InVivoSIM anti-human EGFR (Cetuximab Biosimilar)
Product Details
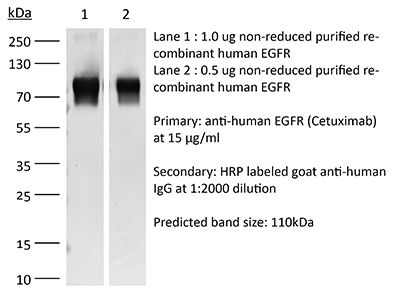
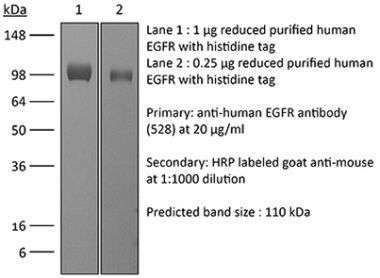
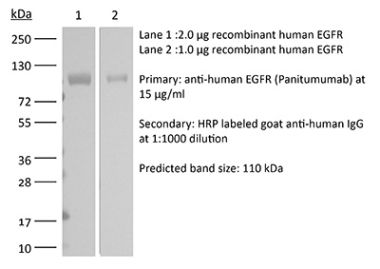
This non-therapeutic biosimilar antibody uses the same variable regions from the therapeutic antibody Cetuximab making it ideal for research use. This Cetuximab biosimilar reacts with human EGFR (epidermal growth factor receptor) also known as ErbB-1. EGFR is a 170 kDa cell-surface receptor and belongs to the ErbB family of receptors. EGFR signaling is activated upon binding one of its ligands including epidermal growth factor (EGF), transforming growth factor α (TGF α), Amphiregulin, and heparin binding-EGF (HB-EGF). Upon activation, EGFR transitions from an inactive monomeric form to an active homodimer. This initiates several downstream signal transduction cascades including the MAPK, Akt and JNK pathways, leading to DNA synthesis and cell proliferation. EGFR overexpression or constitutive activation are associated with many cancers. For this reason, anti-EGFR monoclonal antibody mediated immunotherapies are currently being explored as cancer treatments. Cetuximab inhibits tumor cell proliferation by blocking the interaction of EGF with EGFR.Specifications
| Isotype | Human IgG1 |
|---|---|
| Recommended Isotype Control(s) | RecombiMAb human IgG1 isotype control, anti-hen egg lysozyme |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Human EGFR (ErbB1) |
| Reported Applications |
in vitro EGFR2+ cell depletion (ADCC assay) in vitro functional assay EGFR blockade in vivo imaging Immunohistochemistry (frozen) Immunofluorescence Flow Cytometry ELISA |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<0.5EU/mg (<0.0005EU/μg) Determined by LAL gel clotting assay |
| Aggregation |
<5% Determined by SEC |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_2894723 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
Immunohistochemistry (frozen), Immunofluorescence, in vivo imaging
Gupta S, Pal R, Schmidt EJ, Krishnamoorthy M, Leporati A, Kumar ATN, Bogdanov A. (2024). "Miniaturized Fab' imaging probe derived from a clinical antibody: Characterization and imaging in CRISPRi-attenuated mammary tumor models" iScience 27(8):110102. PubMed
Clinical imaging-assisted oncosurgical navigation requires cancer-specific miniaturized optical imaging probes. We report a near-infrared (NIR) Fab'-based epidermal growth factor receptor (EGFR)-specific probe carrying 3 NIR fluorophores (Fab'-800CW), which retained high-affinity binding to EGFR ectodomain (equilibrium KD E = 1 nM). Fab'-800CW showed a robust 4-times gain of fluorescence intensity (FI) and a 20% lifetime (FLT) increase under the conditions mimicking intracellular degradation. The probe was tested by using triple-negative breast cancer (TNBC) cell lines obtained by applying CRISPR interference (CRISPRi) effect of EGFR-targeting sgRNA and dCas9-KRAB chimera coexpression in MDA-MB-231 cells (WT cells). FI imaging in cell culture proved a 50% EGFR expression attenuation by CRISPRi. FI imaging in animals harboring attenuated or WT TNBC tumors with ex vivo corroboration identified differences between WT and CRISPRi tumors FI at 30 min post injection. Our results suggest the feasibility of EGFR expression imaging using a Fab'-based probe relevant for imaging-guided cancer surgery.
in vitro EGFR blockade
Linde IL, Prestwood TR, Qiu J, Pilarowski G, Linde MH, Zhang X, Shen L, Reticker-Flynn NE, Chiu DK, Sheu LY, Van Deursen S, Tolentino LL, Song WC, Engleman EG. (2023). "Neutrophil-activating therapy for the treatment of cancer" Cancer Cell 41(2):356-372.e10. PubMed
Despite their cytotoxic capacity, neutrophils are often co-opted by cancers to promote immunosuppression, tumor growth, and metastasis. Consequently, these cells have received little attention as potential cancer immunotherapeutic agents. Here, we demonstrate in mouse models that neutrophils can be harnessed to induce eradication of tumors and reduce metastatic seeding through the combined actions of tumor necrosis factor, CD40 agonist, and tumor-binding antibody. The same combination activates human neutrophils in vitro, enabling their lysis of human tumor cells. Mechanistically, this therapy induces rapid mobilization and tumor infiltration of neutrophils along with complement activation in tumors. Complement component C5a activates neutrophils to produce leukotriene B4, which stimulates reactive oxygen species production via xanthine oxidase, resulting in oxidative damage and T cell-independent clearance of multiple tumor types. These data establish neutrophils as potent anti-tumor immune mediators and define an inflammatory pathway that can be harnessed to drive neutrophil-mediated eradication of cancer.
in vitro EGFR blockade
Linde IL, Prestwood TR, Qiu J, Pilarowski G, Linde MH, Zhang X, Shen L, Reticker-Flynn NE, Chiu DK, Sheu LY, Van Deursen S, Tolentino LL, Song WC, Engleman EG. (2023). "Neutrophil-activating therapy for the treatment of cancer" Cancer Cell 41(2):356-372.e10. PubMed
Despite their cytotoxic capacity, neutrophils are often co-opted by cancers to promote immunosuppression, tumor growth, and metastasis. Consequently, these cells have received little attention as potential cancer immunotherapeutic agents. Here, we demonstrate in mouse models that neutrophils can be harnessed to induce eradication of tumors and reduce metastatic seeding through the combined actions of tumor necrosis factor, CD40 agonist, and tumor-binding antibody. The same combination activates human neutrophils in vitro, enabling their lysis of human tumor cells. Mechanistically, this therapy induces rapid mobilization and tumor infiltration of neutrophils along with complement activation in tumors. Complement component C5a activates neutrophils to produce leukotriene B4, which stimulates reactive oxygen species production via xanthine oxidase, resulting in oxidative damage and T cell-independent clearance of multiple tumor types. These data establish neutrophils as potent anti-tumor immune mediators and define an inflammatory pathway that can be harnessed to drive neutrophil-mediated eradication of cancer.
in vitro EGFR2+ cell depletion (ADCC assay)
Lee DH, Ahn H, Sim HI, Choi E, Choi S, Jo Y, Yun B, Song HK, Oh SJ, Denda-Nagai K, Park CS, Irimura T, Park Y, Jin HS. (2023). "A CRISPR activation screen identifies MUC-21 as critical for resistance to NK and T cell-mediated cytotoxicity" J Exp Clin Cancer Res 42(1):272. PubMed
Background: Immunotherapy has significantly advanced cancer treatments, but many patients do not respond to it, partly due to immunosuppressive mechanisms used by tumor cells. These cells employ immunosuppressive ligands to evade detection and elimination by the immune system. Therefore, the discovery and characterization of novel immunosuppressive ligands that facilitate immune evasion are crucial for developing more potent anti-cancer therapies. Methods: We conducted gain-of-function screens using a CRISPRa (CRISPR activation) library that covered the entire human transmembrane sub-genome to identify surface molecules capable of hindering NK-mediated cytotoxicity. The immunosuppressive role and mechanism of MUC21 were validated using NK and T cell mediated cytotoxicity assays. Bioinformatics tools were employed to assess the clinical implications of mucin-21 (MUC21) in cancer cell immunity. Results: Our genetic screens revealed that MUC21 expression on cancer cell surfaces inhibits both the cytotoxic activity of NK cells and antibody-dependent cellular cytotoxicity, but not affecting complement-dependent cytotoxicity. Additionally, MUC21 expression hinders T cell activation by impeding antigen recognition, thereby diminishing the effectiveness of the immune checkpoint inhibitor, anti-PD-L1. Moreover, MUC21 expression suppress the antitumor function of both CAR-T cells and CAR-NK cells. Mechanistically, MUC21 facilitates immune evasion by creating steric hindrance, preventing interactions between cancer and immune cells. Bioinformatics analysis revealed elevated MUC21 expression in lung cancer, which correlated with reduced infiltration and activation of cytotoxic immune cells. Intriguingly, MUC21 expression was higher in non-small cell lung cancer (NSCLC) tumors that were non-responsive to anti-PD-(L)1 treatment compared to responsive tumors. Conclusions: These findings indicate that surface MUC21 serves as a potent immunosuppressive ligand, shielding cancer cells from NK and CD8+T cell attacks. This suggests that inhibiting MUC21 could be a promising strategy to improve cancer immunotherapy.
in vitro functional assay
Iadonato S, Ovechkina Y, Lustig K, Cross J, Eyde N, Frazier E, Kabi N, Katz C, Lance R, Peckham D, Sridhar S, Talbaux C, Tihista I, Xu M, Guillaudeux T. (2023). "A highly potent anti-VISTA antibody KVA12123 - a new immune checkpoint inhibitor and a promising therapy against poorly immunogenic tumors" Front Immunol . PubMed
Background: Immune checkpoint therapies have led to significant breakthroughs in cancer patient treatment in recent years. However, their efficiency is variable, and resistance to immunotherapies is common. VISTA is an immune-suppressive checkpoint inhibitor of T cell response belonging to the B7 family and a promising novel therapeutic target. VISTA is expressed in the immuno-suppressive tumor microenvironment, primarily by myeloid lineage cells, and its genetic knockout or antibody blockade restores an efficient antitumor immune response. Methods: Fully human monoclonal antibodies directed against VISTA were produced after immunizing humanized Trianni mice and single B cell sequencing. Anti-VISTA antibodies were evaluated for specificity, cross-reactivity, monocyte and T cell activation, Fc-effector functions, and antitumor efficacy using in vitro and in vivo models to select the KVA12123 antibody lead candidate. The pharmacokinetics and safety profiles of KVA12123 were evaluated in cynomolgus monkeys. Results: Here, we report the development of a clinical candidate anti-VISTA monoclonal antibody, KVA12123. KVA12123 showed high affinity binding to VISTA through a unique epitope distinct from other clinical-stage anti-VISTA monoclonal antibodies. This clinical candidate demonstrated high specificity against VISTA with no cross-reactivity detected against other members of the B7 family. KVA12123 blocked VISTA binding to its binding partners. KVA12123 induced T cell activation and demonstrated NK-mediated monocyte activation. KVA12123 treatment mediated strong single-agent antitumor activity in several syngeneic tumor models and showed enhanced efficacy in combination with anti-PD-1 treatment. This clinical candidate was engineered to improve its pharmacokinetic characteristics and reduce Fc-effector functions. It was well-tolerated in preclinical toxicology studies in cynomolgus monkeys, where hematology, clinical chemistry evaluations, and clinical observations revealed no indicators of toxicity. No cytokines associated with cytokine release syndrome were elevated. Conclusion: These results establish that KVA12123 is a promising drug candidate with a distinct but complementary mechanism of action of the first generation of immune checkpoint inhibitors. This antibody is currently evaluated alone and in combination with pembrolizumab in a Phase 1/2 open-label clinical trial in patients with advanced solid tumors.
in vitro functional assay
Müller T, Tasser C, Tesar M, Fucek I, Schniegler-Mattox U, Koch J, Ellwanger K. (2023). "Selection of bispecific antibodies with optimal developability using FcRn‑Ph‑HPLC as an optimized FcRn affinity chromatography method" MAbs 15(1):2245519. PubMed
A challenge when developing therapeutic antibodies is the identification of candidates with favorable pharmacokinetics (PK) early in development. A key determinant of immunoglobulin (IgG) serum half‑life in vivo is the efficiency of pH-dependent binding to the neonatal Fc receptor (FcRn). Numerous studies have proposed techniques to assess FcRn binding of IgG-based therapeutics in vitro, enabling prediction of serum half-life prior to clinical assessment. FcRn high-performance liquid chromatography (HPLC) assays FcRn binding of therapeutic IgGs across a pH gradient, allowing the correlation of IgG column retention time to the half‑life of a therapeutic IgG in vivo. However, as FcRn retention time cannot be directly compared to an in vivo parameter, modifications to FcRn-HPLC are required to enable interpretation of the data within a physiological context, to provide more accurate estimations of serum half-life. This study presents an important modification to this method, FcRn-pH-HPLC, which reproducibly measures FcRn dissociation pH, allowing correlation with previously established half-lives of therapeutic antibodies. Furthermore, the influence of incorporating various antibody modifications, binding modules, and their orientations within IgGs and bispecifics on FcRn dissociation pH was evaluated using antibodies from the redirected optimized cell killing (ROCK®) platform. Target and effector antigen-binding domain sequences, their presentation format and orientation within a bispecific antibody alter FcRn retention; tested Fc domain modifications and incorporating stabilizing disulfide bonds had minimal effect. This study may inform the generation of mono-, bi- and multi-specific antibodies with tailored half-lives based on FcRn binding properties in vitro, to differentiate antibody-based therapeutic candidates with optimal developability.
- Cancer Research,
- Immunology and Microbiology
A highly potent anti-VISTA antibody KVA12123 - a new immune checkpoint inhibitor and a promising therapy against poorly immunogenic tumors.
In Frontiers in Immunology on 28 December 2023 by Iadonato, S., Ovechkina, Y., et al.
PubMed
Immune checkpoint therapies have led to significant breakthroughs in cancer patient treatment in recent years. However, their efficiency is variable, and resistance to immunotherapies is common. VISTA is an immune-suppressive checkpoint inhibitor of T cell response belonging to the B7 family and a promising novel therapeutic target. VISTA is expressed in the immuno-suppressive tumor microenvironment, primarily by myeloid lineage cells, and its genetic knockout or antibody blockade restores an efficient antitumor immune response. Fully human monoclonal antibodies directed against VISTA were produced after immunizing humanized Trianni mice and single B cell sequencing. Anti-VISTA antibodies were evaluated for specificity, cross-reactivity, monocyte and T cell activation, Fc-effector functions, and antitumor efficacy using in vitro and in vivo models to select the KVA12123 antibody lead candidate. The pharmacokinetics and safety profiles of KVA12123 were evaluated in cynomolgus monkeys. Here, we report the development of a clinical candidate anti-VISTA monoclonal antibody, KVA12123. KVA12123 showed high affinity binding to VISTA through a unique epitope distinct from other clinical-stage anti-VISTA monoclonal antibodies. This clinical candidate demonstrated high specificity against VISTA with no cross-reactivity detected against other members of the B7 family. KVA12123 blocked VISTA binding to its binding partners. KVA12123 induced T cell activation and demonstrated NK-mediated monocyte activation. KVA12123 treatment mediated strong single-agent antitumor activity in several syngeneic tumor models and showed enhanced efficacy in combination with anti-PD-1 treatment. This clinical candidate was engineered to improve its pharmacokinetic characteristics and reduce Fc-effector functions. It was well-tolerated in preclinical toxicology studies in cynomolgus monkeys, where hematology, clinical chemistry evaluations, and clinical observations revealed no indicators of toxicity. No cytokines associated with cytokine release syndrome were elevated. These results establish that KVA12123 is a promising drug candidate with a distinct but complementary mechanism of action of the first generation of immune checkpoint inhibitors. This antibody is currently evaluated alone and in combination with pembrolizumab in a Phase 1/2 open-label clinical trial in patients with advanced solid tumors. Copyright © 2023 Iadonato, Ovechkina, Lustig, Cross, Eyde, Frazier, Kabi, Katz, Lance, Peckham, Sridhar, Talbaux, Tihista, Xu and Guillaudeux.
- FC/FACS,
- Mus musculus (House mouse),
- Cancer Research
Neutrophil-activating therapy for the treatment of cancer.
In Cancer Cell on 13 February 2023 by Linde, I. L., Prestwood, T. R., et al.
PubMed
Despite their cytotoxic capacity, neutrophils are often co-opted by cancers to promote immunosuppression, tumor growth, and metastasis. Consequently, these cells have received little attention as potential cancer immunotherapeutic agents. Here, we demonstrate in mouse models that neutrophils can be harnessed to induce eradication of tumors and reduce metastatic seeding through the combined actions of tumor necrosis factor, CD40 agonist, and tumor-binding antibody. The same combination activates human neutrophils in vitro, enabling their lysis of human tumor cells. Mechanistically, this therapy induces rapid mobilization and tumor infiltration of neutrophils along with complement activation in tumors. Complement component C5a activates neutrophils to produce leukotriene B4, which stimulates reactive oxygen species production via xanthine oxidase, resulting in oxidative damage and T cell-independent clearance of multiple tumor types. These data establish neutrophils as potent anti-tumor immune mediators and define an inflammatory pathway that can be harnessed to drive neutrophil-mediated eradication of cancer. Copyright © 2023 Elsevier Inc. All rights reserved.