumor cytotoxic T-cell immune response.
Pro-phagocytic function and structural basis of GPR84 signaling.
In Nature Communications on 14 September 2023 by Zhang, X., Wang, Y., et al.
PubMed
GPR84 is a unique orphan G protein-coupled receptor (GPCR) that can be activated by endogenous medium-chain fatty acids (MCFAs). The signaling of GPR84 is largely pro-inflammatory, which can augment inflammatory response, and GPR84 also functions as a pro-phagocytic receptor to enhance phagocytic activities of macrophages. In this study, we show that the activation of GPR84 by the synthetic agonist 6-OAU can synergize with the blockade of CD47 on cancer cells to induce phagocytosis of cancer cells by macrophages. We also determine a high-resolution structure of the GPR84-Gi signaling complex with 6-OAU. This structure reveals an occluded binding pocket for 6-OAU, the molecular basis of receptor activation involving non-conserved structural motifs of GPR84, and an unusual Gi-coupling interface. Together with computational docking and simulations studies, this structure also suggests a mechanism for the high selectivity of GPR84 for MCFAs and a potential routes of ligand binding and dissociation. These results provide a framework for understanding GPR84 signaling and developing new drugs targeting GPR84. © 2023. Springer Nature Limited.
- Biochemistry and Molecular biology,
Blockade of CD47 function attenuates restenosis by promoting smooth muscle cell efferocytosis and inhibiting their migration and proliferation.
In The Journal of Biological Chemistry on 1 April 2023 by Govatati, S., Pichavaram, P., et al.
PubMed
Cluster of differentiation 47 (CD47) plays an important role in the pathophysiology of various diseases including atherosclerosis but its role in neointimal hyperplasia which contributes to restenosis has not been studied. Using molecular approaches in combination with a mouse vascular endothelial denudation model, we studied the role of CD47 in injury-induced neointimal hyperplasia. We determined that thrombin-induced CD47 expression both in human aortic smooth muscle cells (HASMCs) and mouse aortic smooth muscle cells. In exploring the mechanisms, we found that the protease-activated receptor 1-Gα protein q/11 (Gαq/11)-phospholipase Cβ3-nuclear factor of activated T cells c1 signaling axis regulates thrombin-induced CD47 expression in HASMCs. Depletion of CD47 levels using its siRNA or interference of its function by its blocking antibody (bAb) blunted thrombin-induced migration and proliferation of HASMCs and mouse aortic smooth muscle cells. In addition, we found that thrombin-induced HASMC migration requires CD47 interaction with integrin β3. On the other hand, thrombin-induced HASMC proliferation was dependent on CD47's role in nuclear export and degradation of cyclin-dependent kinase-interacting protein 1. In addition, suppression of CD47 function by its bAb rescued HASMC efferocytosis from inhibition by thrombin. We also found that vascular injury induces CD47 expression in intimal SMCs and that inhibition of CD47 function by its bAb, while alleviating injury-induced inhibition of SMC efferocytosis, attenuated SMC migration, and proliferation resulting in reduced neointima formation. Thus, these findings reveal a pathological role for CD47 in neointimal hyperplasia. Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
- Cancer Research,
- Immunology and Microbiology
BND-22, a first-in-class humanized ILT2-blocking antibody, promotes antitumor immunity and tumor regression.
In Journal for Immunotherapy of Cancer on 1 September 2022 by Mandel, I., Haves Ziv, D., et al.
PubMed
Cancer immunotherapy has revolutionized cancer treatment. However, considering the limited success of immunotherapy to only some cancer types and patient cohorts, there is an unmet need for developing new treatments that will result in higher response rates in patients with cancer. Immunoglobulin-like transcript 2 (ILT2), a LILRB family member, is an inhibitory receptor expressed on a variety of immune cells including T cells, natural killer (NK) cells and different myeloid cells. In the tumor microenvironment, binding of class I MHC (in particular HLA-G) to ILT2 on immune cells mediates a strong inhibitory effect, which manifests in inhibition of antitumor cytotoxicity of T and NK cells, and prevention of phagocytosis of the tumor cells by macrophages. We describe here the development and characteristics of BND-22, a novel, humanized monoclonal antibody that selectively binds to ILT2 and blocks its interaction with classical MHC I and HLA-G. BND-22 was evaluated for its binding and blocking characteristics as well as its ability to increase the antitumor activity of macrophages, T cells and NK cells in various in vitro, ex vivo and in vivo systems. Collectively, our data suggest that BND-22 enhances activity of both innate and adaptive immune cells, thus generating robust and comprehensive antitumor immunity. In humanized mice models, blocking ILT2 with BND-22 decreased the growth of human tumors, hindered metastatic spread to the lungs, and prolonged survival of the tumor-bearing mice. In addition, BND-22 improved the antitumor immune response of approved therapies such as anti-PD-1 or anti-EGFR antibodies. BND-22 is a first-in-human ILT2 blocking antibody which has demonstrated efficient antitumor activity in various preclinical models as well as a favorable safety profile. Clinical evaluation of BND-22 as a monotherapy or in combination with other therapeutics is under way in patients with cancer. NCT04717375. © Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
- In Vitro,
- Homo sapiens (Human),
- Cancer Research,
- Immunology and Microbiology
Dendritic cells can prime anti-tumor CD8+ T cell responses through major histocompatibility complex cross-dressing.
In Immunity on 14 June 2022 by MacNabb, B. W., Tumuluru, S., et al.
PubMed
Antigen cross-presentation, wherein dendritic cells (DCs) present exogenous antigen on major histocompatibility class I (MHC-I) molecules, is considered the primary mechanism by which DCs initiate tumor-specific CD8+ T cell responses. Here, we demonstrate that MHC-I cross-dressing, an antigen presentation pathway in which DCs acquire and display intact tumor-derived peptide:MHC-I molecules, is also important in orchestrating anti-tumor immunity. Cancer cell MHC-I expression was required for optimal CD8+ T cell activation in two subcutaneous tumor models. In vivo acquisition of tumor-derived peptide:MHC-I molecules by DCs was sufficient to induce antigen-specific CD8+ T cell priming. Transfer of tumor-derived human leukocyte antigen (HLA) molecules to myeloid cells was detected in vitro and in human tumor xenografts. In conclusion, MHC-I cross-dressing is crucial for anti-tumor CD8+ T cell priming by DCs. In addition to quantitatively enhancing tumor antigen presentation, MHC cross-dressing might also enable DCs to more faithfully and efficiently mirror the cancer cell peptidome. Copyright © 2022 Elsevier Inc. All rights reserved.
- Cancer Research,
- Pharmacology
Blocking CD47 Shows Superior Anti-tumor Therapeutic Effects of Bevacizumab in Gastric Cancer.
In Frontiers in Pharmacology on 14 June 2022 by Shi, C., Li, J., et al.
PubMed
Background: Bevacizumab (Avastin®), a humanized antiangiogenic monoclonal antibody, is widely used in the clinical treatment of tumour diseases. However, recent research has shown that the beneficial antiangiogenic effects of these agents have been limited in a number of patients due to complex immunosuppressive mechanisms. Here, we report a synergistic antitumour strategy through simultaneous blockade of VEGF and CD47 signalling to enhance the curative effect of advanced gastric cancer. Method: A BGC-823 gastric tumour model was chosen to evaluate antitumour efficacy. Macrophage migration and phagocytosis were evaluated to determine immune-related resistance to bevacizumab therapy. Synergistic antitumour activity was observed on the basis of tumour volume, tumour weight, tumour inhibition rate, tumour angiogenesis and tumour metastasis when bevacizumab was combined with an anti-CD47 monoclonal antibody. Results: Our study demonstrated that synergistic therapy targeting CD47 and VEGF reversed macrophage migration and phagocytosis, which were inhibited by antiangiogenic therapy and enhanced antitumour effects. Moreover, blockade of CD47 induced by antiangiogenic therapy inhibited tumour metastasis. Conclusion: Our data provide an effective strategy to attenuate resistance to bevacizumab therapy, promoting clinical cancer treatment with antiangiogenic drugs in combination with CD47-targeting inhibitors. Copyright © 2022 Shi, Li, Fan and Liu.
- Immunology and Microbiology
Preclinical characterization of the novel anti-SIRPα antibody BR105 that targets the myeloid immune checkpoint.
In Journal for Immunotherapy of Cancer on 1 March 2022 by Wu, Z. H., Li, N., et al.
PubMed
The CD47-SIRPα pathway acts as an important myeloid cell immune checkpoint and targeting the CD47/SIRPα axis represents a promising strategy to promote antitumor immunity. Several CD47-targeting agents show encouraging early activity in clinical trials. However, due to ubiquitous expression of CD47, the antigen sink and hematologic toxicity, such as anemia and thrombocytopenia, are main problems for developing CD47-targeting therapies. Considering the limited expression of SIRPα, targeting SIRPα is an alternative approach to block the CD47-SIRPα pathway, which may result in differential efficacy and safety profiles. SIRPα-targeting antibody BR105 was generated by hybridoma fusion and following humanization. BR105 was characterized for binding to human SIRPα alleles and blockade of the interaction with CD47. The functional activity was determined in in vitro phagocytosis assays by using human macrophages. The effect of BR105 on human T cell activation was studied using an OKT3-induced T-cell proliferation assay and an allogeneic mixed lymphocyte reaction. Human SIRPα-humanized immunodeficient mice were used in cancer models for evaluating the in vivo antitumor efficacy of BR105. Safety was addressed in a repeat-dose toxicity study in cynomolgus monkeys, and toxicokinetic analysis was further evaluated. BR105 shows broad binding activity across various SIRPα variants, and potently blocks the interaction of SIRPα and CD47. In vitro functional assays demonstrated that BR105 synergizes with therapeutic antibodies to promote phagocytosis of tumor cells. Moreover, the combination of BR105 and therapeutic antibody significantly inhibits tumor growth in a xenograft tumor model. Although BR105 may slightly bind to SIRPγ, it does not inhibit T cell activation, unlike other non-selective SIRPα-targeting antibody and CD47-targeting agents. Toxicity studies in non-human primates show that BR105 is well tolerated with no treatment-related adverse effects noted. The novel and differentiated SIRPα-targeting antibody, BR105, was discovered and displays promising antitumor efficacy in vitro and in vivo. BR105 has a favorable safety profile and shows no adverse effects on T cell functionality. These data support further clinical development of BR105, especially as a therapeutic agent to enhance efficacy when used in combination with tumor-targeting antibodies or antibodies that target other immune checkpoints. © Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
- Homo sapiens (Human),
- Cancer Research,
- Immunology and Microbiology
Immunotherapy of glioblastoma explants induces interferon-γ responses and spatial immune cell rearrangements in tumor center, but not periphery
Preprint on BioRxiv : the Preprint Server for Biology on 21 January 2022 by Shekarian, T., Zinner, C. P., et al.
PubMed
Recent therapeutic strategies for glioblastoma (GBM) aim at targeting immune tumor microenvironment (iTME) components to induce antitumoral immunity. A patient-tailored, ex vivo drug testing and response analysis platform for GBM would facilitate personalized therapy planning, provide insights into treatment-induced immune mechanisms in the iTME, and enable the discovery of biomarkers of therapy response and resistance. We cultured 47 GBM explants from tumor center and periphery from 7 patients in perfusion bioreactors to assess iTME responses to immunotherapy. Explants were exposed to antibodies blocking the immune checkpoints CD47, PD-1 or or their combination, and were analyzed by highly multiplexed microscopy (CODEX, co -detection by in dex ing) using an immune-focused 55-marker panel. Culture media were examined for changes of soluble factors including cytokines, chemokines and metabolites. CODEX enabled the spatially resolved identification and quantification of >850,000 single cells in explants, which were classified into 10 cell types by clustering. Explants from center and periphery differed significantly in their cell type composition, their levels of soluble factors, and their responses to immunotherapy. In a subset of explants, culture media displayed increased interferon-γ levels, which correlated with shifts in immune cell composition within specific tissue compartments, including the enrichment of CD4 + and CD8 + T cells within an adaptive immune compartment. Furthermore, significant differences in the expression levels of functional molecules in innate and adaptive immune cell types were found between explants responding or not to immunotherapy. In non-responder explants, T cells showed higher expression of PD-1, LAG-3, TIM-3 and VISTA, whereas in responders, macrophages and microglia showed higher cathepsin D levels. Our study demonstrates that ex vivo immunotherapy of GBM explants enables an active antitumoral immune response within the tumor center in a subset of patients, and provides a framework for multidimensional personalized assessment of tumor response to immunotherapy.
- WB,
- Homo sapiens (Human),
- Immunology and Microbiology
Signal-regulatory protein alpha is an anti-viral entry factor targeting viruses using endocytic pathways.
In PLoS Pathogens on 1 June 2021 by Sarute, N., Cheng, H., et al.
PubMed
Signal-regulatory protein alpha (SIRPA) is a well-known inhibitor of phagocytosis when it complexes with CD47 expressed on target cells. Here we show that SIRPA decreased in vitro infection by a number of pathogenic viruses, including New World and Old World arenaviruses, Zika virus, vesicular stomatitis virus and pseudoviruses bearing the Machupo virus, Ebola virus and SARS-CoV-2 glycoproteins, but not HSV-1, MLV or mNoV. Moreover, mice with targeted mutation of the Sirpa gene that renders it non-functional were more susceptible to infection with the New World arenaviruses Junín virus vaccine strain Candid 1 and Tacaribe virus, but not MLV or mNoV. All SIRPA-inhibited viruses have in common the requirement for trafficking to a low pH endosomal compartment. This was clearly demonstrated with SARS-CoV-2 pseudovirus, which was only inhibited by SIRPA in cells in which it required trafficking to the endosome. Similar to its role in phagocytosis inhibition, SIRPA decreased virus internalization but not binding to cell surface receptors. We also found that increasing SIRPA levels via treatment with IL-4 led to even greater anti-viral activity. These data suggest that enhancing SIRPA's activity could be a target for anti-viral therapies.
- Cancer Research
An antitumor peptide RS17-targeted CD47, design, synthesis, and antitumor activity.
In Cancer Medicine on 1 March 2021 by Wang, X., Wang, Y., et al.
PubMed
CD47 is a widely expressed transmembrane protein located on the surface of somatic cells. It mediates a variety of cellular processes including apoptosis, proliferation, adhesion, and migration. An important role for CD47 is the transmission of a "Don't eat me" signal by interacting with SIRPα on the macrophage surface membrane, thereby preventing the phagocytosis of normal cells. However, cancer cells can take advantage of this autogenous signal to protect themselves from phagocytosis, thus enabling immune escape. Blocking the interaction between CD47 and SIRPα has proven to be effective in removing cancer cells. The treatment of various cancers with CD47 monoclonal antibodies has also been validated. We designed and synthesized a peptide (RS17), which can specifically bind to CD47 and block CD47-SIRPα signaling. The affinity of RS17 for CD47-expressing tumor cells was determined, while the inhibition of CD47-SIRPα signaling was evaluated in vitro and in vivo. The results indicated that RS17 significantly promotes the phagocytosis of tumor cells by macrophages and had a similar therapeutic effect compared with a positive control (CD47 monoclonal antibodies). In addition, a cancer xenograft mouse model was established using CD47-expressing HepG2 cells to evaluate the effect of RS17 on tumor growth in vivo. Using ex vivo and in vivo mouse models, RS17 demonstrated a high inhibitory effect on tumor growth. Based on our results, RS17 may represent a novel therapeutic peptide for cancer therapy. © 2021 The Authors. Cancer Medicine published by John Wiley Sons Ltd.
- Cancer Research,
- Immunology and Microbiology
Effect of cabazitaxel on macrophages improves CD47-targeted immunotherapy for triple-negative breast cancer.
In Journal for Immunotherapy of Cancer on 1 March 2021 by Cao, X., Li, B., et al.
PubMed
Limited therapeutic options are available for triple-negative breast cancer (TNBC), emphasizing an urgent need for more effective treatment approaches. The development of strategies by targeting tumor-associated macrophages (TAMs) to stimulate their ability of Programmed Cell Removal (PrCR) provides a promising new immunotherapy for TNBC treatment. CD47 is a critical self-protective "don't eat me" signal on multiple human cancers against macrophage immunosurveillance. Using human and mouse TNBC preclinical models, we evaluated the efficacy of PrCR-based immunotherapy by blocking CD47. We performed high-throughput screens on FDA-approved anti-cancer small molecule compounds for agents potentiating PrCR and enhancing the efficacy of CD47-targeted therapy for TNBC treatment. We showed that CD47 was widely expressed on TNBC cells and TAMs represented the most abundant immune cell population in TNBC tumors. Blockade of CD47 enabled PrCR of TNBC cells, but the efficacy was not satisfactory. Our high-throughput screens identified cabazitaxel in enhancing PrCR-based immunotherapy. A combination of CD47 blockade and cabazitaxel treatment yielded a highly effective treatment strategy, promoting PrCR of TNBC cells and inhibiting tumor development and metastasis in preclinical models. We demonstrated that cabazitaxel potentiated PrCR by activating macrophages, independent of its cytotoxicity toward cancer cells. When treated with cabazitaxel, the molecular and phenotypic signatures of macrophages were polarized toward M1 state, and the NF-kB signaling pathway became activated. The combination of CD47 blockade and macrophage activation by cabazitaxel synergizes to vastly enhance the elimination of TNBC cells. Our results show that targeting macrophages is a promising and effective strategy for TNBC treatment. © Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
- Cancer Research,
- Immunology and Microbiology
Warburg Effect Is a Cancer Immune Evasion Mechanism Against Macrophage Immunosurveillance.
In Frontiers in Immunology on 20 February 2021 by Chen, J., Cao, X., et al.
PubMed
Evasion of immunosurveillance is critical for cancer initiation and development. The expression of "don't eat me" signals protects cancer cells from being phagocytosed by macrophages, and the blockade of such signals demonstrates therapeutic potential by restoring the susceptibility of cancer cells to macrophage-mediated phagocytosis. However, whether additional self-protective mechanisms play a role against macrophage surveillance remains unexplored. Here, we derived a macrophage-resistant cancer model from cells deficient in the expression of CD47, a major "don't eat me" signal, via a macrophage selection assay. Comparative studies performed between the parental and resistant cells identified self-protective traits independent of CD47, which were examined with both pharmacological or genetic approaches in in vitro phagocytosis assays and in vivo tumor models for their roles in protecting against macrophage surveillance. Here we demonstrated that extracellular acidification resulting from glycolysis in cancer cells protected them against macrophage-mediated phagocytosis. The acidic tumor microenvironment resulted in direct inhibition of macrophage phagocytic ability and recruitment of weakly phagocytic macrophages. Targeting V-ATPase which transports excessive protons in cancer cells to acidify extracellular medium elicited a pro-phagocytic microenvironment with an increased ratio of M1-/M2-like macrophage populations, therefore inhibiting tumor development and metastasis. In addition, blockade of extracellular acidification enhanced cell surface exposure of CD71, targeting which by antibodies promoted cancer cell phagocytosis. Our results reveal that extracellular acidification due to the Warburg effect confers immune evasion ability on cancer cells. This previously unrecognized role highlights the components mediating the Warburg effect as potential targets for new immunotherapy harnessing the tumoricidal capabilities of macrophages. Copyright © 2021 Chen, Cao, Li, Zhao, Chen, Lai, Muend, Nossa, Wang, Guo, Ye, Lee and Feng.
- Cancer Research
Characterization of cluster of differentiation 47 expression and its potential as a therapeutic target in esophageal squamous cell cancer.
In Oncology Letters on 1 February 2018 by Zhao, C. L., Yu, S., et al.
PubMed
The increased expression of cluster of differentiation (CD)47 has been identified in a number of different tumor types and is recognized as an adverse prognostic factor that indicates an increased risk of mortality in patients. The binding of CD47 to signal regulatory protein α (SIRPα) inhibits the macrophage phagocytosis of tumor cells by triggering an inhibitory 'do not eat me' signal. This is one of the mechanisms used by tumor cells to evade immune surveillance. In the present study, CD47 levels and macrophage infiltration were assessed in patients with esophageal squamous cell cancer (ESCC). CD47-overexpressing ESCC cell lines were selected and human M2 macrophage phagocytic activity was measured. The results revealed that CD47 is highly expressed and macrophages are markedly infiltrated in cancerous tissue compared with non-cancerous tissue. High CD47 expression was detected in ESCC cell lines and the results of a phagocytosis assay indicated that human M2 macrophages phagocytized tumor cells in a dose-dependent manner following the blocking of CD47-SIRPα signaling by anti-CD47 antibodies. The results of the present study therefore support the use of anti-CD47 immunotherapy to treat patients with ESCC.
- Cancer Research
Anti-CD47 antibody suppresses tumour growth and augments the effect of chemotherapy treatment in hepatocellular carcinoma.
In Liver International : Official Journal of the International Association for the Study of the Liver on 1 May 2016 by Lo, J., Lau, E. Y., et al.
PubMed
Hepatocellular carcinoma (HCC) is often associated with metastasis and recurrence leading to a poor prognosis. Therefore, development of novel treatment regimens is urgently needed to improve the survival of HCC patients. In this study, we aimed to investigate the in vitro and in vivo effects of anti-CD47 antibody alone and in combination with chemotherapy in HCC. In this study, we examined the functional effects of anti-CD47 antibody (B6H12) on cell proliferation, sphere formation, migration and invasion, chemosensitivity, macrophage-mediated phagocytosis and tumourigenicity both in vitro and in vivo. The therapeutic efficacy of anti-CD47 antibody alone or in combination with doxorubicin was examined in patient-derived HCC xenograft. Blocking CD47 with anti-CD47 monoclonal antibody (B6H12) at 10 μg/ml could suppress self-renewal, tumourigenicity and migration and invasion abilities of MHCC-97L and Huh-7 cells. Interestingly, anti-CD47 antibody synergized the effect of HCC cells to chemotherapeutic drugs including doxorubicin and cisplatin. Blockade of CD47 by anti-CD47 antibody induced macrophage-mediated phagocytosis. Using a patient-derived HCC xenograft mouse model, we found that anti-CD47 antibody (400 μg/mouse) in combination with doxorubicin (2 mg/kg) exerted maximal effects on tumour suppression, as compared with doxorubicin and anti-CD47 antibody alone. Anti-CD47 antibody treatment could complement chemotherapy which may be a promising therapeutic strategy for the treatment of HCC patients. © 2015 John Wiley Sons A/S. Published by John Wiley Sons Ltd.
- Cancer Research,
- Immunology and Microbiology
Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response.
In Proceedings of the National Academy of Sciences of the United States of America on 2 July 2013 by Tseng, D., Volkmer, J. P., et al.
PubMed
Mobilization of the T-cell response against cancer has the potential to achieve long-lasting cures. However, it is not known how to harness antigen-presenting cells optimally to achieve an effective antitumor T-cell response. In this study, we show that anti-CD47 antibody-mediated phagocytosis of cancer by macrophages can initiate an antitumor T-cell immune response. Using the ovalbumin model antigen system, anti-CD47 antibody-mediated phagocytosis of cancer cells by macrophages resulted in increased priming of OT-I T cells [cluster of differentiation 8-positive (CD8(+))] but decreased priming of OT-II T cells (CD4(+)). The CD4(+) T-cell response was characterized by a reduction in forkhead box P3-positive (Foxp3(+)) regulatory T cells. Macrophages following anti-CD47-mediated phagocytosis primed CD8(+) T cells to exhibit cytotoxic function in vivo. This response protected animals from tumor challenge. We conclude that anti-CD47 antibody treatment not only enables macrophage phagocytosis of cancer but also can initiate an antitumor cytotoxic T-cell immune response.