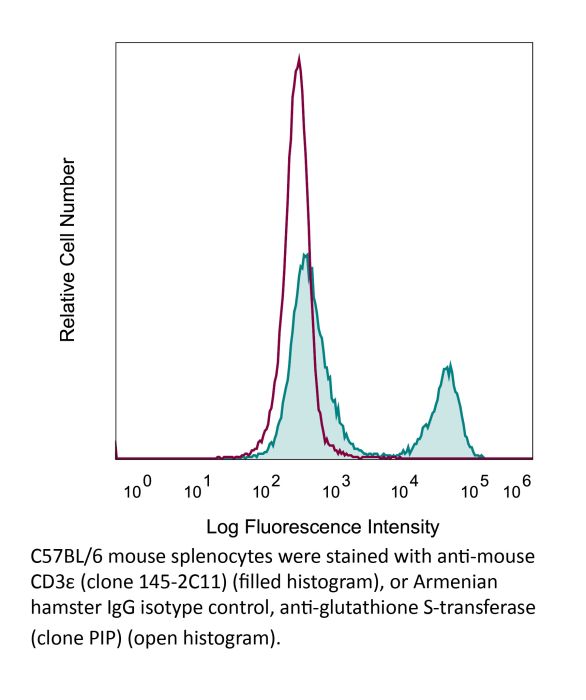
InVivoPlus anti-mouse CD3ε
Product Description
Specifications
| Isotype | Armenian Hamster IgG1 |
|---|---|
| Recommended Isotype Control(s) | InVivoPlus polyclonal Armenian hamster IgG |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse BM10-37 cytotoxic T cells |
| Reported Applications |
in vivo T cell depletion in vitro T cell stimulation/activation Immunofluorescence Flow cytometry Western blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin* |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Aggregation* |
<5% Determined by SEC |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_1107634 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests* |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo T cell depletion
Glasner, A., et al (2018). "NKp46 Receptor-Mediated Interferon-gamma Production by Natural Killer Cells Increases Fibronectin 1 to Alter Tumor Architecture and Control Metastasis" Immunity 48(1): 107-119 e104.
PubMed
Natural killer (NK) cells are innate lymphoid cells, and their presence within human tumors correlates with better prognosis. However, the mechanisms by which NK cells control tumors in vivo are unclear. Here, we used reflectance confocal microscopy (RCM) imaging in humans and in mice to visualize tumor architecture in vivo. We demonstrated that signaling via the NK cell receptor NKp46 (human) and Ncr1 (mouse) induced interferon-gamma (IFN-gamma) secretion from intratumoral NK cells. NKp46- and Ncr1-mediated IFN-gamma production led to the increased expression of the extracellular matrix protein fibronectin 1 (FN1) in the tumors, which altered primary tumor architecture and resulted in decreased metastases formation. Injection of IFN-gamma into tumor-bearing mice or transgenic overexpression of Ncr1 in NK cells in mice resulted in decreased metastasis formation. Thus, we have defined a mechanism of NK cell-mediated control of metastases in vivo that may help develop NK cell-dependent cancer therapies.
in vitro T cell stimulation/activation
Lacher, S. M., et al (2018). "NF-kappaB inducing kinase (NIK) is an essential post-transcriptional regulator of T-cell activation affecting F-actin dynamics and TCR signaling" J Autoimmun 94: 110-121.
PubMed
NF-kappaB inducing kinase (NIK) is the key protein of the non-canonical NF-kappaB pathway and is important for the development of lymph nodes and other secondary immune organs. We elucidated the specific role of NIK in T cells using T-cell specific NIK-deficient (NIK(DeltaT)) mice. Despite showing normal development of lymphoid organs, NIK(DeltaT) mice were resistant to induction of CNS autoimmunity. T cells from NIK(DeltaT) mice were deficient in late priming, failed to up-regulate T-bet and to transmigrate into the CNS. Proteomic analysis of activated NIK(-/-) T cells showed de-regulated expression of proteins involved in the formation of the immunological synapse: in particular, proteins involved in cytoskeleton dynamics. In line with this we found that NIK-deficient T cells were hampered in phosphorylation of Zap70, LAT, AKT, ERK1/2 and PLCgamma upon TCR engagement. Hence, our data disclose a hitherto unknown function of NIK in T-cell priming and differentiation.
in vitro T cell stimulation/activation
Wendland, K., et al (2018). "Retinoic Acid Signaling in Thymic Epithelial Cells Regulates Thymopoiesis" J Immunol 201(2): 524-532.
PubMed
Despite the essential role of thymic epithelial cells (TEC) in T cell development, the signals regulating TEC differentiation and homeostasis remain incompletely understood. In this study, we show a key in vivo role for the vitamin A metabolite, retinoic acid (RA), in TEC homeostasis. In the absence of RA signaling in TEC, cortical TEC (cTEC) and CD80(lo)MHC class II(lo) medullary TEC displayed subset-specific alterations in gene expression, which in cTEC included genes involved in epithelial proliferation, development, and differentiation. Mice whose TEC were unable to respond to RA showed increased cTEC proliferation, an accumulation of stem cell Ag-1(hi) cTEC, and, in early life, a decrease in medullary TEC numbers. These alterations resulted in reduced thymic cellularity in early life, a reduction in CD4 single-positive and CD8 single-positive numbers in both young and adult mice, and enhanced peripheral CD8(+) T cell survival upon TCR stimulation. Collectively, our results identify RA as a regulator of TEC homeostasis that is essential for TEC function and normal thymopoiesis.
in vitro T cell stimulation/activation
Ron-Harel, N., et al (2016). "Mitochondrial Biogenesis and Proteome Remodeling Promote One-Carbon Metabolism for T Cell Activation" Cell Metab 24(1): 104-117.
PubMed
Naive T cell stimulation activates anabolic metabolism to fuel the transition from quiescence to growth and proliferation. Here we show that naive CD4(+) T cell activation induces a unique program of mitochondrial biogenesis and remodeling. Using mass spectrometry, we quantified protein dynamics during T cell activation. We identified substantial remodeling of the mitochondrial proteome over the first 24 hr of T cell activation to generate mitochondria with a distinct metabolic signature, with one-carbon metabolism as the most induced pathway. Salvage pathways and mitochondrial one-carbon metabolism, fed by serine, contribute to purine and thymidine synthesis to enable T cell proliferation and survival. Genetic inhibition of the mitochondrial serine catabolic enzyme SHMT2 impaired T cell survival in culture and antigen-specific T cell abundance in vivo. Thus, during T cell activation, mitochondrial proteome remodeling generates specialized mitochondria with enhanced one-carbon metabolism that is critical for T cell activation and survival.
in vitro T cell stimulation/activation
Gu, A. D., et al (2015). "A critical role for transcription factor Smad4 in T cell function that is independent of transforming growth factor beta receptor signaling" Immunity 42(1): 68-79.
PubMed
Transforming growth factor-beta (TGF-beta) suppresses T cell function to maintain self-tolerance and to promote tumor immune evasion. Yet how Smad4, a transcription factor component of TGF-beta signaling, regulates T cell function remains unclear. Here we have demonstrated an essential role for Smad4 in promoting T cell function during autoimmunity and anti-tumor immunity. Smad4 deletion rescued the lethal autoimmunity resulting from transforming growth factor-beta receptor (TGF-betaR) deletion and compromised T-cell-mediated tumor rejection. Although Smad4 was dispensable for T cell generation, homeostasis, and effector function, it was essential for T cell proliferation after activation in vitro and in vivo. The transcription factor Myc was identified to mediate Smad4-controlled T cell proliferation. This study thus reveals a requirement of Smad4 for T-cell-mediated autoimmunity and tumor rejection, which is beyond the current paradigm. It highlights a TGF-betaR-independent role for Smad4 in promoting T cell function, autoimmunity, and anti-tumor immunity.
in vitro T cell stimulation/activation
Awe, O., et al (2015). "PU.1 Expression in T Follicular Helper Cells Limits CD40L-Dependent Germinal Center B Cell Development" J Immunol .
PubMed
PU.1 is an ETS family transcription factor that is important for the development of multiple hematopoietic cell lineages. Previous work demonstrated a critical role for PU.1 in promoting Th9 development and in limiting Th2 cytokine production. Whether PU.1 has functions in other Th lineages is not clear. In this study, we examined the effects of ectopic expression of PU.1 in CD4+ T cells and observed decreased expression of genes involved with the function of T follicular helper (Tfh) cells, including Il21 and Tnfsf5 (encoding CD40L). T cells from conditional mutant mice that lack expression of PU.1 in T cells (Sfpi1lck-/-) demonstrated increased production of CD40L and IL-21 in vitro. Following adjuvant-dependent or adjuvant-independent immunization, we observed that Sfpi1lck-/- mice had increased numbers of Tfh cells, increased germinal center B cells (GCB cells), and increased Ab production in vivo. This correlated with increased expression of IL-21 and CD40L in Tfh cells from Sfpi1lck-/- mice compared with control mice. Finally, although blockade of IL-21 did not affect GCB cells in Sfpi1lck-/- mice, anti-CD40L treatment of immunized Sfpi1lck-/- mice decreased GCB cell numbers and Ag-specific Ig concentrations. Together, these data indicate an inhibitory role for PU.1 in the function of Tfh cells, germinal centers, and Tfh-dependent humoral immunity.
in vitro T cell stimulation/activation
Xu, H., et al (2015). "Regulation of bifurcating B cell trajectories by mutual antagonism between transcription factors IRF4 and IRF8" Nat Immunol .
PubMed
Upon recognition of antigen, B cells undertake a bifurcated response in which some cells rapidly differentiate into plasmablasts while others undergo affinity maturation in germinal centers (GCs). Here we identified a double-negative feedback loop between the transcription factors IRF4 and IRF8 that regulated the initial developmental bifurcation of activated B cells as well as the GC response. IRF8 dampened signaling via the B cell antigen receptor (BCR), facilitated antigen-specific interaction with helper T cells, and promoted antibody affinity maturation while antagonizing IRF4-driven differentiation of plasmablasts. Genomic analysis revealed concentration-dependent actions of IRF4 and IRF8 in regulating distinct gene-expression programs. Stochastic modeling suggested that the double-negative feedback was sufficient to initiate bifurcation of the B cell developmental trajectories.
in vitro T cell stimulation/activation
Huang, Y., et al (2015). "CRK proteins selectively regulate T cell migration into inflamed tissues" J Clin Invest 125(3): 1019-1032.
PubMed
Effector T cell migration into inflamed sites greatly exacerbates tissue destruction and disease severity in inflammatory diseases, including graft-versus-host disease (GVHD). T cell migration into such sites depends heavily on regulated adhesion and migration, but the signaling pathways that coordinate these functions downstream of chemokine receptors are largely unknown. Using conditional knockout mice, we found that T cells lacking the adaptor proteins CRK and CRK-like (CRKL) exhibit reduced integrin-dependent adhesion, chemotaxis, and diapedesis. Moreover, these two closely related proteins exhibited substantial functional redundancy, as ectopic expression of either protein rescued defects in T cells lacking both CRK and CRKL. We determined that CRK proteins coordinate with the RAP guanine nucleotide exchange factor C3G and the adhesion docking molecule CASL to activate the integrin regulatory GTPase RAP1. CRK proteins were required for effector T cell trafficking into sites of inflammation, but not for migration to lymphoid organs. In a murine bone marrow transplantation model, the differential migration of CRK/CRKL-deficient T cells resulted in efficient graft-versus-leukemia responses with minimal GVHD. Together, the results from our studies show that CRK family proteins selectively regulate T cell adhesion and migration at effector sites and suggest that these proteins have potential as therapeutic targets for preventing GVHD.
in vitro T cell stimulation/activation
Immunofluorescence
Kim, Y. U., et al (2015). "Regulation of autoimmune germinal center reactions in lupus-prone BXD2 mice by follicular helper T cells" PLoS One 10(3): e0120294.
PubMed
BXD2 mice spontaneously develop autoantibodies and subsequent glomerulonephritis, offering a useful animal model to study autoimmune lupus. Although initial studies showed a critical contribution of IL-17 and Th17 cells in mediating autoimmune B cell responses in BXD2 mice, the role of follicular helper T (Tfh) cells remains incompletely understood. We found that both the frequency of Th17 cells and the levels of IL-17 in circulation in BXD2 mice were comparable to those of wild-type. By contrast, the frequency of PD-1+ CXCR5+ Tfh cells was significantly increased in BXD2 mice compared with wild-type mice, while the frequency of PD-1+ CXCR5+ Foxp3+ follicular regulatory T (Tfr) cells was reduced in the former group. The frequency of Tfh cells rather than that of Th17 cells was positively correlated with the frequency of germinal center B cells as well as the levels of autoantibodies to dsDNA. More importantly, CXCR5+ CD4+ T cells isolated from BXD2 mice induced the production of IgG from naive B cells in an IL-21-dependent manner, while CCR6+ CD4+ T cells failed to do so. These results together demonstrate that Tfh cells rather than Th17 cells contribute to the autoimmune germinal center reactions in BXD2 mice.
in vitro T cell stimulation/activation
Liu, H., et al (2015). "The Immune Adaptor SLP-76 Binds to SUMO-RANGAP1 at Nuclear Pore Complex Filaments to Regulate Nuclear Import of Transcription Factors in T Cells" Mol Cell 59(5): 840-849.
PubMed
While immune cell adaptors regulate proximal T cell signaling, direct regulation of the nuclear pore complex (NPC) has not been reported. NPC has cytoplasmic filaments composed of RanGAP1 and RanBP2 with the potential to interact with cytoplasmic mediators. Here, we show that the immune cell adaptor SLP-76 binds directly to SUMO-RanGAP1 of cytoplasmic fibrils of the NPC, and that this interaction is needed for optimal NFATc1 and NF-kappaB p65 nuclear entry in T cells. Transmission electron microscopy showed anti-SLP-76 cytoplasmic labeling of the majority of NPCs in anti-CD3 activated T cells. Further, SUMO-RanGAP1 bound to the N-terminal lysine 56 of SLP-76 where the interaction was needed for optimal RanGAP1-NPC localization and GAP exchange activity. While the SLP-76-RanGAP1 (K56E) mutant had no effect on proximal signaling, it impaired NF-ATc1 and p65/RelA nuclear entry and in vivo responses to OVA peptide. Overall, we have identified SLP-76 as a direct regulator of nuclear pore function in T cells.
in vitro T cell stimulation/activation
Flow Cytometry
Tang, W., et al (2014). "The oncoprotein and transcriptional regulator Bcl-3 governs plasticity and pathogenicity of autoimmune T cells" Immunity 41(4): 555-566.
PubMed
Bcl-3 is an atypical member of the IkappaB family that modulates transcription in the nucleus via association with p50 (NF-kappaB1) or p52 (NF-kappaB2) homodimers. Despite evidence attesting to the overall physiologic importance of Bcl-3, little is known about its cell-specific functions or mechanisms. Here we demonstrate a T-cell-intrinsic function of Bcl-3 in autoimmunity. Bcl-3-deficient T cells failed to induce disease in T cell transfer-induced colitis and experimental autoimmune encephalomyelitis. The protection against disease correlated with a decrease in Th1 cells that produced the cytokines IFN-gamma and GM-CSF and an increase in Th17 cells. Although differentiation into Th1 cells was not impaired in the absence of Bcl-3, differentiated Th1 cells converted to less-pathogenic Th17-like cells, in part via mechanisms involving expression of the RORgammat transcription factor. Thus, Bcl-3 constrained Th1 cell plasticity and promoted pathogenicity by blocking conversion to Th17-like cells, revealing a unique type of regulation that shapes adaptive immunity.
in vitro T cell stimulation/activation
Rabenstein, H., et al (2014). "Differential kinetics of antigen dependency of CD4+ and CD8+ T cells" J Immunol 192(8): 3507-3517.
PubMed
Ag recognition via the TCR is necessary for the expansion of specific T cells that then contribute to adaptive immunity as effector and memory cells. Because CD4+ and CD8+ T cells differ in terms of their priming APCs and MHC ligands we compared their requirements of Ag persistence during their expansion phase side by side. Proliferation and effector differentiation of TCR transgenic and polyclonal mouse T cells were thus analyzed after transient and continuous TCR signals. Following equally strong stimulation, CD4+ T cell proliferation depended on prolonged Ag presence, whereas CD8+ T cells were able to divide and differentiate into effector cells despite discontinued Ag presentation. CD4+ T cell proliferation was neither affected by Th lineage or memory differentiation nor blocked by coinhibitory signals or missing inflammatory stimuli. Continued CD8+ T cell proliferation was truly independent of self-peptide/MHC-derived signals. The subset divergence was also illustrated by surprisingly broad transcriptional differences supporting a stronger propensity of CD8+ T cells to programmed expansion. These T cell data indicate an intrinsic difference between CD4+ and CD8+ T cells regarding the processing of TCR signals for proliferation. We also found that the presentation of a MHC class II-restricted peptide is more efficiently prolonged by dendritic cell activation in vivo than a class I bound one. In summary, our data demonstrate that CD4+ T cells require continuous stimulation for clonal expansion, whereas CD8+ T cells can divide following a much shorter TCR signal.
in vitro T cell stimulation/activation
Vegran, F., et al (2014). "The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells" Nat Immunol 15(8): 758-766.
PubMed
The TH9 subset of helper T cells was initially shown to contribute to the induction of autoimmune and allergic diseases, but subsequent evidence has suggested that these cells also exert antitumor activities. However, the molecular events that account for their effector properties are elusive. Here we found that the transcription factor IRF1 enhanced the effector function of TH9 cells and dictated their anticancer properties. Under TH9-skewing conditions, interleukin 1beta (IL-1beta) induced phosphorylation of the transcription factor STAT1 and subsequent expression of IRF1, which bound to the promoters of Il9 and Il21 and enhanced secretion of the cytokines IL-9 and IL-21 from TH9 cells. Furthermore, IL-1beta-induced TH9 cells exerted potent anticancer functions in an IRF1- and IL-21-dependent manner. Our findings thus identify IRF1 as a target for controlling the function of TH9 cells.
in vitro T cell stimulation/activation
Bertin, S., et al (2014). "The ion channel TRPV1 regulates the activation and proinflammatory properties of CD4(+) T cells" Nat Immunol 15(11): 1055-1063.
PubMed
TRPV1 is a Ca(2+)-permeable channel studied mostly as a pain receptor in sensory neurons. However, its role in other cell types is poorly understood. Here we found that TRPV1 was functionally expressed in CD4(+) T cells, where it acted as a non-store-operated Ca(2+) channel and contributed to T cell antigen receptor (TCR)-induced Ca(2+) influx, TCR signaling and T cell activation. In models of T cell-mediated colitis, TRPV1 promoted colitogenic T cell responses and intestinal inflammation. Furthermore, genetic and pharmacological inhibition of TRPV1 in human CD4(+) T cells recapitulated the phenotype of mouse Trpv1(-/-) CD4(+) T cells. Our findings suggest that inhibition of TRPV1 could represent a new therapeutic strategy for restraining proinflammatory T cell responses.
in vitro T cell stimulation/activation
Berger, H., et al (2013). "SOCS3 transactivation by PPARgamma prevents IL-17-driven cancer growth" Cancer Res 73(12): 3578-3590.
PubMed
Activation of the transcription factor PPARgamma by the n-3 fatty acid docosahexaenoic acid (DHA) is implicated in controlling proinflammatory cytokine secretion, but the intracellular signaling pathways engaged by PPARgamma are incompletely characterized. Here, we identify the adapter-encoding gene SOCS3 as a critical transcriptional target of PPARgamma. SOCS3 promoter binding and gene transactivation by PPARgamma was associated with a repression in differentiation of proinflammatory T-helper (TH)17 cells. Accordingly, TH17 cells induced in vitro displayed increased SOCS3 expression and diminished capacity to produce interleukin (IL)-17 following activation of PPARgamma by DHA. Furthermore, naive CD4 T cells derived from mice fed a DHA-enriched diet displayed less capability to differentiate into TH17 cells. In two different mouse models of cancer, DHA prevented tumor outgrowth and angiogenesis in an IL-17-dependent manner. Altogether, our results uncover a novel molecular pathway by which PPARgamma-induced SOCS3 expression prevents IL-17-mediated cancer growth.
in vitro T cell stimulation/activation
Sledzinska, A., et al (2013). "TGF-beta signalling is required for CD4(+) T cell homeostasis but dispensable for regulatory T cell function" PLoS Biol 11(10): e1001674.
PubMed
TGF-beta is widely held to be critical for the maintenance and function of regulatory T (T(reg)) cells and thus peripheral tolerance. This is highlighted by constitutive ablation of TGF-beta receptor (TR) during thymic development in mice, which leads to a lethal autoimmune syndrome. Here we describe that TGF-beta-driven peripheral tolerance is not regulated by TGF-beta signalling on mature CD4(+) T cells. Inducible TR2 ablation specifically on CD4(+) T cells did not result in a lethal autoinflammation. Transfer of these TR2-deficient CD4(+) T cells to lymphopenic recipients resulted in colitis, but not overt autoimmunity. In contrast, thymic ablation of TR2 in combination with lymphopenia led to lethal multi-organ inflammation. Interestingly, deletion of TR2 on mature CD4(+) T cells does not result in the collapse of the T(reg) cell population as observed in constitutive models. Instead, a pronounced enlargement of both regulatory and effector memory T cell pools was observed. This expansion is cell-intrinsic and seems to be caused by increased T cell receptor sensitivity independently of common gamma chain-dependent cytokine signals. The expression of Foxp3 and other regulatory T cells markers was not dependent on TGF-beta signalling and the TR2-deficient T(reg) cells retained their suppressive function both in vitro and in vivo. In summary, absence of TGF-beta signalling on mature CD4(+) T cells is not responsible for breakdown of peripheral tolerance, but rather controls homeostasis of mature T cells in adult mice.
in vitro T cell stimulation/activation
Goswami, R., et al (2012). "STAT6-dependent regulation of Th9 development" J Immunol 188(3): 968-975.
PubMed
Th cell effector subsets develop in response to specific cytokine environments. The development of a particular cytokine-secreting pattern requires an integration of signals that may promote the development of opposing pathways. A recent example of this paradigm is the IL-9-secreting Th9 cell that develops in response to TGF-beta and IL-4, cytokines that, in isolation, promote the development of inducible regulatory T cells and Th2 cells, respectively. To determine how the balance of these factors results in priming for IL-9 secretion, we examined the effects of each pathway on transcription factors that regulate Th cell differentiation. We demonstrated that TGF-beta induces the PU.1-encoding Sfpi1 locus and that this is independent of IL-4-induced STAT6 activation. IL-4-activated STAT6 is required for repressing the expression of T-bet and Foxp3 in Th9 cells, transcription factors that inhibit IL-9 production, and STAT6 is required for the induction of IRF4, which promotes Th9 development. These data established a transcription factor network that regulates IL-9 and demonstrated how combinations of cytokine signals generate cytokine-secreting potential by altering the expression of a panel of transcription factors.
in vivo T cell depletion
Peng, B., et al (2009). "Anti-CD3 antibodies modulate anti-factor VIII immune responses in hemophilia A mice after factor VIII plasmid-mediated gene therapy" Blood 114(20): 4373-4382.
PubMed
One major obstacle in gene therapy is the generation of immune responses directed against transgene product. Five consecutive anti-CD3 treatments concomitant with factor VIII (FVIII) plasmid injection prevented the formation of inhibitory antibodies against FVIII and achieved persistent, therapeutic levels of FVIII gene expression in treated hemophilia A mice. Repeated plasmid gene transfer is applicable in tolerized mice without eliciting immune responses. Anti-CD3 treatment significantly depleted both CD4+ and CD8+ T cells, whereas increased transforming growth factor-beta levels in plasma and the frequency of both CD4+CD25+FoxP3+ and CD4+CD25-Foxp3+ regulatory T cells in the initial few weeks after treatment. Although prior depletion of CD4+CD25+ cells did not abrogate tolerance induction, adoptive transfer of CD4+ cells from tolerized mice at 6 weeks after treatment protected recipient mice from anti-FVIII immune responses. Anti-CD3-treated mice mounted immune responses against both T-dependent and T-independent neo-antigens, indicating that anti-CD3 did not hamper the immune systems in the long term. Concomitant FVIII plasmid + anti-CD3 treatment induced long-term tolerance specific to FVIII via a mechanism involving the increase in transforming growth factor-beta levels and the generation of adaptive FVIII-specific CD4+Foxp3+ regulatory T cells at the periphery. Furthermore, anti-CD3 can reduce the titers of preexisting anti-FVIII inhibitory antibodies in hemophilia A mice.
in vitro T cell stimulation/activation
Dardalhon, V., et al (2008). "IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells" Nat Immunol 9(12): 1347-1355.
PubMed
Transcription factor Foxp3 is critical for generating regulatory T cells (T(reg) cells). Transforming growth factor-beta (TGF-beta) induces Foxp3 and suppressive T(reg) cells from naive T cells, whereas interleukin 6 (IL-6) inhibits the generation of inducible T(reg) cells. Here we show that IL-4 blocked the generation of TGF-beta-induced Foxp3(+) T(reg) cells and instead induced a population of T helper cells that produced IL-9 and IL-10. The IL-9(+)IL-10(+) T cells demonstrated no regulatory properties despite producing abundant IL-10. Adoptive transfer of IL-9(+)IL-10(+) T cells into recombination-activating gene 1-deficient mice induced colitis and peripheral neuritis, the severity of which was aggravated if the IL-9(+)IL-10(+) T cells were transferred with CD45RB(hi) CD4(+) effector T cells. Thus IL-9(+)IL-10(+) T cells lack suppressive function and constitute a distinct population of helper-effector T cells that promote tissue inflammation.
Product Citations
-
-
Cell Biology
-
Immunology and Microbiology
TMEM41B is an endoplasmic reticulum Ca2+ release channel maintaining naive T cell quiescence and responsiveness.
In Cell Discov on 4 March 2025 by Ma, Y., Wang, Y., et al.
PubMed
In mammalian cells, endoplasmic reticulum (ER) passively releases Ca2+ under steady state, but channels involved remain elusive. Here, we report that TMEM41B, an ER-resident membrane protein critical for autophagy, lipid metabolism, and viral infection, functions as an ER Ca2+ release channel. Biochemically, purified recombinant TMEM41B forms a concentration-dependent Ca2+ channel in single-channel electrophysiology assays. Cellularly, TMEM41B deficiency causes ER Ca2+ overload, while overexpression of TMEM41B depletes ER Ca2+. Immunologically, ER Ca2+ overload leads to upregulation of IL-2 and IL-7 receptors in naive T cells, which in turn increases basal signaling of JAK-STAT, AKT-mTOR, and MAPK pathways. This dysregulation drives TMEM41B-deficient naive T cells into a metabolically activated yet immunologically naive state. ER Ca2+ overload also downregulates CD5, lowering the activation threshold of TMEM41B-deficient T cells and leading to heightened T cell responses during infections. In summary, we identify TMEM41B as a concentration-dependent ER Ca2+ release channel, revealing an unexpected role of ER Ca2+ in naive T cell quiescence and responsiveness.
-
-
-
Immunology and Microbiology
Short-chain fatty acids are a key mediator of gut microbial regulation of T cell trafficking and differentiation after traumatic brain injury
In Research Square on 21 November 2024 by Celorrio, M., Shumilov, K., et al.
-
-
-
Immunology and Microbiology
IL-34 empowers regulatory T cells with novel non-canonical function to safeguard brain barrier integrity during neuro-inflammation
In bioRxiv on 13 September 2024 by Van Hoecke, L., Verreycken, J., et al.
-
-
-
Immunology and Microbiology
IL-34 empowers regulatory T cells with novel non-canonical function to safeguard brain barrier integrity during neuro-inflammation
In BioRxiv : the Preprt Server for Biology on 13 September 2024 by Van Hoecke, L., Verreycken, J., et al.
In efforts to find reparative strategies for brain damage, brain-associated regulatory T cells (Tregs) have gained increasing attention in recent years. Beyond their textbook immunoregulatory function, Tregs have emerged as key players in the response to brain trauma and the restoration of damaged brain tissue. Here, we are the first to describe a novel, non-canonical function of Tregs in maintaining the sealing capacity of both the blood-brain barrier (BBB) and the blood-cerebrospinal fluid (CSF) barrier. Moreover, we identified the cytokine IL-34 as a critical determinant in this newly unveiled Treg function. Mechanistically, IL-34 exerts its influence by modulating the expression and localization of the tight junction protein ZO-1 in both BBB endothelial cells and choroid plexus epithelial cells, thereby reinforcing the strength of the brain barriers. Given the well-established notion of leaky brain barriers and the involvement of immunological components in neurological diseases such as Alzheimer’s disease (AD) and multiple sclerosis (MS), we further demonstrate diminished IL-34 expression in Tregs derived from patients with relapsing-remitting MS (RR-MS) and patients with AD and even mild cognitive impairment (MCI). Remarkably, our study reveals the potential of IL-34 treatment in reinstating the integrity of brain barriers within murine models mimicking these neurological disorders. These ground-breaking findings shed light on the intricate relationship between Tregs, IL-34, and the integrity of brain barriers. They offer novel avenues for therapeutic approaches to ameliorate brain barrier dysfunction in the context of neurological disorders.
-
-
-
Immunology and Microbiology
-
Neuroscience
Early depletion of gut microbiota shape oligodendrocyte response after traumatic brain injury.
In J Neuroinflammation on 15 July 2024 by Shumilov, K., Ni, A., et al.
PubMed
White matter injury (WMI) is thought to be a major contributor to long-term cognitive dysfunctions after traumatic brain injury (TBI). This damage occurs partly due to apoptotic death of oligodendrocyte lineage cells (OLCs) after the injury, triggered directly by the trauma or in response to degenerating axons. Recent research suggests that the gut microbiota modulates the inflammatory response through the regulation of peripheral immune cell infiltration after TBI. Additionally, T-cells directly impact OLCs differentiation and proliferation. Therefore, we hypothesized that the gut microbiota plays a critical role in regulating the OLC response to WMI influencing T-cells differentiation and activation. Gut microbial depletion early after TBI chronically reduced re-myelination, acutely decreased OLCs proliferation, and was associated with increased myelin debris accumulation. Surprisingly, the absence of T-cells in gut microbiota depleted mice restored OLC proliferation and remyelination after TBI. OLCs co-cultured with T-cells derived from gut microbiota depleted mice resulted in impaired proliferation and increased expression of MHC-II compared with T cells from control-injured mice. Furthermore, MHC-II expression in OLCs appears to be linked to impaired proliferation under gut microbiota depletion and TBI conditions. Collectively our data indicates that depletion of the gut microbiota after TBI impaired remyelination, reduced OLCs proliferation with concomitantly increased OLC MHCII expression, and required the presence of T cells. This data suggests that T cells are an important mechanistic link by which the gut microbiota modulate the oligodendrocyte response and white matter recovery after TBI.
-
-
-
Immunology and Microbiology
-
Neuroscience
Early depletion of gut microbiota shape oligodendrocyte response after traumatic brain injury.
In Journal of Neuroflammation on 15 July 2024 by Shumilov, K., Ni, A., et al.
White matter injury (WMI) is thought to be a major contributor to long-term cognitive dysfunctions after traumatic brain injury (TBI). This damage occurs partly due to apoptotic death of oligodendrocyte lineage cells (OLCs) after the injury, triggered directly by the trauma or in response to degenerating axons. Recent research suggests that the gut microbiota modulates the inflammatory response through the regulation of peripheral immune cell infiltration after TBI. Additionally, T-cells directly impact OLCs differentiation and proliferation. Therefore, we hypothesized that the gut microbiota plays a critical role in regulating the OLC response to WMI influencing T-cells differentiation and activation. Gut microbial depletion early after TBI chronically reduced re-myelination, acutely decreased OLCs proliferation, and was associated with increased myelin debris accumulation. Surprisingly, the absence of T-cells in gut microbiota depleted mice restored OLC proliferation and remyelination after TBI. OLCs co-cultured with T-cells derived from gut microbiota depleted mice resulted in impaired proliferation and increased expression of MHC-II compared with T cells from control-injured mice. Furthermore, MHC-II expression in OLCs appears to be linked to impaired proliferation under gut microbiota depletion and TBI conditions. Collectively our data indicates that depletion of the gut microbiota after TBI impaired remyelination, reduced OLCs proliferation with concomitantly increased OLC MHCII expression, and required the presence of T cells. This data suggests that T cells are an important mechanistic link by which the gut microbiota modulate the oligodendrocyte response and white matter recovery after TBI. © 2024. The Author(s).
-
-
-
Cancer Research
-
Immunology and Microbiology
Proteobacteria impair anti-tumor immunity in the omentum by consuming arginine.
In Cell Host Microbe on 10 July 2024 by Meza-Perez, S., Liu, M., et al.
PubMed
Gut microbiota influence anti-tumor immunity, often by producing immune-modulating metabolites. However, microbes consume a variety of metabolites that may also impact host immune responses. We show that tumors grow unchecked in the omenta of microbe-replete mice due to immunosuppressive Tregs. By contrast, omental tumors in germ-free, neomycin-treated mice or mice colonized with altered Schaedler's flora (ASF) are spontaneously eliminated by CD8+ T cells. These mice lack Proteobacteria capable of arginine catabolism, causing increases in serum arginine that activate the mammalian target of the rapamycin (mTOR) pathway in Tregs to reduce their suppressive capacity. Transfer of the Proteobacteria, Escherichia coli (E. coli), but not a mutant unable to catabolize arginine, to ASF mice reduces arginine levels, restores Treg suppression, and prevents tumor clearance. Supplementary arginine similarly decreases Treg suppressive capacity, increases CD8+ T cell effectiveness, and reduces tumor burden. Thus, microbial consumption of arginine alters anti-tumor immunity, offering potential therapeutic strategies for tumors in visceral adipose tissue.
-
-
Stage-specific GATA3 induction promotes ILC2 development after lineage commitment.
In Nat Commun on 5 July 2024 by Furuya, H., Toda, Y., et al.
PubMed
Group 2 innate lymphoid cells (ILC2s) are a subset of innate lymphocytes that produce type 2 cytokines, including IL-4, IL-5, and IL-13. GATA3 is a critical transcription factor for ILC2 development at multiple stages. However, when and how GATA3 is induced to the levels required for ILC2 development remains unclear. Herein, we identify ILC2-specific GATA3-related tandem super-enhancers (G3SE) that induce high GATA3 in ILC2-committed precursors. G3SE-deficient mice exhibit ILC2 deficiency in the bone marrow, lung, liver, and small intestine with minimal impact on other ILC lineages or Th2 cells. Single-cell RNA-sequencing and subsequent flow cytometry analysis show that GATA3 induction mechanism, which is required for entering the ILC2 stage, is lost in IL-17RB+PD-1- late ILC2-committed precursor stage in G3SE-deficient mice. Cnot6l, part of the CCR4-NOT deadenylase complex, is a possible GATA3 target during ILC2 development. Our findings implicate a stage-specific regulatory mechanism for GATA3 expression during ILC2 development.
-
-
Immunology and Microbiology
Induction of immortal-like and functional CAR T cells by defined factors.
In J Exp Med on 6 May 2024 by Wang, L., Jin, G., et al.
PubMed
Long-term antitumor efficacy of chimeric antigen receptor (CAR) T cells depends on their functional persistence in vivo. T cells with stem-like properties show better persistence, but factors conferring bona fide stemness to T cells remain to be determined. Here, we demonstrate the induction of CAR T cells into an immortal-like and functional state, termed TIF. The induction of CARTIF cells depends on the repression of two factors, BCOR and ZC3H12A, and requires antigen or CAR tonic signaling. Reprogrammed CARTIF cells possess almost infinite stemness, similar to induced pluripotent stem cells while retaining the functionality of mature T cells, resulting in superior antitumor effects. Following the elimination of target cells, CARTIF cells enter a metabolically dormant state, persisting in vivo with a saturable niche and providing memory protection. TIF represents a novel state of T cells with unprecedented stemness, which confers long-term functional persistence of CAR T cells in vivo and holds broad potential in T cell therapies.
-
-
-
Biochemistry and Molecular biology
-
Cell Biology
-
Cell Biology
Sphingolipid biosynthesis is essential for metabolic rewiring during TH17 cell differentiation.
In Sci Adv on 26 April 2024 by Abimannan, T., Parthibane, V., et al.
PubMed
T helper 17 (TH17) cells are implicated in autoimmune diseases, and several metabolic processes are shown to be important for their development and function. In this study, we report an essential role for sphingolipids synthesized through the de novo pathway in TH17 cell development. Deficiency of SPTLC1, a major subunit of serine palmitoyl transferase enzyme complex that catalyzes the first and rate-limiting step of de novo sphingolipid synthesis, impaired glycolysis in differentiating TH17 cells by increasing intracellular reactive oxygen species (ROS) through enhancement of nicotinamide adenine dinucleotide phosphate oxidase 2 activity. Increased ROS leads to impaired activation of mammalian target of rapamycin C1 and reduced expression of hypoxia-inducible factor 1-alpha and c-Myc-induced glycolytic genes. SPTLCI deficiency protected mice from developing experimental autoimmune encephalomyelitis and experimental T cell transfer colitis. Our results thus show a critical role for de novo sphingolipid biosynthetic pathway in shaping adaptive immune responses with implications in autoimmune diseases.
-
-
-
Neuroscience
Gut Microbiota Shape Oligodendrocyte Response after Traumatic Brain Injury
In Research Square on 24 April 2024 by Shumilov, K., Ni, A., et al.
-
-
-
Immunology and Microbiology
-
Cell Biology
-
Biochemistry and Molecular biology
TMEM41B is an endoplasmic reticulum Ca2+release channel maintaining T cell metabolic quiescence and responsiveness
In bioRxiv on 26 December 2023 by Ma, Y., Wang, Y., et al.
-
-
-
Immunology and Microbiology
SIKs Regulate HDAC7 Stabilization and Cytokine Recall in Late-Stage T Cell Effector Differentiation.
In J Immunol on 15 December 2023 by Helms, R. S., Marin-Gonzalez, A., et al.
PubMed
Understanding the mechanisms underlying the acquisition and maintenance of effector function during T cell differentiation is important to unraveling how these processes can be dysregulated in the context of disease and manipulated for therapeutic intervention. In this study, we report the identification of a previously unappreciated regulator of murine T cell differentiation through the evaluation of a previously unreported activity of the kinase inhibitor, BioE-1197. Specifically, we demonstrate that liver kinase B1 (LKB1)-mediated activation of salt-inducible kinases epigenetically regulates cytokine recall potential in effector CD8+ and Th1 cells. Evaluation of this phenotype revealed that salt-inducible kinase-mediated phosphorylation-dependent stabilization of histone deacetylase 7 (HDAC7) occurred during late-stage effector differentiation. HDAC7 stabilization increased nuclear HDAC7 levels, which correlated with total and cytokine loci-specific reductions in the activating transcription mark histone 3 lysine 27 acetylation (H3K27Ac). Accordingly, HDAC7 stabilization diminished transcriptional induction of cytokine genes upon restimulation. Inhibition of this pathway during differentiation produced effector T cells epigenetically poised for enhanced cytokine recall. This work identifies a previously unrecognized target for enhancing effector T cell functionality.
-
-
-
Immunology and Microbiology
Kruppel-like factor 2+ CD4 T cells avert microbiota-induced intestinal inflammation.
In Cell Rep on 28 November 2023 by Shao, T. Y., Jiang, T. T., et al.
PubMed
Intestinal colonization by antigenically foreign microbes necessitates expanded peripheral immune tolerance. Here we show commensal microbiota prime expansion of CD4 T cells unified by the Kruppel-like factor 2 (KLF2) transcriptional regulator and an essential role for KLF2+ CD4 cells in averting microbiota-driven intestinal inflammation. CD4 cells with commensal specificity in secondary lymphoid organs and intestinal tissues are enriched for KLF2 expression, and distinct from FOXP3+ regulatory T cells or other differentiation lineages. Mice with conditional KLF2 deficiency in T cells develop spontaneous rectal prolapse and intestinal inflammation, phenotypes overturned by eliminating microbiota or reconstituting with donor KLF2+ cells. Activated KLF2+ cells selectively produce IL-10, and eliminating IL-10 overrides their suppressive function in vitro and protection against intestinal inflammation in vivo. Together with reduced KLF2+ CD4 cell accumulation in Crohn's disease, a necessity for the KLF2+ subpopulation of T regulatory type 1 (Tr1) cells in sustaining commensal tolerance is demonstrated.
-
-
-
Immunology and Microbiology
Induction of immortal-like and functional CAR-T cells by defined factors
In bioRxiv on 6 November 2023 by Wang, L., Jin, G., et al.
-
-
-
Immunology and Microbiology
One infusion of engineered long-lasting and multifunctional T cells cures asthma in mice
In bioRxiv on 6 November 2023 by Jin, G., Liu, Y., et al.
-
-
-
Immunology and Microbiology
An essential role for miR-15/16 in Treg suppression and restriction of proliferation.
In Cell Rep on 31 October 2023 by Johansson, K., Gagnon, J. D., et al.
PubMed
The miR-15/16 family targets a large network of genes in T cells to restrict their cell cycle, memory formation, and survival. Upon T cell activation, miR-15/16 are downregulated, allowing rapid expansion of differentiated effector T cells to mediate a sustained response. Here, we used conditional deletion of miR-15/16 in regulatory T cells (Tregs) to identify immune functions of the miR-15/16 family in T cells. miR-15/16 are indispensable to maintain peripheral tolerance by securing efficient suppression by a limited number of Tregs. miR-15/16 deficiency alters expression of critical Treg proteins and results in accumulation of functionally impaired FOXP3loCD25loCD127hi Tregs. Excessive proliferation in the absence of miR-15/16 shifts Treg fate and produces an effector Treg phenotype. These Tregs fail to control immune activation, leading to spontaneous multi-organ inflammation and increased allergic inflammation in a mouse model of asthma. Together, our results demonstrate that miR-15/16 expression in Tregs is essential to maintain immune tolerance.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Cell Biology
-
Biochemistry and Molecular biology
Molecular, metabolic, and functional CD4 T cell paralysis in the lymph node impedes tumor control.
In Cell Rep on 26 September 2023 by Guo, M., Abd-Rabbo, D., et al.
PubMed
CD4 T cells are central effectors of anti-cancer immunity and immunotherapy, yet the regulation of CD4 tumor-specific T (TTS) cells is unclear. We demonstrate that CD4 TTS cells are quickly primed and begin to divide following tumor initiation. However, unlike CD8 TTS cells or exhaustion programming, CD4 TTS cell proliferation is rapidly frozen in place by a functional interplay of regulatory T cells and CTLA4. Together these mechanisms paralyze CD4 TTS cell differentiation, redirecting metabolic circuits, and reducing their accumulation in the tumor. The paralyzed state is actively maintained throughout cancer progression and CD4 TTS cells rapidly resume proliferation and functional differentiation when the suppressive constraints are alleviated. Overcoming their paralysis established long-term tumor control, demonstrating the importance of rapidly crippling CD4 TTS cells for tumor progression and their potential restoration as therapeutic targets.
-
-
-
Cancer Research
-
Immunology and Microbiology
Tumor immunogenicity dictates reliance on TCF1 in CD8+ T cells for response to immunotherapy.
In Cancer Cell on 11 September 2023 by Escobar, G., Tooley, K., et al.
PubMed
Stem-like CD8+ T cells are regulated by T cell factor 1 (TCF1) and are considered requisite for immune checkpoint blockade (ICB) response. However, recent findings indicate that reliance on TCF1+CD8+ T cells for ICB efficacy may differ across tumor contexts. We find that TCF1 is essential for optimal priming of tumor antigen-specific CD8+ T cells and ICB response in poorly immunogenic tumors that accumulate TOX+ dysfunctional T cells, but is dispensable for T cell priming and therapy response in highly immunogenic tumors that efficiently expand transitory effectors. Importantly, improving T cell priming by vaccination or by enhancing antigen presentation on tumors rescues the defective responses of TCF1-deficient CD8+ T cells upon ICB in poorly immunogenic tumors. Our study highlights TCF1's role during the early stages of anti-tumor CD8+ T cell responses with important implications for guiding optimal therapeutic interventions in cancers with low TCF1+CD8+ T cells and low-neo-antigen expression.
-
-
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Molecular, metabolic and functional CD4 T cell paralysis impedes tumor control
In bioRxiv on 17 April 2023 by Guo, M., Abd-Rabbo, D., et al.
-