InVivoMAb anti-human CD3
Product Details
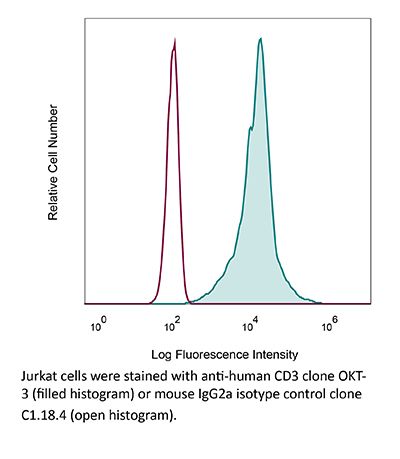
The OKT-3 monoclonal antibody reacts with human CD3ε, a 20 kDa transmembrane cell-surface protein that belongs to the immunoglobulin superfamily. CD3ε is one of five polypeptide chains that combine to form the TCR complex. CD3ε is expressed on T lymphocytes, NK-T cells, and to varying degrees on developing thymocytes. CD3 plays roles in TCR signaling, T lymphocyte activation, and antigen recognition. The OKT-3 antibody has immunosuppressive properties in vivo and has been shown to effectively treat renal, heart and liver allograft rejection.Specifications
| Isotype | Mouse IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG2a isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Not available or unknown |
| Reported Applications |
in vitro T cell stimulation/activation in vivo T cell depletion in humanized mice ex vivo T cell inhibition for xenografts Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107632 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
in vitro T cell stimulation/activation
Rochman, Y., et al. (2015). "Functional characterization of human T cell hyporesponsiveness induced by CTLA4-Ig" PLoS One 10(4): e0122198. PubMed
During activation, T cells integrate multiple signals from APCs and cytokine milieu. The blockade of these signals can have clinical benefits as exemplified by CTLA4-Ig, which blocks interaction of B7 co-stimulatory molecules on APCs with CD28 on T cells. Variants of CTLA4-Ig, abatacept and belatacept are FDA approved as immunosuppressive agents in arthritis and transplantation, yet murine studies suggested that CTLA4-Ig could be beneficial in a number of other diseases. However, detailed analysis of human CD4 cell hyporesponsivness induced by CTLA4-Ig has not been performed. Herein, we established a model to study the effect of CTLA4-Ig on the activation of human naive T cells in a human mixed lymphocytes system. Comparison of human CD4 cells activated in the presence or absence of CTLA4-Ig showed that co-stimulation blockade during TCR activation does not affect NFAT signaling but results in decreased activation of NF-kappaB and AP-1 transcription factors followed by a profound decrease in proliferation and cytokine production. The resulting T cells become hyporesponsive to secondary activation and, although capable of receiving TCR signals, fail to proliferate or produce cytokines, demonstrating properties of anergic cells. However, unlike some models of T cell anergy, these cells did not possess increased levels of the TCR signaling inhibitor CBLB. Rather, the CTLA4-Ig-induced hyporesponsiveness was associated with an elevated level of p27kip1 cyclin-dependent kinase inhibitor.
in vitro T cell stimulation/activation
Hill, E. V., et al. (2015). "Glycogen synthase kinase-3 controls IL-10 expression in CD4(+) effector T-cell subsets through epigenetic modification of the IL-10 promoter" Eur J Immunol 45(4): 1103-1115. PubMed
The serine/threonine kinase glycogen synthase kinase-3 (GSK3) plays an important role in balancing pro- and anti-inflammatory cytokines. We have examined the role of GSK3 in production of IL-10 by subsets of CD4(+) T helper cells. Treatment of naive murine CD4(+) T cells with GSK3 inhibitors did not affect their production of IL-10. However, treatment of Th1 and Th2 cells with GSK3 inhibitors dramatically increased production of IL-10. GSK3 inhibition also led to upregulation of IL-10 among Th1, Th2, and Th17 subsets isolated from human blood. The encephalitogenic potential of GSK3 inhibitor treated murine Th1 cells was significantly reduced in adoptive transfer experiments by an IL-10-dependent mechanism. Analysis of the murine IL-10 promoter in response to inhibition of GSK3 in Th1 cells showed modification to a transcriptionally active state indicated by changes in histone H3 acetylation and methylation. Additionally, GSK3 inhibition increased expression of the transcription factors c-Maf, Nfil3, and GATA3, correlating with the increase in IL-10. These findings are important in the context of autoimmune disease since they show that it is possible to reprogram disease-causing cells through GSK3 inhibition.
in vitro T cell stimulation/activation
Liu, H., et al. (2015). "The Immune Adaptor SLP-76 Binds to SUMO-RANGAP1 at Nuclear Pore Complex Filaments to Regulate Nuclear Import of Transcription Factors in T Cells" Mol Cell 59(5): 840-849. PubMed
While immune cell adaptors regulate proximal T cell signaling, direct regulation of the nuclear pore complex (NPC) has not been reported. NPC has cytoplasmic filaments composed of RanGAP1 and RanBP2 with the potential to interact with cytoplasmic mediators. Here, we show that the immune cell adaptor SLP-76 binds directly to SUMO-RanGAP1 of cytoplasmic fibrils of the NPC, and that this interaction is needed for optimal NFATc1 and NF-kappaB p65 nuclear entry in T cells. Transmission electron microscopy showed anti-SLP-76 cytoplasmic labeling of the majority of NPCs in anti-CD3 activated T cells. Further, SUMO-RanGAP1 bound to the N-terminal lysine 56 of SLP-76 where the interaction was needed for optimal RanGAP1-NPC localization and GAP exchange activity. While the SLP-76-RanGAP1 (K56E) mutant had no effect on proximal signaling, it impaired NF-ATc1 and p65/RelA nuclear entry and in vivo responses to OVA peptide. Overall, we have identified SLP-76 as a direct regulator of nuclear pore function in T cells.
in vitro T cell stimulation/activation
Sturner, K. H., et al. (2014). "A multiple sclerosis-associated variant of CBLB links genetic risk with type I IFN function" J Immunol 193(9): 4439-4447. PubMed
Multiple sclerosis (MS) is an autoimmune disease of the CNS, and autoreactive CD4(+) T cells are considered important for its pathogenesis. The etiology of MS involves a complex genetic trait and environmental triggers that include viral infections, particularly the EBV. Among the risk alleles that have repeatedly been identified by genome-wide association studies, three are located near the Casitas B-lineage lymphoma proto-oncogene b gene (CBLB). The CBLB protein (CBL-B) is a key regulator of peripheral immune tolerance by limiting T cell activation and expansion and hence T cell-mediated autoimmunity through its ubiquitin E3-ligase activity. In this study, we show that CBL-B expression is reduced in CD4(+) T cells from relapsing-remitting MS (RR-MS) patients during relapse. The MS risk-related single nucleotide polymorphism of CBLB rs12487066 is associated with diminished CBL-B expression levels and alters the effects of type I IFNs on human CD4(+) T cell proliferation. Mechanistically, the CBLB rs12487066 risk allele mediates increased binding of the transcription factor C/EBPbeta and reduced CBL-B expression in human CD4(+) T cells. Our data suggest a role of the CBLB rs12487066 variant in the interactions of a genetic risk factor and IFN function during viral infections in MS.
in vitro T cell stimulation/activation, Flow Cytometry
Willing, A., et al. (2014). "CD8(+) MAIT cells infiltrate into the CNS and alterations in their blood frequencies correlate with IL-18 serum levels in multiple sclerosis" Eur J Immunol 44(10): 3119-3128. PubMed
Recent findings indicate a pathogenic involvement of IL-17-producing CD8(+) T cells in multiple sclerosis (MS). IL-17 production has been attributed to a subset of CD8(+) T cells that belong to the mucosal-associated invariant T (MAIT) cell population. Here, we report a reduction of CD8(+) MAIT cells in the blood of MS patients compared with healthy individuals, which significantly correlated with IL-18 serum levels in MS patients. In vitro stimulation of peripheral blood mononuclear cells from healthy individuals and MS patients with IL-18 specifically activated CD8(+) MAIT cells. Moreover, IL-18 together with T-cell receptor stimulation induced, specifically on CD8(+) MAIT cells, an upregulation of the integrin very late antigen-4 that is essential for the infiltration of CD8(+) T cells into the CNS. Notably, we were able to identify CD8(+) MAIT cells in MS brain lesions by immunohistochemistry while they were almost absent in the cerebrospinal fluid (CSF). In summary, our findings indicate that an IL-18-driven activation of CD8(+) MAIT cells contributes to their CNS infiltration in MS, in turn leading to reduced CD8(+) MAIT-cell frequencies in the blood. Therefore, CD8(+) MAIT cells seem to play a role in the innate arm of immunopathology in MS.
in vitro T cell stimulation/activation
Esposito, L., et al. (2014). "Investigation of soluble and transmembrane CTLA-4 isoforms in serum and microvesicles" J Immunol 193(2): 889-900. PubMed
Expression of the CTLA-4 gene is absolutely required for immune homeostasis, but aspects of its molecular nature remain undefined. In particular, the characterization of the soluble CTLA-4 (sCTLA-4) protein isoform generated by an alternatively spliced mRNA of CTLA4 lacking transmembrane-encoding exon 3 has been hindered by the difficulty in distinguishing it from the transmembrane isoform of CTLA-4, Tm-CTLA-4. In the current study, sCTLA-4 has been analyzed using novel mAbs and polyclonal Abs specific for its unique C-terminal amino acid sequence. We demonstrate that the sCTLA-4 protein is secreted at low levels following the activation of primary human CD4(+) T cells and is increased only rarely in the serum of autoimmune patients. Unexpectedly, during our studies aimed to define the kinetics of sCTLA-4 produced by activated human CD4(+) T cells, we discovered that Tm-CTLA-4 is associated with microvesicles produced by the activated cells. The functional roles of sCTLA-4 and microvesicle-associated Tm-CTLA-4 warrant further investigation, especially as they relate to the multiple mechanisms of action described for the more commonly studied cell-associated Tm-CTLA-4.
in vivo T cell depletion in humanized mice, ex vivo T cell inhibtion for xenografts
Wunderlich, M., et al. (2014). "OKT3 prevents xenogeneic GVHD and allows reliable xenograft initiation from unfractionated human hematopoietic tissues" Blood 123(24): e134-144. PubMed
Immunodeficient mice are now readily engrafted with human hematopoietic cells. However, these mice are susceptible to graft-versus-host disease (GVHD) induced by the engraftment and rapid expansion of coinjected human T cells. Therefore, highly purified sample populations must be used, adding significant time, expense, and effort. Here, we have explored in vivo and in vitro methods utilizing anti-T-cell antibodies to circumvent this problem. Intraperitoneal injection of the antibody within 48 hours prevented GVHD. Alternatively, short-term in vitro incubation of cells with antibody immediately before transplant was equally effective. Although in vitro antithymocyte globulin treatment resulted in a dramatic loss of SCID-repopulating cells (SRCs), treatment with OKT3 or UCHT1 abrogated GVHD risk and preserved engraftment potential. Leukemia samples that presented with substantial human T-cell contamination were effectively rescued from GVHD. In addition, OKT3 treatment of unfractionated cord blood resulted in robust engraftment of primary and secondary mice that was indistinguishable from grafts obtained using purified CD34(+) cells. Limiting dilution analysis of unfractionated blood demonstrated a SRC frequency of 1 in 300 to 500 CD34(+) cells, similar to that of purified hematopoietic stem and progenitor cells. This protocol streamlines xenograft studies while significantly reducing the cost and time of the procedure.
in vitro T cell stimulation/activation
Lines, J. L., et al. (2014). "VISTA is an immune checkpoint molecule for human T cells" Cancer Res 74(7): 1924-1932. PubMed
V-domain Ig suppressor of T cell activation (VISTA) is a potent negative regulator of T-cell function that is expressed on hematopoietic cells. VISTA levels are heightened within the tumor microenvironment, in which its blockade can enhance antitumor immune responses in mice. In humans, blockade of the related programmed cell death 1 (PD-1) pathway has shown great potential in clinical immunotherapy trials. Here, we report the structure of human VISTA and examine its function in lymphocyte negative regulation in cancer. VISTA is expressed predominantly within the hematopoietic compartment with highest expression within the myeloid lineage. VISTA-Ig suppressed proliferation of T cells but not B cells and blunted the production of T-cell cytokines and activation markers. Our results establish VISTA as a negative checkpoint regulator that suppresses T-cell activation, induces Foxp3 expression, and is highly expressed within the tumor microenvironment. By analogy to PD-1 and PD-L1 blockade, VISTA blockade may offer an immunotherapeutic strategy for human cancer.
IL-21-armored B7H3 CAR-iNKT cells exert potent antitumor effects.
In IScience on 19 January 2024 by Liu, Y., Dang, Y., et al.
PubMed
CD1d-restricted invariant NKT (iNKT) cells play a critical role in tumor immunity. However, the scarcity and limited persistence restricts their development and clinical application. Here, we demonstrated that iNKT cells could be efficiently expanded using modified cytokines combination from peripheral blood mononuclear cells. Introduction of IL-21 significantly increased the frequency of CD62L-positive memory-like iNKT cells. iNKT cells armoring with B7H3-targeting second generation CAR and IL-21 showed potent tumor cell killing activity. Moreover, co-expression of IL-21 promoted the activation of Stat3 signaling and reduced the expression of exhaustion markers in CAR-iNKT cells in vitro. Most importantly, IL-21-arming significantly prolonged B7H3 CAR-iNKT cell proliferation and survival in vivo, thus improving their therapeutic efficacy in mouse renal cancer xerograph models without observed cytokine-related adverse events. In summary, these results suggest that B7H3 CAR-iNKT armored with IL-21 is a promising therapeutic strategy for cancer treatment. © 2023 The Authors.
- Cancer Research,
Facile generation of biepitopic antibodies with intrinsic agonism for activating receptors in the tumor necrosis factor superfamily
Preprint on BioRxiv : the Preprint Server for Biology on 12 December 2023 by Jhajj, H. S., Schardt, J. S., et al.
PubMed
Summary Agonist antibodies that activate cellular receptors are being pursued for therapeutic applications ranging from neurodegenerative diseases to cancer. For the tumor necrosis factor (TNF) receptor superfamily, higher-order clustering of three or more receptors is key to their potent activation. This can be achieved using antibodies that recognize two unique epitopes on the same receptor and mediate receptor superclustering. However, identifying compatible pairs of antibodies to generate biepitopic antibodies (also known as biparatopic antibodies) for activating TNF receptors typically requires animal immunization and is a laborious and unpredictable process. Here, we report a simple method for systematically identifying biepitopic antibodies that potently activate TNF receptors without the need for additional animal immunization. Our approach uses off-the-shelf, receptor-specific IgG antibodies, which lack intrinsic (Fc-gamma receptor-independent) agonist activity, to first block their corresponding epitopes. Next, we perform selections for single-chain antibodies from human nonimmune libraries that bind accessible epitopes on the same ectodomains using yeast surface display and fluorescence-activated cell sorting. The selected single-chain antibodies are finally fused to the light chains of IgGs to generate human tetravalent antibodies that engage two different receptor epitopes and mediate potent receptor activation. We highlight the broad utility of this approach by converting several existing clinical-stage antibodies against TNF receptors, including ivuxolimab and pogalizumab against OX40 and utomilumab against CD137, into biepitopic antibodies with highly potent agonist activity. We expect that this widely accessible methodology can be used to systematically generate biepitopic antibodies for activating other receptors in the TNF receptor superfamily and many other receptors whose activation is dependent on strong receptor clustering.
- Homo sapiens (Human),
- Immunology and Microbiology
Precise surface functionalization of PLGA particles for human T cell modulation.
In Nature Protocols on 1 November 2023 by Hadley, P., Chen, Y., et al.
PubMed
The biofunctionalization of synthetic materials has extensive utility for biomedical applications, but approaches to bioconjugation typically show insufficient efficiency and controllability. We recently developed an approach by building synthetic DNA scaffolds on biomaterial surfaces that enables the precise control of cargo density and ratio, thus improving the assembly and organization of functional cargos. We used this approach to show that the modulation and phenotypic adaptation of immune cells can be regulated using our precisely functionalized biomaterials. Here, we describe the three key procedures, including the fabrication of polymeric particles engrafted with short DNA scaffolds, the attachment of functional cargos with complementary DNA strands, and the surface assembly control and quantification. We also explain the critical checkpoints needed to ensure the overall quality and expected characteristics of the biological product. We provide additional experimental design considerations for modifying the approach by varying the material composition, size or cargo types. As an example, we cover the use of the protocol for human primary T cell activation and for the identification of parameters that affect ex vivo T cell manufacturing. The protocol requires users with diverse expertise ranging from synthetic materials to bioconjugation chemistry to immunology. The fabrication procedures and validation assays to design high-fidelity DNA-scaffolded biomaterials typically require 8 d. © 2023. Springer Nature Limited.
- In Vitro,
- Homo sapiens (Human),
- Immunology and Microbiology,
- Pathology
Loss of CD4+ T cell-intrinsic arginase 1 accelerates Th1 response kinetics and reduces lung pathology during influenza infection.
In Immunity on 12 September 2023 by West, E. E., Merle, N. S., et al.
PubMed
Arginase 1 (Arg1), the enzyme catalyzing the conversion of arginine to ornithine, is a hallmark of IL-10-producing immunoregulatory M2 macrophages. However, its expression in T cells is disputed. Here, we demonstrate that induction of Arg1 expression is a key feature of lung CD4+ T cells during mouse in vivo influenza infection. Conditional ablation of Arg1 in CD4+ T cells accelerated both virus-specific T helper 1 (Th1) effector responses and its resolution, resulting in efficient viral clearance and reduced lung pathology. Using unbiased transcriptomics and metabolomics, we found that Arg1-deficiency was distinct from Arg2-deficiency and caused altered glutamine metabolism. Rebalancing this perturbed glutamine flux normalized the cellular Th1 response. CD4+ T cells from rare ARG1-deficient patients or CRISPR-Cas9-mediated ARG1-deletion in healthy donor cells phenocopied the murine cellular phenotype. Collectively, CD4+ T cell-intrinsic Arg1 functions as an unexpected rheostat regulating the kinetics of the mammalian Th1 lifecycle with implications for Th1-associated tissue pathologies. Published by Elsevier Inc.
- Cell Biology,
- Immunology and Microbiology
LC3B conjugation machinery promotes autophagy-independent HIV-1 entry in CD4+ T lymphocytes
Preprint on BioRxiv : the Preprint Server for Biology on 11 July 2023 by Pradel, B., Deffieu, M. S., et al.
PubMed
HIV-1 entry into CD4+ T lymphocytes relies on the viral and cellular membranes’ fusion, leading to viral capsid delivery in the cytoplasm of target cells. The conjugation of ATG8/LC3B protein, process referred to as ATG8ylation and mainly studied in the context of autophagy, occurs transiently in the early stages of the HIV-1 replication cycle in CD4+ T lymphocytes. Despite numerous studies investigating the interplays of HIV-1 with autophagy machinery, the impact of ATG8ylation in the early stages of HIV-1 infection remains unknown. Here we found that HIV-1 exposure leads to the rapid enrichment of LC3B towards the target cell plasma membrane, in close proximity with the incoming viral particles. Furthermore, we demonstrated that ATG8ylation is a key event that facilitates HIV-1 fusion with target CD4+ T cells. Interestingly, this effect is independent of the canonical autophagy pathway as ATG13 silencing does not prevent HIV-1 entry. Together, our results provide an unconventional role of LC3B conjugation subverted by HIV-1 to achieve a critical early step of its replication cycle. Teaser HIV-1 induces LC3B enrichment towards its target cell entry site and uses the conjugation of this protein to favor its entry step.
- FC/FACS,
- Homo sapiens (Human),
- Cancer Research,
- Immunology and Microbiology
STAT4, a potential predictor of prognosis, promotes CD8 T‑cell infiltration in ovarian serous carcinoma by inducing CCL5 secretion.
In Oncology Reports on 1 July 2023 by Wang, W., Liu, S., et al.
PubMed
Ovarian serous carcinoma (OC) is a common cause of mortality among gynecological malignancies. Although tumor‑infiltrating CD8 T cells are associated with a favorable prognosis of OC, the underlying mechanisms are not clearly understood. The present study identified the key genes and potential molecular mechanisms associated with CD8 T‑cell infiltration in OC. The score of CD8 T cells in The Cancer Genome Atlas dataset (376 samples from patients with OC) was estimated using the quanTIseq and MCP‑counter algorithms. Thereafter, a protein‑protein interaction network of differentially expressed genes was constructed and the hub genes were identified using cytoHubba in Cytoscape. The results revealed that signal transducer and activator of transcription 4 (STAT4) was strongly correlated with CD8 T‑cell infiltration in OC. Furthermore, the prognostic value of STAT4 in OC was verified by Kaplan‑Meier curve, and univariate and multivariate analyses. The biological functions of STAT4 were determined by Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway analyses, which revealed that STAT4 is closely related to cytokines in OC. Moreover, Spearman correlation analysis suggested that STAT4 was most positively correlated with CC chemokine ligand 5 (CCL5). CCL5 was revealed to be critical for orchestrating T‑cell infiltration in tumors. Moreover, immunohistochemistry and reverse transcription‑quantitative PCR showed that STAT4, CCL5 and CD8A (a marker for CD8 T cells) were closely related in OC. Moreover, in vitro analysis revealed that STAT4 knockdown led to a decrease in CCL5 expression and CD8 T‑cell migration. Taken together, the present study suggested that STAT4 may regulate CD8 T‑cell infiltration in OC tissues by inducing CCL5 secretion. Furthermore, STAT4 may be considered a promising prognostic biomarker for OC.
- Biochemistry and Molecular biology,
- Immunology and Microbiology
Surfaces for Study of Receptor Dynamics on T Cells.
In Methods in Molecular Biology (Clifton, N.J.) on 28 April 2023 by McColl, J. & Klenerman, D.
PubMed
Microscopy developments since the turn of the decade have seen an abundance of imaging modalities emerge that are revolutionizing the way we image the immune system. We are now able to image faster and utilize techniques that can image individual receptors, in real time, on live T cells. Total internal reflection fluorescence (TIRF) microscopy is one such technique, although it has one problem. The imaging must be carried out close to the glass interface. There are clearly issues with live cell imaging at glass surfaces as these are not biologically relevant. Manipulating the surface is key for maintaining biologically relevant imaging conditions. Here, we describe a simple approach to generate substrates for cell attachment and imaging of receptor dynamics and outline a guide for imaging and tracking T cell, surface receptors using TIRF microscopy. © 2023. The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature.
- Cancer Research,
- Cell Biology,
- Stem Cells and Developmental Biology
Autophagy inhibition impairs leukemia stem cell function in FLT3-ITD AML but has antagonistic interactions with tyrosine kinase inhibition.
In Leukemia on 1 November 2022 by Qiu, S., Kumar, H., et al.
PubMed
The FLT3-ITD mutation is associated with poor prognosis in acute myeloid leukemia (AML). FLT3 tyrosine kinase inhibitors (TKIs) demonstrate clinical efficacy but fail to target leukemia stem cells (LSC) and do not generate sustained responses. Autophagy is an important cellular stress response contributing to hematopoietic stem cells (HSC) maintenance and promoting leukemia development. Here we investigated the role of autophagy in regulating FLT3-ITD AML stem cell function and response to TKI treatment. We show that autophagy inhibition reduced quiescence and depleted repopulating potential of FLT3-ITD AML LSC, associated with mitochondrial accumulation and increased oxidative phosphorylation. However, TKI treatment reduced mitochondrial respiration and unexpectedly antagonized the effects of autophagy inhibition on LSC attrition. We further show that TKI-mediated targeting of AML LSC and committed progenitors was p53-dependent, and that autophagy inhibition enhanced p53 activity and increased TKI-mediated targeting of AML progenitors, but decreased p53 activity in LSC and reduced TKI-mediated LSC inhibition. These results provide new insights into the role of autophagy in differentially regulating AML stem and progenitor cells, reveal unexpected antagonistic effects of combined oncogenic tyrosine kinase inhibition and autophagy inhibition in AML LSC, and suggest an alternative approach to target AML LSC quiescence and regenerative potential. © 2022. The Author(s), under exclusive licence to Springer Nature Limited.
- In Vitro,
- FC/FACS,
- Homo sapiens (Human),
- Biochemistry and Molecular biology,
- Cancer Research,
- Immunology and Microbiology
WNK3 inhibition elicits antitumor immunity by suppressing PD-L1 expression on tumor cells and activating T-cell function.
In Experimental & Molecular Medicine on 1 November 2022 by Yoon, H. J., Kim, G. C., et al.
PubMed
Immune checkpoint therapies, such as programmed cell death ligand 1 (PD-L1) blockade, have shown remarkable clinical benefit in many cancers by restoring the function of exhausted T cells. Hence, the identification of novel PD-L1 regulators and the development of their inhibition strategies have significant therapeutic advantages. Here, we conducted pooled shRNA screening to identify regulators of membrane PD-L1 levels in lung cancer cells targeting druggable genes and cancer drivers. We identified WNK lysine deficient protein kinase 3 (WNK3) as a novel positive regulator of PD-L1 expression. The kinase-dead WNK3 mutant failed to elevate PD-L1 levels, indicating the involvement of its kinase domain in this function. WNK3 perturbation increased cancer cell death in cancer cell-immune cell coculture conditions and boosted the secretion of cytokines and cytolytic enzymes, promoting antitumor activities in CD4+ and CD8+ T cells. WNK463, a pan-WNK inhibitor, enhanced CD8+ T-cell-mediated antitumor activity and suppressed tumor growth as a monotherapy as well as in combination with a low-dose anti-PD-1 antibody in the MC38 syngeneic mouse model. Furthermore, we demonstrated that the c-JUN N-terminal kinase (JNK)/c-JUN pathway underlies WNK3-mediated transcriptional regulation of PD-L1. Our findings highlight that WNK3 inhibition might serve as a potential therapeutic strategy for cancer immunotherapy through its concurrent impact on cancer cells and immune cells. © 2022. The Author(s).
- Cancer Research,
- Immunology and Microbiology
Targeting PD-L1 and TIGIT could restore intratumoral CD8 T cell function in human colorectal cancer.
In Cancer Immunology, Immunotherapy : CII on 1 October 2022 by Thibaudin, M., Limagne, E., et al.
PubMed
Microsatellite stable colorectal cancers (MSS-CRC) are resistant to anti-PD-1/PD-L1 therapy but the combination of immune checkpoints inhibitors (ICI) could be a clue to reverse resistance. Our aim was to evaluate ex vivo the capacity of the combination of atezolizumab (anti-PD-L1) and tiragolumab (anti-TIGIT) to reactivate the immune response of tumor infiltrating lymphocytes (TILs) in MSS-CRC. We analysed CRC tumor tissue and the associated blood sample in parallel. For each patient sample, extensive immunomonitoring and cytokine production were tested. We generated an ex vivo assay to study immune reactivity following immune stimulation with checkpoint inhibitors of tumor cell suspensions. Three microsatellite instable (MSI) and 13 MSS-CRC tumors were analysed. To generalize our observations, bioinformatics analyses were performed on public data of single cell RNA sequencing of CRC TILs and RNA sequencing data of TCGA. Atezolizumab alone could only reactivate T cells from MSI tumors. Atezolizumab and tiragolumab reactivated T cells in 46% of MSS-CRC samples. Reactivation by ICK was observed in patients with higher baseline frequency of Th1 and Tc1 cells, and was also associated with higher baseline T cell polyfunctionality and higher CD96 expression. We showed that a high frequency of CD96 expression on T cells could be a surrogate marker of atezolizumab and tiragolumab efficacy. Together these data suggest that the association of atezolizumab and tiragolumab could restore function of CD4 and CD8 TILs in MSS-CRC and could be tested in a clinical trial in colorectal cancer patients with MSS status. © 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
- Homo sapiens (Human),
- COVID-19,
- Immunology and Microbiology
IL-6 drives T cell death to participate in lymphopenia in COVID-19.
In International Immunopharmacology on 1 October 2022 by Zhou, X., Ye, G., et al.
PubMed
Lymphopenia is a common observation in patients with COVID-19. To explore the cause of T cell lymphopenia in the disease, laboratory results of 64 hospitalized COVID-19 patients were retrospectively analyzed and six patients were randomly selected to trace their changes of T lymphocytes and plasma concentration of IL-6 for the course of disease. Results confirmed that the T-cell lymphopenia, especially CD4+ T cell reduction in COVID-19 patients, was a reliable indicator of severity and hospitalization in infected patients. And CD4+ T cell count below 200 cells/μL predicts critical illness in COVID-19 patients. In vitro assay supported that exposure to key contributors (IL-1β, IL-6, TNF-α and IFN-γ) of COVID-19 cytokine storm caused substantial death of activated T cells. Among these contributors, IL-6 level was found to probably reversely correlate with T cell counts in patients. And IL-6 alone was potent to induce T cell reduction by gasderminE-mediated pyroptosis, inferring IL-6 took a part in affecting the function and status of T cells in COVID-19 patients. Intervention of IL-6 mediated T cell pryprotosis may effectively delay disease progression, maintain normal immune status at an early stage of infection. Copyright © 2022 Elsevier B.V. All rights reserved.
- Binding,
- Homo sapiens (Human),
- Biochemistry and Molecular biology
Discovery of Aptamers Against Cell Surface Markers Using Ligand-Guided Selection.
In Methods in Molecular Biology (Clifton, N.J.) on 27 September 2022 by Williams, N., Patel, R., et al.
PubMed
Oligonucleotide ligands (DNA, RNA, or XNA), also known as aptamers, are selected against various target molecules using an iterative, evolutionary process called systematic evolution of ligands by exponential enrichment (SELEX). To select aptamers against complex cell surface proteins in their native state, a variant of SELEX termed ligand-guided selection (LIGS) was recently introduced. The significance of LIGS is rooted in its strategy of exploiting the selection step in SELEX to identify highly specific aptamers against known cell surface markers. Thus, in LIGS, a higher-affinity secondary ligand, such as a monoclonal antibody (mAb) to a whole-cell bound to an evolved SELEX library, is introduced to outcompete sequences against the mAb targeting cell surface protein or induce a conformational switch to destabilize the aptamer-surface cell surface protein resulting in elution of the sequences. Here, we describe the detailed method of LIGS utilized in identifying aptamers against T-cell receptor cluster of differentiation three complex (TCR-CD3) expressed in human T-cells and T-cell leukemia. © 2023. The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature.
- Cancer Research,
- Immunology and Microbiology
Balance between immunoregulatory B cells and plasma cells drives pancreatic tumor immunity.
In Cell Reports Medicine on 20 September 2022 by Mirlekar, B., Wang, Y., et al.
PubMed
Plasma cell responses are associated with anti-tumor immunity and favorable response to immunotherapy. B cells can amplify anti-tumor immune responses through antibody production; yet B cells in patients and tumor-bearing mice often fail to support this effector function. We identify dysregulated transcriptional program in B cells that disrupts differentiation of naive B cells into anti-tumor plasma cells. The signaling network contributing to this dysfunction is driven by interleukin (IL) 35 stimulation of a STAT3-PAX5 complex that upregulates the transcriptional regulator BCL6 in naive B cells. Transient inhibition of BCL6 in tumor-educated naive B cells is sufficient to reverse the dysfunction in B cell differentiation, stimulating the intra-tumoral accumulation of plasma cells and effector T cells and rendering pancreatic tumors sensitive to anti-programmed cell death protein 1 (PD-1) blockade. Our findings argue that B cell effector dysfunction in cancer can be due to an active systemic suppression program that can be targeted to synergize with T cell-directed immunotherapy.Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
- Immunology and Microbiology
Bioinstructive implantable scaffolds for rapid in vivo manufacture and release of CAR-T cells.
In Nature Biotechnology on 1 August 2022 by Agarwalla, P., Ogunnaike, E. A., et al.
PubMed
Despite their clinical success, chimeric antigen receptor (CAR)-T cell therapies for B cell malignancies are limited by lengthy, costly and labor-intensive ex vivo manufacturing procedures that might lead to cell products with heterogeneous composition. Here we describe an implantable Multifunctional Alginate Scaffold for T Cell Engineering and Release (MASTER) that streamlines in vivo CAR-T cell manufacturing and reduces processing time to a single day. When seeded with human peripheral blood mononuclear cells and CD19-encoding retroviral particles, MASTER provides the appropriate interface for viral vector-mediated gene transfer and, after subcutaneous implantation, mediates the release of functional CAR-T cells in mice. We further demonstrate that in vivo-generated CAR-T cells enter the bloodstream and control distal tumor growth in a mouse xenograft model of lymphoma, showing greater persistence than conventional CAR-T cells. MASTER promises to transform CAR-T cell therapy by fast-tracking manufacture and potentially reducing the complexity and resources needed for provision of this type of therapy. © 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
- WB,
- Immunology and Microbiology
Tetraspanin CD53 controls T cell immunity through regulation of CD45RO stability, mobility, and function.
In Cell Reports on 28 June 2022 by Dunlock, V. E., Arp, A. B., et al.
PubMed
T cells depend on the phosphatase CD45 to initiate T cell receptor signaling. Although the critical role of CD45 in T cells is established, the mechanisms controlling function and localization in the membrane are not well understood. Moreover, the regulation of specific CD45 isoforms in T cell signaling remains unresolved. By using unbiased mass spectrometry, we identify the tetraspanin CD53 as a partner of CD45 and show that CD53 controls CD45 function and T cell activation. CD53-negative T cells (Cd53-/-) exhibit substantial proliferation defects, and Cd53-/- mice show impaired tumor rejection and reduced IFNγ-producing T cells compared with wild-type mice. Investigation into the mechanism reveals that CD53 is required for CD45RO expression and mobility. In addition, CD53 is shown to stabilize CD45 on the membrane and is required for optimal phosphatase activity and subsequent Lck activation. Together, our findings reveal CD53 as a regulator of CD45 activity required for T cell immunity.Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
- Cancer Research,
- Immunology and Microbiology
Neuropilin-1 cooperates with PD-1 in CD8+ T cells predicting outcomes in melanoma patients treated with anti-PD1.
In IScience on 17 June 2022 by Rossignol, J., Belaid, Z., et al.
PubMed
Targeting immune checkpoints, such as Programmed cell Death 1 (PD1), has improved survival in cancer patients by restoring antitumor immune responses. Most patients, however, relapse or are refractory to immune checkpoint blocking therapies. Neuropilin-1 (NRP1) is a transmembrane glycoprotein required for nervous system and angiogenesis embryonic development, also expressed in immune cells. We hypothesized that NRP1 could be an immune checkpoint co-receptor modulating CD8+ T cells activity in the context of the antitumor immune response. Here, we show that NRP1 is recruited in the cytolytic synapse of PD1+CD8+ T cells, cooperates and enhances PD-1 activity. In mice, CD8+ T cells specific deletion of Nrp1 improves anti-PD1 antibody antitumor immune responses. Likewise, in human metastatic melanoma, the expression of NRP1 in tumor infiltrating CD8+ T cells predicts poor outcome of patients treated with anti-PD1. NRP1 is a promising target to overcome resistance to anti-PD1 therapies.© 2022.
- Cell Culture,
- Homo sapiens (Human)
Ursodeoxycholic acid reduces antitumor immunosuppression by inducing CHIP-mediated TGF-β degradation.
In Nature Communications on 14 June 2022 by Shen, Y., Lu, C., et al.
PubMed
TGF-β is essential for inducing systemic tumor immunosuppression; thus, blocking TGF-β can greatly enhance antitumor immunity. However, there are still no effective TGF-β inhibitors in clinical use. Here, we show that the clinically approved compound ursodeoxycholic acid (UDCA), by degrading TGF-β, enhances antitumor immunity through restraining Treg cell differentiation and activation in tumor-bearing mice. Furthermore, UDCA synergizes with anti-PD-1 to enhance antitumor immunity and tumor-specific immune memory in tumor-bearing mice. UDCA phosphorylates TGF-β at T282 site via TGR5-cAMP-PKA axis, causing increased binding of TGF-β to carboxyl terminus of Hsc70-interacting protein (CHIP). Then, CHIP ubiquitinates TGF-β at the K315 site, initiating p62-dependent autophagic sorting and subsequent degradation of TGF-β. Notably, results of retrospective analysis shows that combination therapy with anti-PD-1 or anti-PD-L1 and UDCA has better efficacy in tumor patients than anti-PD-1 or anti-PD-L1 alone. Thus, our results show a mechanism for TGF-β regulation and implicate UDCA as a potential TGF-β inhibitor to enhance antitumor immunity. © 2022. The Author(s).
- FC/FACS,
- Cancer Research
In vivo anti-tumor effect of PARP inhibition in IDH1/2 mutant MDS/AML resistant to targeted inhibitors of mutant IDH1/2.
In Leukemia on 1 May 2022 by Gbyli, R., Song, Y., et al.
PubMed
Treatment options for patients with relapsed/refractory acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) are scarce. Recurring mutations, such as mutations in isocitrate dehydrogenase-1 and -2 (IDH1/2) are found in subsets of AML and MDS, are therapeutically targeted by mutant enzyme-specific small molecule inhibitors (IDHmi). IDH mutations induce diverse metabolic and epigenetic changes that drive malignant transformation. IDHmi alone are not curative and resistance commonly develops, underscoring the importance of alternate therapeutic options. We were first to report that IDH1/2 mutations induce a homologous recombination (HR) defect, which confers sensitivity to poly (ADP)-ribose polymerase inhibitors (PARPi). Here, we show that the PARPi olaparib is effective against primary patient-derived IDH1/2-mutant AML/ MDS xeno-grafts (PDXs). Olaparib efficiently reduced overall engraftment and leukemia-initiating cell frequency as evident in serial transplantation assays in IDH1/2-mutant but not -wildtype AML/MDS PDXs. Importantly, we show that olaparib is effective in both IDHmi-naïve and -resistant AML PDXs, critical given the high relapse and refractoriness rates to IDHmi. Our pre-clinical studies provide a strong rationale for the translation of PARP inhibition to patients with IDH1/2-mutant AML/ MDS, providing an additional line of therapy for patients who do not respond to or relapse after targeted mutant IDH inhibition. © 2022. The Author(s), under exclusive licence to Springer Nature Limited.
- Immunology and Microbiology
CD137 (4-1BB) costimulation of CD8+ T cells is more potent when provided in cis than in trans with respect to CD3-TCR stimulation.
In Nature Communications on 15 December 2021 by Otano, I., Azpilikueta, A., et al.
PubMed
CD137 (4-1BB; TNFSR9) is an activation-induced surface receptor that through costimulation effects provide antigen-primed T cells with augmented survival, proliferation and effector functions as well as metabolic advantages. These immunobiological mechanisms are being utilised for cancer immunotherapy with agonist CD137-binding and crosslinking-inducing agents that elicit CD137 intracellular signaling. In this study, side-by-side comparisons show that provision of CD137 costimulation in-cis with regard to the TCR-CD3-ligating cell is superior to that provided in-trans in terms of T cell activation, proliferation, survival, cytokine secretion and mitochondrial fitness in mouse and human. Cis ligation of CD137 relative to the TCR-CD3 complex results in more intense canonical and non-canonical NF-κB signaling and provides a more robust induction of cell cycle and DNA damage repair gene expression programs. Here we report that the superiority of cis versus trans CD137-costimulation is readily observed in vivo and is relevant for understanding the immunotherapeutic effects of CAR T cells and CD137 agonistic therapies currently undergoing clinical trials, which may provide costimulation either in cis or in trans. © 2021. The Author(s).
- Cell Culture,
- Homo sapiens (Human),
- COVID-19,
- Immunology and Microbiology
Effective chimeric antigen receptor T cells against SARS-CoV-2.
In IScience on 19 November 2021 by Guo, X., Kazanova, A., et al.
PubMed
Current therapies to treat coronavirus disease 2019 (COVID-19) involve vaccines against the spike protein S1 of SARS-CoV-2. Here, we outline an alternative approach involving chimeric antigen receptors (CARs) in T cells (CAR-Ts). CAR-T recognition of the SARS-CoV-2 receptor-binding domain (RBD) peptide induced ribosomal protein S6 phosphorylation, the increased expression of activation antigen, CD69 and effectors, interferon-γ, granzyme B, perforin, and Fas-ligand on overlapping subsets of CAR-Ts. CAR-Ts further showed potent in vitro killing of target cells loaded with RBD, S1 peptide, or expressing the S1 protein. The efficacy of killing varied with different sized hinge regions, whereas time-lapse microscopy showed CAR-T cluster formation around RBD-expressing targets. Cytolysis of targets was mediated primarily by the GZMB/perforin pathway. Lastly, we showed in vivo killing of S1-expressing cells by our SARS-CoV-2 CAR-Ts in mice. The successful generation of SARS-CoV-2 CAR-Ts represents a living vaccine approach for the treatment of COVID-19.Crown Copyright © 2021.