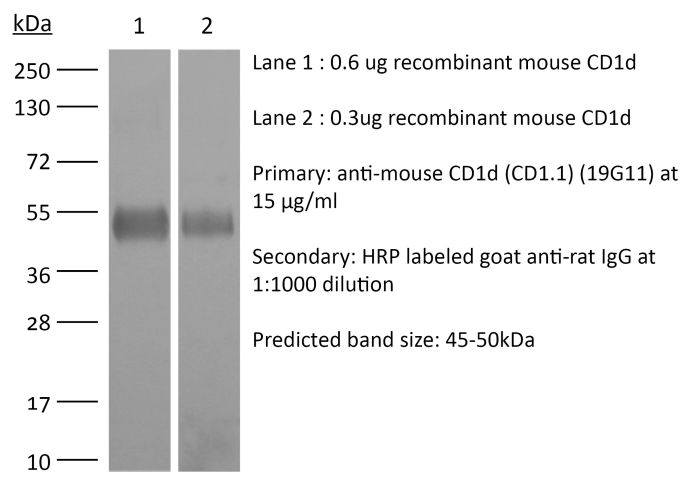
InVivoMAb anti-mouse CD1d (CD1.1)
Product Details
The 19G11 monoclonal antibody reacts with mouse CD1d also known as CD1.1. CD1d is a 48 kDa type I membrane glycoprotein and a member of the CD1 family of glycoproteins. CD1d is a non-classical MHC protein with structural homology to class I MHC molecules. CD1d is expressed on the surface of various antigen-presenting cells and is involved in the presentation of non-peptide glycolipid antigens to to CD1d-restricted T cells. CD1d-presented glycolipid antigens activate invariant natural killer T (iNKT) cells, through the interaction with the T-cell receptor present on iNKT cell membranes. When activated, iNKT cells rapidly produce Th1 and Th2 cytokines.Specifications
| Isotype | Rat IgG1 |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG1 isotype control, anti-horseradish peroxidase |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse CD1d |
| Reported Applications |
in vivo CD1d neutralization in vitro CD1d neutralization |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107568 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
in vitro CD1d neutralization
Melum, E., et al. (2019). "Control of CD1d-restricted antigen presentation and inflammation by sphingomyelin" Nat Immunol 20(12): 1644-1655. PubMed
Invariant natural killer T (iNKT) cells recognize activating self and microbial lipids presented by CD1d. CD1d can also bind non-activating lipids, such as sphingomyelin. We hypothesized that these serve as endogenous regulators and investigated humans and mice deficient in acid sphingomyelinase (ASM), an enzyme that degrades sphingomyelin. We show that ASM absence in mice leads to diminished CD1d-restricted antigen presentation and iNKT cell selection in the thymus, resulting in decreased iNKT cell levels and resistance to iNKT cell-mediated inflammatory conditions. Defective antigen presentation and decreased iNKT cells are also observed in ASM-deficient humans with Niemann-Pick disease, and ASM activity in healthy humans correlates with iNKT cell phenotype. Pharmacological ASM administration facilitates antigen presentation and restores the levels of iNKT cells in ASM-deficient mice. Together, these results demonstrate that control of non-agonistic CD1d-associated lipids is critical for iNKT cell development and function in vivo and represents a tight link between cellular sphingolipid metabolism and immunity.
in vivo CD1d neutralization
Olszak, T., et al. (2014). "Protective mucosal immunity mediated by epithelial CD1d and IL-10" Nature 509(7501): 497-502. PubMed
The mechanisms by which mucosal homeostasis is maintained are of central importance to inflammatory bowel disease. Critical to these processes is the intestinal epithelial cell (IEC), which regulates immune responses at the interface between the commensal microbiota and the host. CD1d presents self and microbial lipid antigens to natural killer T (NKT) cells, which are involved in the pathogenesis of colitis in animal models and human inflammatory bowel disease. As CD1d crosslinking on model IECs results in the production of the important regulatory cytokine interleukin (IL)-10 (ref. 9), decreased epithelial CD1d expression–as observed in inflammatory bowel disease–may contribute substantially to intestinal inflammation. Here we show in mice that whereas bone-marrow-derived CD1d signals contribute to NKT-cell-mediated intestinal inflammation, engagement of epithelial CD1d elicits protective effects through the activation of STAT3 and STAT3-dependent transcription of IL-10, heat shock protein 110 (HSP110; also known as HSP105), and CD1d itself. All of these epithelial elements are critically involved in controlling CD1d-mediated intestinal inflammation. This is demonstrated by severe NKT-cell-mediated colitis upon IEC-specific deletion of IL-10, CD1d, and its critical regulator microsomal triglyceride transfer protein (MTP), as well as deletion of HSP110 in the radioresistant compartment. Our studies thus uncover a novel pathway of IEC-dependent regulation of mucosal homeostasis and highlight a critical role of IL-10 in the intestinal epithelium, with broad implications for diseases such as inflammatory bowel disease.
in vivo CD1d neutralization
An, D., et al. (2014). "Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells" Cell 156(1-2): 123-133. PubMed
Coevolution of beneficial microorganisms with the mammalian intestine fundamentally shapes mammalian physiology. Here, we report that the intestinal microbe Bacteroides fragilis modifies the homeostasis of host invariant natural killer T (iNKT) cells by supplementing the host’s endogenous lipid antigen milieu with unique inhibitory sphingolipids. The process occurs early in life and effectively impedes iNKT cell proliferation during neonatal development. Consequently, total colonic iNKT cell numbers are restricted into adulthood, and hosts are protected against experimental iNKT cell-mediated, oxazolone-induced colitis. In studies with neonatal mice lacking access to bacterial sphingolipids, we found that treatment with B. fragilis glycosphingolipids-exemplified by an isolated peak (MW = 717.6) called GSL-Bf717-reduces colonic iNKT cell numbers and confers protection against oxazolone-induced colitis in adulthood. Our results suggest that the distinctive inhibitory capacity of GSL-Bf717 and similar molecules may prove useful in the treatment of autoimmune and allergic disorders in which iNKT cell activation is destructive.
- Mus musculus (House mouse),
Ultra-low volume intradermal administration of radiation-attenuated sporozoites with the glycolipid adjuvant 7DW8-5 completely protects mice against malaria.
In Scientific Reports on 4 February 2024 by Watson, F. N., Shears, M. J., et al.
PubMed
Radiation-attenuated sporozoite (RAS) vaccines can completely prevent blood stage Plasmodium infection by inducing liver-resident memory CD8+ T cells to target parasites in the liver. Such T cells can be induced by 'Prime-and-trap' vaccination, which here combines DNA priming against the P. yoelii circumsporozoite protein (CSP) with a subsequent intravenous (IV) dose of liver-homing RAS to "trap" the activated and expanding T cells in the liver. Prime-and-trap confers durable protection in mice, and efforts are underway to translate this vaccine strategy to the clinic. However, it is unclear whether the RAS trapping dose must be strictly administered by the IV route. Here we show that intradermal (ID) RAS administration can be as effective as IV administration if RAS are co-administrated with the glycolipid adjuvant 7DW8-5 in an ultra-low inoculation volume. In mice, the co-administration of RAS and 7DW8-5 in ultra-low ID volumes (2.5 µL) was completely protective and dose sparing compared to standard volumes (10-50 µL) and induced protective levels of CSP-specific CD8+ T cells in the liver. Our finding that adjuvants and ultra-low volumes are required for ID RAS efficacy may explain why prior reports about higher volumes of unadjuvanted ID RAS proved less effective than IV RAS. The ID route may offer significant translational advantages over the IV route and could improve sporozoite vaccine development. © 2024. The Author(s).
- Mus musculus (House mouse),
- Immunology and Microbiology
Unique adipose tissue invariant natural killer T cell subpopulations control adipocyte turnover in mice.
In Nature Communications on 21 December 2023 by Han, S. M., Park, E. S., et al.
PubMed
Adipose tissue invariant natural killer T (iNKT) cells are a crucial cell type for adipose tissue homeostasis in obese animals. However, heterogeneity of adipose iNKT cells and their function in adipocyte turnover are not thoroughly understood. Here, we investigate transcriptional heterogeneity in adipose iNKT cells and their hierarchy using single-cell RNA sequencing in lean and obese mice. We report that distinct subpopulations of adipose iNKT cells modulate adipose tissue homeostasis through adipocyte death and birth. We identify KLRG1+ iNKT cells as a unique iNKT cell subpopulation in adipose tissue. Adoptive transfer experiments showed that KLRG1+ iNKT cells are selectively generated within adipose tissue microenvironment and differentiate into a CX3CR1+ cytotoxic subpopulation in obese mice. In addition, CX3CR1+ iNKT cells specifically kill enlarged and inflamed adipocytes and recruit macrophages through CCL5. Furthermore, adipose iNKT17 cells have the potential to secrete AREG, and AREG is involved in stimulating adipose stem cell proliferation. Collectively, our data suggest that each adipose iNKT cell subpopulation plays key roles in the control of adipocyte turnover via interaction with adipocytes, adipose stem cells, and macrophages in adipose tissue. © 2023. The Author(s).
- Biochemistry and Molecular biology,
- Cell Biology,
- Immunology and Microbiology
Aberrant cholesterol metabolic signaling impairs antitumor immunosurveillance through natural killer T cell dysfunction in obese liver.
In Cellular Molecular Immunology on 1 July 2022 by Tang, W., Zhou, J., et al.
PubMed
Obesity is a major risk factor for cancers including hepatocellular carcinoma (HCC) that develops from a background of non-alcoholic fatty liver disease (NAFLD). Hypercholesterolemia is a common comorbidity of obesity. Although cholesterol biosynthesis mainly occurs in the liver, its role in HCC development of obese people remains obscure. Using high-fat high-carbohydrate diet-associated orthotopic and spontaneous NAFLD-HCC mouse models, we found that hepatic cholesterol accumulation in obesity selectively suppressed natural killer T (NKT) cell-mediated antitumor immunosurveillance. Transcriptome analysis of human liver revealed aberrant cholesterol metabolism and NKT cell dysfunction in NAFLD patients. Notably, cholesterol-lowering rosuvastatin restored NKT expansion and cytotoxicity to prevent obesogenic diet-promoted HCC development. Moreover, suppression of hepatic cholesterol biosynthesis by a mammalian target of rapamycin (mTOR) inhibitor vistusertib preceded tumor regression, which was abolished by NKT inactivation but not CD8+ T cell depletion. Mechanistically, sterol regulatory element-binding protein 2 (SREBP2)-driven excessive cholesterol production from hepatocytes induced lipid peroxide accumulation and deficient cytotoxicity in NKT cells, which were supported by findings in people with obesity, NAFLD and NAFLD-HCC. This study highlights mTORC1/SREBP2/cholesterol-mediated NKT dysfunction in the tumor-promoting NAFLD liver microenvironment, providing intervention strategies that invigorating NKT cells to control HCC in the obesity epidemic. © 2022. The Author(s), under exclusive licence to CSI and USTC.
- In Vivo,
- Mus musculus (House mouse)
Cryopreserved Sporozoites with and without the Glycolipid Adjuvant 7DW8-5 Protect in Prime-and-Trap Malaria Vaccination.
In The American Journal of Tropical Medicine and Hygiene on 6 April 2022 by Watson, F., Shears, M., et al.
PubMed
Repeated intravenous (IV) administration of radiation-attenuated sporozoite (RAS) vaccines induces Plasmodium-specific CD8+ liver-resident memory T (Trm) cells in mice and achieves sterile protection against challenge. Our heterologous "prime-and-trap" vaccine strategy was previously shown to simplify and improve upon RAS vaccination. Prime-and-trap vaccination combines epidermal priming by DNA-encoded circumsporozoite protein (CSP) antigen followed by a single IV dose of freshly dissected RAS (fresh-RAS) to direct and trap activated and expanding CD8+ T cells in the liver. Prime-and-trap vaccination protects mice against wild-type sporozoite (spz) challenge. Assessment of prime-and-trap vaccines in nonhuman primate (NHP) models and/or humans would be greatly enabled if fresh-RAS could be replaced by cryopreserved RAS (cryo-RAS). Here, we investigated if fresh-RAS could be replaced with cryo-RAS for prime-and-trap vaccination in BALB/cj mice. Despite a reduction in spz vaccine liver burden following cryo-RAS administration compared with fresh-RAS, cryo-RAS induced a similar level of Plasmodium yoelii (Py) CSP-specific CD8+ liver Trm cells and completely protected mice against Py spz challenge 112 days after vaccination. Additionally, when the glycolipid adjuvant 7DW8-5 was co-administered with cryo-RAS, 7DW8-5 permitted the dose of cryo-RAS to be reduced four-fold while still achieving high rates of sterile protection. In summary, cryo-RAS with and without 7DW8-5 were compatible with prime-and-trap malaria vaccination in a mouse model, which may accelerate the pathway for this vaccine strategy to move to NHPs and humans.
- Immunology and Microbiology,
- Neuroscience
Depletion of NK Cells Improves Cognitive Function in the Alzheimer Disease Mouse Model.
In The Journal of Immunology on 15 July 2020 by Zhang, Y., Fung, I. T. H., et al.
PubMed
Despite mounting evidence suggesting the involvement of the immune system in regulating brain function, the specific role of immune and inflammatory cells in neurodegenerative diseases remain poorly understood. In this study, we report that depletion of NK cells, a type of innate lymphocytes, alleviates neuroinflammation, stimulates neurogenesis, and improves cognitive function in a triple-transgenic Alzheimer disease (AD) mouse model. NK cells in the brains of triple-transgenic AD mouse model (3xTg-AD) mice exhibited an enhanced proinflammatory profile. Depletion of NK cells by anti-NK1.1 Abs drastically improved cognitive function of 3xTg-AD mice. NK cell depletion did not affect amyloid β concentrations but enhanced neurogenesis and reduced neuroinflammation. Notably, in 3xTg-AD mice depleted of NK cells, microglia demonstrated a homeostatic-like morphology, decreased proliferative response and reduced expression of neurodestructive proinflammatory cytokines. Together, our results suggest a proinflammatory role for NK cells in 3xTg-AD mice and indicate that targeting NK cells might unlock novel strategies to combat AD. Copyright © 2020 by The American Association of Immunologists, Inc.
- Mus musculus (House mouse),
- Immunology and Microbiology
Control of CD1d-restricted antigen presentation and inflammation by sphingomyelin.
In Nature Immunology on 1 December 2019 by Melum, E., Jiang, X., et al.
PubMed
Invariant natural killer T (iNKT) cells recognize activating self and microbial lipids presented by CD1d. CD1d can also bind non-activating lipids, such as sphingomyelin. We hypothesized that these serve as endogenous regulators and investigated humans and mice deficient in acid sphingomyelinase (ASM), an enzyme that degrades sphingomyelin. We show that ASM absence in mice leads to diminished CD1d-restricted antigen presentation and iNKT cell selection in the thymus, resulting in decreased iNKT cell levels and resistance to iNKT cell-mediated inflammatory conditions. Defective antigen presentation and decreased iNKT cells are also observed in ASM-deficient humans with Niemann-Pick disease, and ASM activity in healthy humans correlates with iNKT cell phenotype. Pharmacological ASM administration facilitates antigen presentation and restores the levels of iNKT cells in ASM-deficient mice. Together, these results demonstrate that control of non-agonistic CD1d-associated lipids is critical for iNKT cell development and function in vivo and represents a tight link between cellular sphingolipid metabolism and immunity.
- Binding,
- Immunology and Microbiology
TLR9-mediated dendritic cell activation uncovers mammalian ganglioside species with specific ceramide backbones that activate invariant natural killer T cells.
In PLoS Biology on 1 March 2019 by Paget, C., Deng, S., et al.
PubMed
CD1d-restricted invariant natural killer T (iNKT) cells represent a heterogeneous population of lipid-reactive T cells that are involved in many immune responses, mediated through T-cell receptor (TCR)-dependent and/or independent activation. Although numerous microbial lipid antigens (Ags) have been identified, several lines of evidence have suggested the existence of relevant Ags of endogenous origin. However, the identification of their precise nature as well as the molecular mechanisms involved in their generation are still highly controversial and ill defined. Here, we identified two mammalian gangliosides-namely monosialoganglioside GM3 and disialoganglioside GD3-as endogenous activators for mouse iNKT cells. These glycosphingolipids are found in Toll-like receptor-stimulated dendritic cells (DC) as several species varying in their N-acyl fatty chain composition. Interestingly, their ability to activate iNKT cells is highly dependent on the ceramide backbone structure. Thus, both synthetic GM3 and GD3 comprising a d18:1-C24:1 ceramide backbone were able to activate iNKT cells in a CD1d-dependent manner. GM3 and GD3 are not directly recognized by the iNKT TCR and required the Ag presenting cell intracellular machinery to reveal their antigenicity. We propose a new concept in which iNKT cells can rapidly respond to pre-existing self-molecules after stress-induced structural changes in CD1d-expressing cells. Moreover, these gangliosides conferred partial protection in the context of bacterial infection. Thus, this report identified new biologically relevant lipid self-Ags for iNKT cells.
- In Vivo,
- Mus musculus (House mouse),
- Immunology and Microbiology
Regulatory T Cells Promote Natural Killer Cell Education in Mixed Chimeras.
In American Journal of Transplantation : Official Journal of the American Society of Transplantation and the American Society of Transplant Surgeons on 1 December 2017 by Mahr, B., Pilat, N., et al.
PubMed
Therapeutic administration of regulatory T cells (Tregs) leads to engraftment of conventional doses of allogeneic bone marrow (BM) in nonirradiated recipient mice conditioned with costimulation blockade and mammalian target of rapamycin inhibition. The mode of action responsible for this Treg effect is poorly understood but may encompass the control of costimulation blockade-resistant natural killer (NK) cells. We show that transient NK cell depletion at the time of BM transplantation led to BM engraftment and persistent chimerism without Treg transfer but failed to induce skin graft tolerance. In contrast, the permanent absence of anti-donor NK reactivity in mice grafted with F1 BM was associated with both chimerism and tolerance comparable to Treg therapy, implying that NK cell tolerization is a critical mechanism of Treg therapy. Indeed, NK cells of Treg-treated BM recipients reshaped their receptor repertoire in the presence of donor MHC in a manner suggesting attenuated donor reactivity. These results indicate that adoptively transferred Tregs prevent BM rejection, at least in part, by suppressing NK cells and promote tolerance by regulating the appearance of NK cells expressing activating receptors to donor class I MHC. © 2017 The American Society of Transplantation and the American Society of Transplant Surgeons.
- In Vivo,
- Mus musculus (House mouse),
- Immunology and Microbiology
Influenza A virus-induced release of interleukin-10 inhibits the anti-microbial activities of invariant natural killer T cells during invasive pneumococcal superinfection.
In Mucosal Immunology on 1 March 2017 by Barthelemy, A., Ivanov, S., et al.
PubMed
During influenza A virus (IAV) infection, changes in the lung's physical and immunological defenses predispose the host to bacterial superinfections. Invariant natural killer T (iNKT) cells are innate-like T lymphocytes that have beneficial or harmful functions during infection. We investigated the iNKT cells' role in a model of invasive pneumococcal superinfection. The use of Jα18-/- mice indicated that iNKT cells limited susceptibility to influenza-pneumococcal infection and reduced the lethal synergism. This role did not depend on immune-based anti-bacterial mechanisms. At the time of bacterial exposure, iNKT cells from IAV-experienced mice failed to produce antipneumococcal interferon-γ and adoptive transfer of fresh iNKT cells before Streptococcus pneumoniae challenge did not restore anti-bacterial host defenses. Impaired iNKT cell activation in superinfected animals was related to the IAV-induced immunosuppressive cytokine interleukin-10 (IL-10), rather than to an intrinsic functional defect. IL-10 dampened the activation of iNKT cells in response to pneumococci by inhibiting the production of IL-12 by pulmonary monocyte-derived dendritic cells. Neutralization of IL-10 restored iNKT cell activation and tends to increase resistance to secondary bacterial infection. Overall, iNKT cells have a beneficial role (upstream of bacterial colonization) in controlling influenza-pneumococcal superinfection, although they represent novel targets of immunosuppression at the time of bacterial challenge.