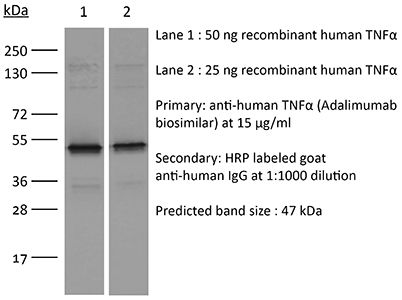
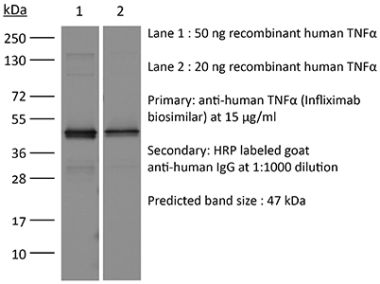
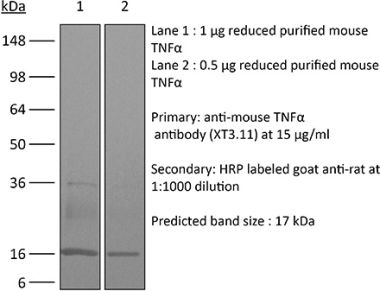
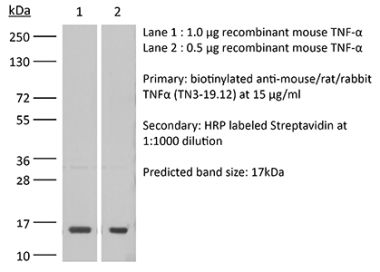
InVivoSIM anti-human TNFα (Adalimumab Biosimilar)
Product Details
This non-therapeutic biosimilar antibody uses the same variable regions from the therapeutic antibody Adalimumab making it ideal for research use. This Adalimumab biosimilar reacts with human TNFα (tumor necrosis factor-alpha) a multifunctional proinflammatory cytokine. TNFα belongs to the TNF superfamily of cytokines and signals through its two receptors, TNFR1 and TNFR2 which can be activated by both the soluble trimeric and membrane-bound and forms of TNFα. TNFα is primarily produced by macrophages in response to foreign antigens such as bacteria (lipopolysaccharides), viruses, and parasites as well as mitogens and other cytokines but can also be expressed by monocytes, neutrophils, NK cells, CD4 T cells and some specialized dendritic cells. TNFα is known to play key roles in a wide spectrum of biological processes including immunoregulation, cell proliferation, differentiation, apoptosis, antitumor activity, inflammation, anorexia, cachexia, septic shock, hematopoiesis, and viral replication. TNFα dysregulation has been implicated in a variety of diseases, including autoimmune diseases, insulin resistance, and cancer. Adalimumab blocks the interaction of TNFα with the TNFR1 (p55) and TNFR2 (p75) resulting in a down-regulation of the inflammatory response associated with autoimmune diseases.Specifications
| Isotype | Human IgG1 |
|---|---|
| Recommended Isotype Control(s) | RecombiMAb human IgG1 isotype control, anti-hen egg lysozyme |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Human TNFα |
| Reported Applications |
in vitro TNFα neutralization in vitro functional assay Flow Cytometry ELISA Immunofluoresence Immunoprecipitation Immunohistochemistry Western Blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<0.5EU/mg (<0.0005EU/μg) Determined by LAL gel clotting assay |
| Aggregation |
<5% Determined by SEC |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_2894722 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
in vitro TNFα neutralization
Wang F, Zhou F, Peng J, Chen H, Xie J, Liu C, Xiong H, Chen S, Xue G, Zhou X, Xie Y. (2024). "Macrophage Tim-3 maintains intestinal homeostasis in DSS-induced colitis by suppressing neutrophil necroptosis" Redox Biol . PubMed
T-cell immunoglobulin domain and mucin domain-3 (Tim-3) is a versatile immunomodulator that protects against intestinal inflammation. Necroptosis is a type of cell death that regulates intestinal homeostasis and inflammation. The mechanism(s) underlying the protective role of macrophage Tim-3 in intestinal inflammation is unclear; thus, we investigated whether specific Tim-3 knockdown in macrophages drives intestinal inflammation via necroptosis. Tim-3 protein and mRNA expression were assessed via double immunofluorescence staining and single-cell RNA sequencing (sc-RNA seq), respectively, in the colonic tissues of patients with inflammatory bowel disease (IBD) and healthy controls. Macrophage-specific Tim3-knockout (Tim-3M-KO) mice were generated to explore the function and mechanism of Tim-3 in dextran sodium sulfate (DSS)-induced colitis. Necroptosis was blocked by pharmacological inhibitors of receptor-interacting protein kinase (RIP)1, RIP3, and reactive oxygen species (ROS). Additionally, in vitro experiments were performed to assess the mechanisms of neutrophil necroptosis induced by Tim-3 knockdown macrophages. Although Tim-3 is relatively inactive in macrophages during colon homeostasis, it is highly active during colitis. Compared to those in controls, Tim-3M-KO mice showed increased susceptibility to colitis, higher colitis scores, and increased pro-inflammatory mediator expression. Following the administration of RIP1/RIP3 or ROS inhibitors, a significant reduction in intestinal inflammation symptoms was observed in DSS-treated Tim-3M-KO mice. Further analysis indicated the TLR4/NF-κB pathway in Tim-3 knockdown macrophages mediates the TNF-α-induced necroptosis pathway in neutrophils. Macrophage Tim-3 regulates neutrophil necroptosis via intracellular ROS signaling. Tim-3 knockdown macrophages can recruit neutrophils and induce neutrophil necroptosis, thereby damaging the intestinal mucosal barrier and triggering a vicious cycle in the development of colitis. Our results demonstrate a protective role of macrophage Tim-3 in maintaining gut homeostasis by inhibiting neutrophil necroptosis and provide novel insights into the pathogenesis of IBD.
in vitro TNFα neutralization
Fredon M, Poussard M, Biichlé S, Bonnefoy F, Mantion CF, Seffar E, Renosi F, Bôle-Richard E, Boidot R, Chevrier S, Anna F, Loustau M, Caumartin J, Gonçalves-Venturelli M, Robinet E, Saas P, Deconinck E, Daguidau E, Roussel X, Godet Y, Adotévi O, Angelot-Delettre F, Galaine J, Garnache-Ottou F. (2024). "Impact of scFv on Functionality and Safety of Third-Generation CD123 CAR T Cells" Cancer Immunol Res 12(8):1090-1107. PubMed
Chimeric antigen receptor (CAR) T cells express an extracellular domain consisting of a single-chain fragment variable (scFv) targeting a surface tumor-associated antigen. scFv selection should involve safety profiling with evaluation of the efficacy/toxicity balance, especially when the target antigen also is expressed on healthy cells. Here, to assess differences in terms of efficacy and on-target/off-tumor effects, we generated five different CARs targeting CD123 by substituting only the scFv. In in vitro models, T cells engineered to express three of these five CD123 CARs were effectively cytotoxic on leukemic cells without increasing lysis of monocytes or endothelial cells. Using the IncuCyte system, we confirmed the low cytotoxicity of CD123 CAR T cells on endothelial cells. Hematotoxicity evaluation using progenitor culture and CD34 cell lysis showed that two of the five CD123 CAR T cells were less cytotoxic on hematopoietic stem cells. Using a humanized mouse model, we confirmed that CD123- cells were not eliminated by the CD123 CAR T cells. Two CD123 CAR T cells reduced tumor infiltration and increased the overall survival of mice in three in vivo models of blastic plasmacytoid dendritic cell neoplasm. In an aggressive version of this model, bulk RNA sequencing analysis showed that these CD123 CAR T cells upregulated genes associated with cytotoxicity and activation/exhaustion a few days after the injection. Together, these results emphasize the importance of screening different scFvs for the development of CAR constructs to support selection of cells with the optimal risk-benefit ratio for clinical development.
in vitro functional assay
Jarvi NL, Balu-Iyer SV. (2023). "A mechanistic marker-based screening tool to predict clinical immunogenicity of biologics" Commun Med (Lond) 3(1):174. PubMed
Background: The efficacy and safety of therapeutic proteins are undermined by immunogenicity driven by anti-drug antibodies. Immunogenicity risk assessment is critically necessary during drug development, but current methods lack predictive power and mechanistic insight into antigen uptake and processing leading to immune response. A key mechanistic step in T-cell-dependent immune responses is the migration of mature dendritic cells to T-cell areas of lymphoid compartments, and this phenomenon is most pronounced in the immune response toward subcutaneously delivered proteins. Methods: The migratory potential of monocyte-derived dendritic cells is proposed to be a mechanistic marker for immunogenicity screening. Following exposure to therapeutic protein in vitro, dendritic cells are analyzed for changes in activation markers (CD40 and IL-12) in combination with levels of the chemokine receptor CXCR4 to represent migratory potential. Then a transwell assay captures the intensity of dendritic cell migration in the presence of a gradient of therapeutic protein and chemokine ligands. Results: Here, we show that an increased ability of the therapeutic protein to induce dendritic cell migration along a gradient of chemokine CCL21 and CXCL12 predicts higher immunogenic potential. Expression of the chemokine receptor CXCR4 on human monocyte-derived dendritic cells, in combination with activation markers CD40 and IL-12, strongly correlates with clinical anti-drug antibody incidence. Conclusions: Mechanistic understanding of processes driving immunogenicity led to the development of a predictive tool for immunogenicity risk assessment of therapeutic proteins. These predictive markers could be adapted for immunogenicity screening of other biological modalities.
in vitro functional assay
Müller T, Tasser C, Tesar M, Fucek I, Schniegler-Mattox U, Koch J, Ellwanger K. (2023). "Selection of bispecific antibodies with optimal developability using FcRn‑Ph‑HPLC as an optimized FcRn affinity chromatography method" MAbs 15(1):2245519. PubMed
A challenge when developing therapeutic antibodies is the identification of candidates with favorable pharmacokinetics (PK) early in development. A key determinant of immunoglobulin (IgG) serum half‑life in vivo is the efficiency of pH-dependent binding to the neonatal Fc receptor (FcRn). Numerous studies have proposed techniques to assess FcRn binding of IgG-based therapeutics in vitro, enabling prediction of serum half-life prior to clinical assessment. FcRn high-performance liquid chromatography (HPLC) assays FcRn binding of therapeutic IgGs across a pH gradient, allowing the correlation of IgG column retention time to the half‑life of a therapeutic IgG in vivo. However, as FcRn retention time cannot be directly compared to an in vivo parameter, modifications to FcRn-HPLC are required to enable interpretation of the data within a physiological context, to provide more accurate estimations of serum half-life. This study presents an important modification to this method, FcRn-pH-HPLC, which reproducibly measures FcRn dissociation pH, allowing correlation with previously established half-lives of therapeutic antibodies. Furthermore, the influence of incorporating various antibody modifications, binding modules, and their orientations within IgGs and bispecifics on FcRn dissociation pH was evaluated using antibodies from the redirected optimized cell killing (ROCK®) platform. Target and effector antigen-binding domain sequences, their presentation format and orientation within a bispecific antibody alter FcRn retention; tested Fc domain modifications and incorporating stabilizing disulfide bonds had minimal effect. This study may inform the generation of mono-, bi- and multi-specific antibodies with tailored half-lives based on FcRn binding properties in vitro, to differentiate antibody-based therapeutic candidates with optimal developability.
- Immunology and Microbiology,
Macrophage Tim-3 maintains intestinal homeostasis in DSS-induced colitis by suppressing neutrophil necroptosis.
In Redox Biology on 1 April 2024 by Wang, F., Zhou, F., et al.
PubMed
T-cell immunoglobulin domain and mucin domain-3 (Tim-3) is a versatile immunomodulator that protects against intestinal inflammation. Necroptosis is a type of cell death that regulates intestinal homeostasis and inflammation. The mechanism(s) underlying the protective role of macrophage Tim-3 in intestinal inflammation is unclear; thus, we investigated whether specific Tim-3 knockdown in macrophages drives intestinal inflammation via necroptosis. Tim-3 protein and mRNA expression were assessed via double immunofluorescence staining and single-cell RNA sequencing (sc-RNA seq), respectively, in the colonic tissues of patients with inflammatory bowel disease (IBD) and healthy controls. Macrophage-specific Tim3-knockout (Tim-3M-KO) mice were generated to explore the function and mechanism of Tim-3 in dextran sodium sulfate (DSS)-induced colitis. Necroptosis was blocked by pharmacological inhibitors of receptor-interacting protein kinase (RIP)1, RIP3, and reactive oxygen species (ROS). Additionally, in vitro experiments were performed to assess the mechanisms of neutrophil necroptosis induced by Tim-3 knockdown macrophages. Although Tim-3 is relatively inactive in macrophages during colon homeostasis, it is highly active during colitis. Compared to those in controls, Tim-3M-KO mice showed increased susceptibility to colitis, higher colitis scores, and increased pro-inflammatory mediator expression. Following the administration of RIP1/RIP3 or ROS inhibitors, a significant reduction in intestinal inflammation symptoms was observed in DSS-treated Tim-3M-KO mice. Further analysis indicated the TLR4/NF-κB pathway in Tim-3 knockdown macrophages mediates the TNF-α-induced necroptosis pathway in neutrophils. Macrophage Tim-3 regulates neutrophil necroptosis via intracellular ROS signaling. Tim-3 knockdown macrophages can recruit neutrophils and induce neutrophil necroptosis, thereby damaging the intestinal mucosal barrier and triggering a vicious cycle in the development of colitis. Our results demonstrate a protective role of macrophage Tim-3 in maintaining gut homeostasis by inhibiting neutrophil necroptosis and provide novel insights into the pathogenesis of IBD. Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
- Immunology and Microbiology
A mechanistic marker-based screening tool to predict clinical immunogenicity of biologics.
In Commun Med (Lond) on 8 December 2023 by Jarvi, N. L. & Balu-Iyer, S. V.
PubMed
The efficacy and safety of therapeutic proteins are undermined by immunogenicity driven by anti-drug antibodies. Immunogenicity risk assessment is critically necessary during drug development, but current methods lack predictive power and mechanistic insight into antigen uptake and processing leading to immune response. A key mechanistic step in T-cell-dependent immune responses is the migration of mature dendritic cells to T-cell areas of lymphoid compartments, and this phenomenon is most pronounced in the immune response toward subcutaneously delivered proteins. The migratory potential of monocyte-derived dendritic cells is proposed to be a mechanistic marker for immunogenicity screening. Following exposure to therapeutic protein in vitro, dendritic cells are analyzed for changes in activation markers (CD40 and IL-12) in combination with levels of the chemokine receptor CXCR4 to represent migratory potential. Then a transwell assay captures the intensity of dendritic cell migration in the presence of a gradient of therapeutic protein and chemokine ligands. Here, we show that an increased ability of the therapeutic protein to induce dendritic cell migration along a gradient of chemokine CCL21 and CXCL12 predicts higher immunogenic potential. Expression of the chemokine receptor CXCR4 on human monocyte-derived dendritic cells, in combination with activation markers CD40 and IL-12, strongly correlates with clinical anti-drug antibody incidence. Mechanistic understanding of processes driving immunogenicity led to the development of a predictive tool for immunogenicity risk assessment of therapeutic proteins. These predictive markers could be adapted for immunogenicity screening of other biological modalities. © 2023. The Author(s).