InVivoPlus anti-mouse CD25 (IL-2Rα)
Product Details
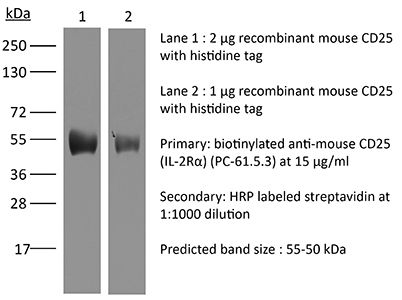
The PC-61.5.3 monoclonal antibody reacts with mouse IL-2Rα also known as CD25, Ly-43, p55, or Tac. IL-2Rα is the 55 kDa ligand-binding subunit of the interleukin 2 receptor alpha chain. IL-2Rα is expressed on activated mature T and B lymphocytes, thymocyte subsets, pre-B cells, and T regulatory cells. IL-2Rα has been shown to play roles in lymphocyte differentiation, activation, and proliferation. Alone, the IL-2Rα binds IL-2 with relatively low affinity however, when IL-2Rα associates with IL-2Rβ (CD122) and the common gamma chain (CD132) the complex binds IL-2 with high affinity. The PC-61.5.3 antibody has been shown to inhibit the binding of IL-2 to both the low and high affinity IL-2 receptor forms. Additionally, the PC-61.5.3 antibody is commonly used to deplete CD4+FoxP3+ T regulatory cells in vivo.Specifications
| Isotype | Rat IgG1, λ |
|---|---|
| Recommended Isotype Control(s) | InVivoPlus rat IgG1 isotype control, anti-horseradish peroxidase |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | IL-2-dependent cytolytic mouse T cell clone B6.1 |
| Reported Applications |
in vivo regulatory T cell depletion Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin* |
<1EU/mg (<0.001EU/μg) Determined by LAL gel clotting assay |
| Aggregation* |
<5% Determined by SEC |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 μM filtered |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A High Salt |
| RRID | AB_1107619 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests* |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Additional Formats
Recommended Products
in vivo regulatory T cell depletion
Goschl, L., et al. (2018). "A T cell-specific deletion of HDAC1 protects against experimental autoimmune encephalomyelitis" J Autoimmun 86: 51-61. PubMed
Multiple sclerosis (MS) is a human neurodegenerative disease characterized by the invasion of autoreactive T cells from the periphery into the CNS. Application of pan-histone deacetylase inhibitors (HDACi) ameliorates experimental autoimmune encephalomyelitis (EAE), an animal model for MS, suggesting that HDACi might be a potential therapeutic strategy for MS. However, the function of individual HDAC members in the pathogenesis of EAE is not known. In this study we report that mice with a T cell-specific deletion of HDAC1 (using the Cd4-Cre deleter strain; HDAC1-cKO) were completely resistant to EAE despite the ability of HDAC1cKO CD4(+) T cells to differentiate into Th17 cells. RNA sequencing revealed STAT1 as a prominent upstream regulator of differentially expressed genes in activated HDAC1-cKO CD4(+) T cells and this was accompanied by a strong increase in phosphorylated STAT1 (pSTAT1). This suggests that HDAC1 controls STAT1 activity in activated CD4(+) T cells. Increased pSTAT1 levels correlated with a reduced expression of the chemokine receptors Ccr4 and Ccr6, which are important for the migration of T cells into the CNS. Finally, EAE susceptibility was restored in WT:HDAC1-cKO mixed BM chimeric mice, indicating a cell-autonomous defect. Our data demonstrate a novel pathophysiological role for HDAC1 in EAE and provide evidence that selective inhibition of HDAC1 might be a promising strategy for the treatment of MS.
in vivo regulatory T cell depletion
Clemente-Casares, X., et al. (2016). "Expanding antigen-specific regulatory networks to treat autoimmunity" Nature 530(7591): 434-440. PubMed
Regulatory T cells hold promise as targets for therapeutic intervention in autoimmunity, but approaches capable of expanding antigen-specific regulatory T cells in vivo are currently not available. Here we show that systemic delivery of nanoparticles coated with autoimmune-disease-relevant peptides bound to major histocompatibility complex class II (pMHCII) molecules triggers the generation and expansion of antigen-specific regulatory CD4(+) T cell type 1 (TR1)-like cells in different mouse models, including mice humanized with lymphocytes from patients, leading to resolution of established autoimmune phenomena. Ten pMHCII-based nanomedicines show similar biological effects, regardless of genetic background, prevalence of the cognate T-cell population or MHC restriction. These nanomedicines promote the differentiation of disease-primed autoreactive T cells into TR1-like cells, which in turn suppress autoantigen-loaded antigen-presenting cells and drive the differentiation of cognate B cells into disease-suppressing regulatory B cells, without compromising systemic immunity. pMHCII-based nanomedicines thus represent a new class of drugs, potentially useful for treating a broad spectrum of autoimmune conditions in a disease-specific manner.
in vivo regulatory T cell depletion
Deligne, C., et al. (2015). "Anti-CD20 therapy induces a memory Th1 response through the IFN-gamma/IL-12 axis and prevents protumor regulatory T-cell expansion in mice" Leukemia 29(4): 947-957. PubMed
The long-lasting clinical response by lymphoma patients to anti-CD20 therapy has been attributed to the induction of an anti-tumor adaptive immunity. We previously demonstrated that a CD4-dependent mechanism is responsible for the long-term protection of CD20(+) tumor-bearing mice by anti-CD20 treatment. Here, we compare tumor immunity in tumor-bearing animals that did or did not receive anti-CD20 treatment. Splenic CD4(+)FoxP3(+) regulatory T cells (Tregs) expanded substantially in untreated mice that exhibited then a reduced survival, whereas Tregs depletion led to long-term survival of the animals, suggesting the establishment of a Treg-dependent immunosuppressive environment after tumor injection. Strikingly, anti-CD20 therapy reversed the initial expansion of Tregs, and was accompanied by a marked increase in the number of Th1 cells, with no detectable change in Th2 and Th17 cell numbers. Interleukin-12 serum level was also increased by the anti-CD20 treatment, and activated myeloid dendritic cells producing interleukin-12 could be detected in lymph nodes of treated animals, while interferon-gamma blockade strongly reduced survival. Also, CD4(+) effector memory T cells were evidenced in surviving animals, and the transfer of CD4(+) T cells induced long-term protection. Thus, anti-CD20 therapy promotes strong anti-tumor adaptive immunity, opposes Treg expansion and inhibits tumor cells from maintaining an immunosuppressive environment.
in vivo regulatory T cell depletion
Christensen, A. D., et al. (2015). "Depletion of regulatory T cells in a hapten-induced inflammation model results in prolonged and increased inflammation driven by T cells" Clin Exp Immunol 179(3): 485-499. PubMed
Regulatory T cells (Tregs ) are known to play an immunosuppressive role in the response of contact hypersensitivity (CHS), but neither the dynamics of Tregs during the CHS response nor the exaggerated inflammatory response after depletion of Tregs has been characterized in detail. In this study we show that the number of Tregs in the challenged tissue peak at the same time as the ear-swelling reaches its maximum on day 1 after challenge, whereas the number of Tregs in the draining lymph nodes peaks at day 2. As expected, depletion of Tregs by injection of a monoclonal antibody to CD25 prior to sensitization led to a prolonged and sustained inflammatory response which was dependent upon CD8 T cells, and co-stimulatory blockade with cytotoxic T lymphocyte antigen-4-immunoglobulin (CTLA-4-Ig) suppressed the exaggerated inflammation. In contrast, blockade of the interleukin (IL)-10-receptor (IL-10R) did not further increase the exaggerated inflammatory response in the Treg -depleted mice. In the absence of Tregs , the response changed from a mainly acute reaction with heavy infiltration of neutrophils to a sustained response with more chronic characteristics (fewer neutrophils and dominated by macrophages). Furthermore, depletion of Tregs enhanced the release of cytokines and chemokines locally in the inflamed ear and augmented serum levels of the systemic inflammatory mediators serum amyloid (SAP) and haptoglobin early in the response.
in vivo regulatory T cell depletion, Flow Cytometry
Glatigny, S., et al. (2015). "Integrin alpha L controls the homing of regulatory T cells during CNS autoimmunity in the absence of integrin alpha 4" Sci Rep 5: 7834. PubMed
Experimental autoimmune encephalomyelitis (EAE), the animal model of multiple sclerosis (MS), results from an autoimmune attack of the central nervous system (CNS) by effector T helper (Th) 1 and Th17 cells. Regulatory T cells (Treg) can control effector T cells and limit the progression of CNS autoimmunity. Integrin alpha 4 (Itga4) is critical for the entry of Th1 but not Th17 cells into the CNS during EAE. Whether Itga4 controls the homing of Tregs in the CNS and whether Tregs can limit Th17-mediated EAE has, however, not been addressed. Through selective elimination of Itga4 in Foxp3-expressing cells, we show here that Tregs can suppress Th17-mediated EAE and enter into the CNS independently of Itga4. Furthermore, similarly to Th17 cells and in contrast to Th1 cells, Tregs depend on LFA-1 for their entry into the CNS in the absence of Itga4. Therefore, these data suggest that the efficacy of Itga4 neutralization on MS progression may be associated with the prevention of Th1 cells and the maintenance of Tregs migration into the CNS.
in vivo regulatory T cell depletion, Flow Cytometry
Park, H. J., et al. (2015). "PD-1 upregulated on regulatory T cells during chronic virus infection enhances the suppression of CD8+ T cell immune response via the interaction with PD-L1 expressed on CD8+ T cells" J Immunol 194(12): 5801-5811. PubMed
Regulatory T (Treg) cells act as terminators of T cell immuniy during acute phase of viral infection; however, their role and suppressive mechanism in chronic viral infection are not completely understood. In this study, we compared the phenotype and function of Treg cells during acute or chronic infection with lymphocytic choriomeningitis virus. Chronic infection, unlike acute infection, led to a large expansion of Treg cells and their upregulation of programmed death-1 (PD-1). Treg cells from chronically infected mice (chronic Treg cells) displayed greater suppressive capacity for inhibiting both CD8(+) and CD4(+) T cell proliferation and subsequent cytokine production than those from naive or acutely infected mice. A contact between Treg and CD8(+) T cells was necessary for the potent suppression of CD8(+) T cell immune response. More importantly, the suppression required cell-specific expression and interaction of PD-1 on chronic Treg cells and PD-1 ligand on CD8(+) T cells. Our study defines PD-1 upregulated on Treg cells and its interaction with PD-1 ligand on effector T cells as one cause for the potent T cell suppression and proposes the role of PD-1 on Treg cells, in addition to that on exhausted T cells, during chronic viral infection.
in vivo regulatory T cell depletion
Miller, M. L., et al. (2015). "Spontaneous restoration of transplantation tolerance after acute rejection" Nat Commun 6: 7566. PubMed
Transplantation is a cure for end-stage organ failure but, in the absence of pharmacological immunosuppression, allogeneic organs are acutely rejected. Such rejection invariably results in allosensitization and accelerated rejection of secondary donor-matched grafts. Transplantation tolerance can be induced in animals and a subset of humans, and enables long-term acceptance of allografts without maintenance immunosuppression. However, graft rejection can occur long after a state of transplantation tolerance has been acquired. When such an allograft is rejected, it has been assumed that the same rules of allosensitization apply as to non-tolerant hosts and that immunological tolerance is permanently lost. Using a mouse model of cardiac transplantation, we show that when Listeria monocytogenes infection precipitates acute rejection, thus abrogating transplantation tolerance, the donor-specific tolerant state re-emerges, allowing spontaneous acceptance of a donor-matched second transplant. These data demonstrate a setting in which the memory of allograft tolerance dominates over the memory of transplant rejection.
in vivo regulatory T cell depletion
Allam, A., et al. (2014). "Dual function of CD70 in viral infection: modulator of early cytokine responses and activator of adaptive responses" J Immunol 193(2): 871-878. PubMed
The role of the TNF family member CD70 in adaptive T cell responses has been intensively studied, but its function in innate responses is still under investigation. In this study, we show that CD70 inhibits the early innate response to murine CMV (MCMV) but is essential for the optimal generation of virus-specific CD8 T cells. CD70(-/-) mice reacted to MCMV infection with a robust type I IFN and proinflammatory cytokine response. This response was sufficient for initial control of MCMV, although at later time points, CD70(-/-) mice became more susceptible to MCMV infection. The heightened cytokine response during the early phase of MCMV infection in CD70(-/-) mice was paralleled by a reduction in regulatory T cells (Treg). Treg from naive CD70(-/-) mice were not as efficient at suppressing T cell proliferation compared with Treg from naive wild-type mice, and depletion of Treg during MCMV infection in Foxp3-diphtheria toxin receptor mice or in wild-type mice recapitulated the phenotype observed in CD70(-/-) mice. Our study demonstrates that although CD70 is required for the activation of the antiviral adaptive response, it has a regulatory role in early cytokine responses to viruses such as MCMV, possibly through maintenance of Treg survival and function.
in vivo regulatory T cell depletion
Sarraj, B., et al. (2014). "Impaired selectin-dependent leukocyte recruitment induces T-cell exhaustion and prevents chronic allograft vasculopathy and rejection" Proc Natl Acad Sci U S A 111(33): 12145-12150. PubMed
Selectin-selectin ligand interactions mediate the initial steps in leukocyte migration, an integral part of immune responses. Fucosyltransferase-VII (FucT-VII), encoded by Fut7, is essential for biosynthesis of selectin ligands. In an established model of cardiac allograft vasculopathy and chronic rejection, Fut7(-/-) recipients exhibited long-term graft survival with minimal vasculopathy compared with WT controls. Graft survival was associated with CD4 T-cell exhaustion in the periphery, characterized by impaired effector cytokine production, defective proliferation, increased expression of inhibitory receptors programmed death-1 (PD-1) and T cell Ig- and mucin-domain-containing molecule-3 (Tim-3), low levels of IL-7Ralpha on CD4 T cells, and reduced migration of polyfunctional CD4 memory T cells to the allograft. Blocking PD-1 triggered rejection only in Fut7(-/-) recipients, whereas depleting regulatory T cells had no effect in either Fut7(-/-) or WT recipients. Adoptive transfer experiments confirmed that this CD4 T cell-exhausted phenotype is seen primarily in Fut7(-/-) CD4 T cells. These data suggest that impaired leukocyte recruitment is a novel mechanism leading to CD4 T-cell exhaustion. Our experimental system serves as an excellent model to study CD4 T-cell exhaustion as a dominant mechanism of transplant tolerance. Further, targeting FucT-VII may serve as a promising strategy to prevent chronic allograft rejection and promote tolerance.
in vivo regulatory T cell depletion
Richter, K., et al. (2013). "Macrophage and T cell produced IL-10 promotes viral chronicity" PLoS Pathog 9(11): e1003735. PubMed
Chronic viral infections lead to CD8(+) T cell exhaustion, characterized by impaired cytokine secretion. Presence of the immune-regulatory cytokine IL-10 promotes chronicity of Lymphocytic Choriomeningitis Virus (LCMV) Clone 13 infection, while absence of IL-10/IL-10R signaling early during infection results in viral clearance and higher percentages and numbers of antiviral, cytokine producing T cells. IL-10 is produced by several cell types during LCMV infection but it is currently unclear which cellular sources are responsible for induction of viral chronicity. Here, we demonstrate that although dendritic cells produce IL-10 and overall IL-10 mRNA levels decrease significantly in absence of CD11c(+) cells, absence of IL-10 produced by CD11c(+) cells failed to improve the LCMV-specific T cell response and control of LCMV infection. Similarly, NK cell specific IL-10 deficiency had no positive impact on the LCMV-specific T cell response or viral control, even though high percentages of NK cells produced IL-10 at early time points after infection. Interestingly, we found markedly improved T cell responses and clearance of normally chronic LCMV Clone 13 infection when either myeloid cells or T cells lacked IL-10 production and mice depleted of monocytes/macrophages or CD4(+) T cells exhibited reduced overall levels of IL-10 mRNA. These data suggest that the decision whether LCMV infection becomes chronic or can be cleared critically depends on early CD4(+) T cell and monocyte/macrophage produced IL-10.
in vivo regulatory T cell depletion
Tang, S., et al. (2012). "Increased CD8+ T-cell function following castration and immunization is countered by parallel expansion of regulatory T cells" Cancer Res 72(8): 1975-1985. PubMed
Although androgen ablation therapy is effective in treating primary prostate cancers, a significant number of patients develop incurable castration-resistant disease. Recent studies have suggested a potential synergy between vaccination and androgen ablation, yet the enhanced T-cell function is transient. Using a defined tumor antigen model, UV-8101-RE, we found that concomitant castration significantly increased the frequency and function of antigen-specific CD8(+) T cells early after the immunization of wild-type mice. However, at a late time point after immunization, effector function was reduced to the same level as noncastrated mice and was accompanied by a concomitant amplification in CD4(+)CD25(+)Foxp3(+) regulatory T cells (Treg) following immunization. We investigated whether Treg expansion occurred following castration of prostate tumor-bearing mice. In the prostate-specific Pten(-/-) mouse model of prostate cancer, we observed an accelerated Treg expansion in mice bearing the castration-resistant endogenous prostate tumor, which prevented effector responses to UV-8101-RE. Treg depletion together with castration elicited a strong CD8(+) T-cell response to UV-8101-RE in Pten(-/-) mice and rescued effector function in castrated and immunized wild-type mice. In addition, Treg expansion in Pten(-/-) mice was prevented by in vivo interleukin (IL)-2 blockade suggesting that increased IL-2 generated by castration and immunization promotes Treg expansion. Our findings therefore suggest that although effector responses are augmented by castration, the concomitant expansion of Tregs is one mechanism responsible for only transient immune potentiation after androgen ablation.
in vivo regulatory T cell depletion, Flow Cytometry
Locatelli, G., et al. (2012). "Primary oligodendrocyte death does not elicit anti-CNS immunity" Nat Neurosci 15(4): 543-550. PubMed
Anti-myelin immunity is commonly thought to drive multiple sclerosis, yet the initial trigger of this autoreactivity remains elusive. One of the proposed factors for initiating this disease is the primary death of oligodendrocytes. To specifically test such oligodendrocyte death as a trigger for anti-CNS immunity, we inducibly killed oligodendrocytes in an in vivo mouse model. Strong microglia-macrophage activation followed oligodendrocyte death, and myelin components in draining lymph nodes made CNS antigens available to lymphocytes. However, even conditions favoring autoimmunity-bystander activation, removal of regulatory T cells, presence of myelin-reactive T cells and application of demyelinating antibodies-did not result in the development of CNS inflammation after oligodendrocyte death. In addition, this lack of reactivity was not mediated by enhanced myelin-specific tolerance. Thus, in contrast with previously reported impairments of oligodendrocyte physiology, diffuse oligodendrocyte death alone or in conjunction with immune activation does not trigger anti-CNS immunity.
in vivo regulatory T cell depletion
Mohamadzadeh, M., et al. (2011). "Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid" Proc Natl Acad Sci U S A 108 Suppl 1: 4623-4630. PubMed
Imbalance in the regulatory immune mechanisms that control intestinal cellular and bacterial homeostasis may lead to induction of the detrimental inflammatory signals characterized in humans as inflammatory bowel disease. Induction of proinflammatory cytokines (i.e., IL-12) induced by dendritic cells (DCs) expressing pattern recognition receptors may skew naive T cells to T helper 1 polarization, which is strongly implicated in mucosal autoimmunity. Recent studies show the ability of probiotic microbes to treat and prevent numerous intestinal disorders, including Clostridium difficile-induced colitis. To study the molecular mechanisms involved in the induction and repression of intestinal inflammation, the phosphoglycerol transferase gene that plays a key role in lipoteichoic acid (LTA) biosynthesis in Lactobacillus acidophilus NCFM (NCK56) was deleted. The data show that the L. acidophilus LTA-negative in LTA (NCK2025) not only down-regulated IL-12 and TNFalpha but also significantly enhanced IL-10 in DCs and controlled the regulation of costimulatory DC functions, resulting in their inability to induce CD4(+) T-cell activation. Moreover, treatment of mice with NCK2025 compared with NCK56 significantly mitigated dextran sulfate sodium and CD4(+)CD45RB(high)T cell-induced colitis and effectively ameliorated dextran sulfate sodium-established colitis through a mechanism that involves IL-10 and CD4(+)FoxP3(+) T regulatory cells to dampen exaggerated mucosal inflammation. Directed alteration of cell surface components of L. acidophilus NCFM establishes a potential strategy for the treatment of inflammatory intestinal disorders.
in vivo regulatory T cell depletion
Akins, E. J., et al. (2010). "In situ vaccination combined with androgen ablation and regulatory T-cell depletion reduces castration-resistant tumor burden in prostate-specific pten knockout mice" Cancer Res 70(9): 3473-3482. PubMed
There is no effective treatment for prostate cancer arising after androgen ablation. Previous studies have analyzed the short-term effects of androgen ablation on the immune system and suggest an abatement of immune suppression by hormone removal. Because castration-resistant disease can arise years after treatment, it is crucial to determine the duration of immune potentiation by castration. Because immunotherapeutic efficacy is determined by the balance of immune cell subsets and their location within the tumor, we assessed the acute and chronic effect of androgen ablation on the localization of T-cell subsets within castration-resistant murine prostate cancer. We observed a transient increase in CD4+ and CD8+ T-cell numbers at the residual tumor after androgen ablation. More than 2 months later, regulatory T cells (Treg) were increasingly found within prostate epithelium, whereas CTLs, which were evenly distributed before androgen ablation, became sequestered within stroma. Anti-CD25 antibody administration along with castration enhanced CTL access to cancerous glands but did not increase effector function. Intraprostatic injection of LIGHT-expressing tumor cells increased the proportion of CD8+ T cells with functional capacity within the cancerous gland. In addition, Treg depletion within the tumor was enhanced. Together, these manipulations significantly reduced castration-resistant tumor burden. Thus, our results indicate that immune modulations, which prevent Treg accumulation and augment effector cell infiltration of prostatic epithelium, may be effective in reducing tumor burden or preventing tumor recurrence after androgen ablation therapy.
in vivo regulatory T cell depletion
D’Alessio, F. R., et al. (2009). "CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury" J Clin Invest 119(10): 2898-2913. PubMed
Acute lung injury (ALI) is characterized by rapid alveolar injury, inflammation, cytokine induction, and neutrophil accumulation. Although early events in the pathogenesis of ALI have been defined, the mechanisms underlying resolution are unknown. As a model of ALI, we administered intratracheal (i.t.) LPS to mice and observed peak lung injury 4 days after the challenge, with resolution by day 10. Numbers of alveolar lymphocytes increased as injury resolved. To examine the role of lymphocytes in this response, lymphocyte-deficient Rag-1-/- and C57BL/6 WT mice were exposed to i.t. LPS. The extent of injury was similar between the groups of mice through day 4, but recovery was markedly impaired in the Rag-1-/- mice. Adoptive transfer studies revealed that infusion of CD4+CD25+Foxp3+ Tregs as late as 24 hours after i.t. LPS normalized resolution in Rag-1-/- mice. Similarly, Treg depletion in WT mice delayed recovery. Treg transfer into i.t. LPS-exposed Rag-1-/- mice also corrected the elevated levels of alveolar proinflammatory cytokines and increased the diminished levels of alveolar TGF-beta and neutrophil apoptosis. Mechanistically, Treg-mediated resolution of lung injury was abrogated by TGF-beta inhibition. Moreover, BAL of patients with ALI revealed dynamic changes in CD3+CD4+CD25hiCD127loFoxp3+ cells. These results indicate that Tregs modify innate immune responses during resolution of lung injury and suggest potential targets for treating ALI, for which there are no specific therapies currently available.
- Biochemistry and Molecular biology,
- Cancer Research,
- Cell Biology,
- Immunology and Microbiology,
- Mus musculus (House mouse)
Lactobacillus gallinarum-derived metabolites boost anti-PD1 efficacy in colorectal cancer by inhibiting regulatory T cells through modulating IDO1/Kyn/AHR axis.
In Gut on 24 November 2023 by Fong, W., Li, Q., et al.
PubMed
Gut microbiota is a key player in dictating immunotherapy response. We aimed to explore the immunomodulatory effect of probiotic Lactobacillus gallinarum and its role in improving anti-programmed cell death protein 1 (PD1) efficacy against colorectal cancer (CRC). The effects of L. gallinarum in anti-PD1 response were assessed in syngeneic mouse models and azoxymethane/dextran sulfate sodium-induced CRC model. The change of immune landscape was identified by multicolour flow cytometry and validated by immunohistochemistry staining and in vitro functional assays. Liquid chromatography-mass spectrometry was performed to identify the functional metabolites. L. gallinarum significantly improved anti-PD1 efficacy in two syngeneic mouse models with different microsatellite instability (MSI) statuses (MSI-high for MC38, MSI-low for CT26). Such effect was confirmed in CRC tumourigenesis model. L. gallinarum synergised with anti-PD1 therapy by reducing Foxp3+ CD25+ regulatory T cell (Treg) intratumoural infiltration, and enhancing effector function of CD8+ T cells. L. gallinarum-derived indole-3-carboxylic acid (ICA) was identified as the functional metabolite. Mechanistically, ICA inhibited indoleamine 2,3-dioxygenase (IDO1) expression, therefore suppressing kynurenine (Kyn) production in tumours. ICA also competed with Kyn for binding site on aryl hydrocarbon receptor (AHR) and antagonised Kyn binding on CD4+ T cells, thereby inhibiting Treg differentiation in vitro. ICA phenocopied L. gallinarum effect and significantly improved anti-PD1 efficacy in vivo, which could be reversed by Kyn supplementation. L. gallinarum-derived ICA improved anti-PD1 efficacy in CRC through suppressing CD4+Treg differentiation and enhancing CD8+T cell function by modulating the IDO1/Kyn/AHR axis. L. gallinarum is a potential adjuvant to augment anti-PD1 efficacy against CRC. © Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
- Cancer Research,
- Immunology and Microbiology
Kindlin-1 regulates IL-6 secretion and modulates the immune environment in breast cancer models.
In eLife on 8 March 2023 by Webb, E. R., Dodd, G. L., et al.
PubMed
The adhesion protein Kindlin-1 is over-expressed in breast cancer where it is associated with metastasis-free survival; however, the mechanisms involved are poorly understood. Here, we report that Kindlin-1 promotes anti-tumor immune evasion in mouse models of breast cancer. Deletion of Kindlin-1 in Met-1 mammary tumor cells led to tumor regression following injection into immunocompetent hosts. This was associated with a reduction in tumor infiltrating Tregs. Similar changes in T cell populations were seen following depletion of Kindlin-1 in the polyomavirus middle T antigen (PyV MT)-driven mouse model of spontaneous mammary tumorigenesis. There was a significant increase in IL-6 secretion from Met-1 cells when Kindlin-1 was depleted and conditioned media from Kindlin-1-depleted cells led to a decrease in the ability of Tregs to suppress the proliferation of CD8+ T cells, which was dependent on IL-6. In addition, deletion of tumor-derived IL-6 in the Kindlin-1-depleted tumors reversed the reduction of tumor-infiltrating Tregs. Overall, these data identify a novel function for Kindlin-1 in regulation of anti-tumor immunity, and that Kindlin-1 dependent cytokine secretion can impact the tumor immune environment. © 2023, Webb et al.
- In Vivo,
- IHC,
- Mus musculus (House mouse),
- Cancer Research
Bile salt hydrolase in non-enterotoxigenic Bacteroides potentiates colorectal cancer.
In Nature Communications on 10 February 2023 by Sun, L., Zhang, Y., et al.
PubMed
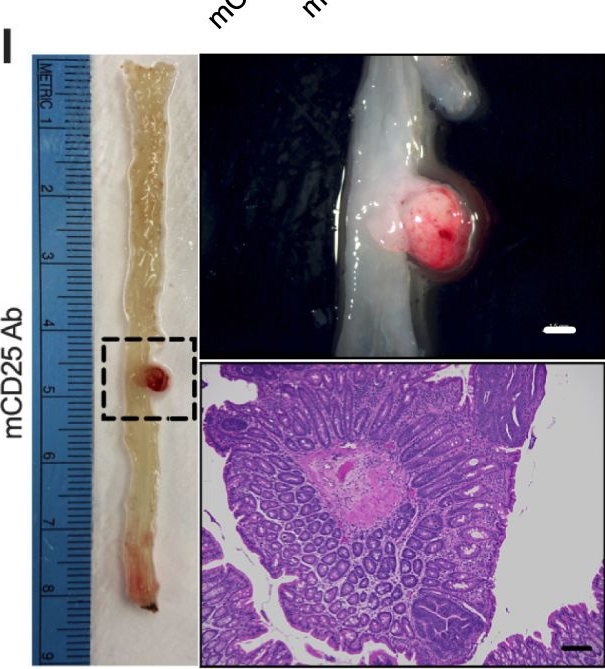
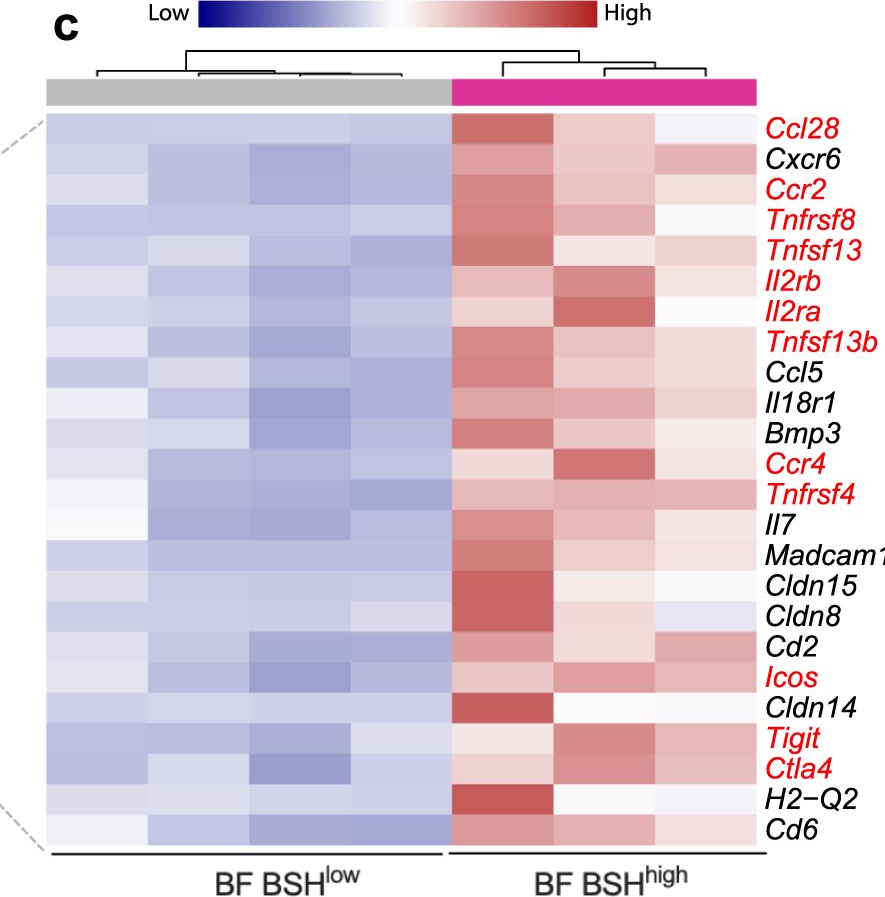
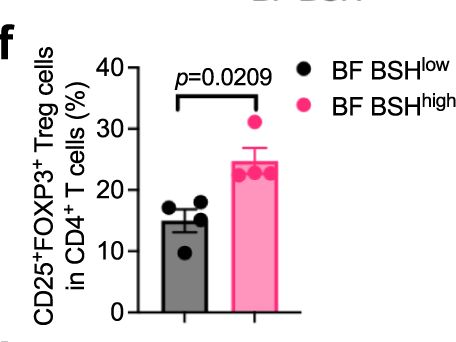
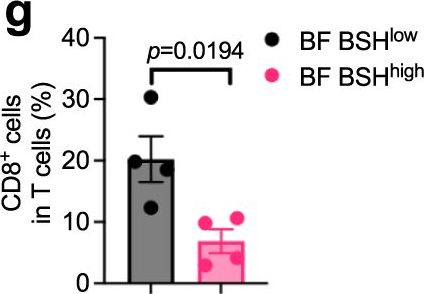
Bile salt hydrolase (BSH) in Bacteroides is considered a potential drug target for obesity-related metabolic diseases, but its involvement in colon tumorigenesis has not been explored. BSH-expressing Bacteroides is found at high abundance in the stools of colorectal cancer (CRC) patients with overweight and in the feces of a high-fat diet (HFD)-induced CRC mouse model. Colonization of B. fragilis 638R, a strain with low BSH activity, overexpressing a recombinant bsh gene from B. fragilis NCTC9343 strain, results in increased unconjugated bile acids in the colon and accelerated progression of CRC under HFD treatment. In the presence of high BSH activity, the resultant elevation of unconjugated deoxycholic acid and lithocholic acid activates the G-protein-coupled bile acid receptor, resulting in increased β-catenin-regulated chemokine (C-C motif) ligand 28 (CCL28) expression in colon tumors. Activation of the β-catenin/CCL28 axis leads to elevated intra-tumoral immunosuppressive CD25+FOXP3+ Treg cells. Blockade of the β-catenin/CCL28 axis releases the immunosuppression to enhance the intra-tumoral anti-tumor response, which decreases CRC progression under HFD treatment. Pharmacological inhibition of BSH reduces HFD-accelerated CRC progression, coincident with suppression of the β-catenin/CCL28 pathway. These findings provide insights into the pro-carcinogenetic role of Bacteroides in obesity-related CRC progression and characterize BSH as a potential target for CRC prevention and treatment. © 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
- Cancer Research
Loss of the adhesion protein Kindlin-1 stimulates tumor clearance via modulation of Tregs
Preprint on BioRxiv : the Preprint Server for Biology on 5 March 2022 by Webb, E. R., Dodd, G., et al.
PubMed
The adhesion protein Kindlin-1 is over-expressed in breast cancer where it has been shown to be associated with metastasis-free survival, however, the mechanisms involved are poorly understood. Here, we report that Kindlin-1 promotes anti-tumor immune evasion in a mouse model of breast cancer. Deletion of Kindlin-1 in Met-1 mammary tumor cells leads to tumor regression following injection into immunocompetent hosts. This was associated with a reduction in tumor infiltrating Tregs and impairment of their immune-suppressive activities in Kindlin-1 depleted tumors. Similar changes in T cell populations were seen following depletion of Kindlin-1 in the polyomavirus middle T antigen (PyV MT)-driven mouse model of mammary tumorigenesis. Analysis of cytokines secreted from the Met-1 cells identified a significant increase in IL-6 secretion when Kindlin-1 was depleted. Conditioned media from Kindlin-1 depleted cells lead to a decrease in the ability of Tregs to suppress the proliferation of CD8 + T cells, which was dependent on IL-6 and depletion of CD25 + Tregs resulted in a reduction of Met-1 tumor growth in mice. Overall, these data identify a novel function for Kindlin-1 in the regulation of anti-tumor immunity through cytokine regulation of Treg number and function.
- Cancer Research,
- Immunology and Microbiology
The augment of regulatory T cells undermines the efficacy of anti-PD-L1 treatment in cervical cancer.
In BMC Immunology on 3 September 2021 by Xu, F., Zhang, F., et al.
PubMed
Immune checkpoint inhibitors have aroused great expectation of tumor eradication. However, the effect of anti-PD-L1 treatment for cervical cancer is unsatisfactory and the underlying antagonist to anti-PD-L1 efficacy is remained to be studied. Here, we investigated the anti-tumor effect of anti-PD-L1 treatment in cervical tumor model and identified the antagonist to the therapeutic efficacy of anti-PD-L1 treatment. We found that PD-L1 exhibited a moderate expression in both cervical tumor cell lines and clinical samples compared to other tumor types and the para-tumor tissue respectively. Interestingly, our results showed that the anti-PD-L1 treated mice were dichotomously divided into responsive and unresponsive group after five cycles of anti-PD-L1 treatment although all the mice had the same genome background. In addition, the unresponsive tumors showed less tumor necrosis area and higher immunosuppression activity induced by regulatory T cells (Tregs) population than the responsive ones. Furthermore, we found that anti-PD-L1 treatment autonomously upregulated Tregs proliferation and frequency in multiple immune organs, and, most importantly, Tregs depletion significantly depressed the tumor growth rate and tumor weight compared with either anti-PD-L1 or anti-CD25 treatment alone. Finally, we observed that the upregulating effector CD8+ T cell is associated with the better therapeutic effect of anti-PD-L1 therapy post Tregs depletion. Anti-PD-L1 treatment upregulates Tregs frequency and proliferation in tumor model, and the depletion of Tregs may be a useful adjuvant strategy for anti-PD-L1 therapy of cervical cancer. © 2021. The Author(s).
- Biochemistry and Molecular biology,
- Cancer Research,
- Cell Biology
GCH1 induces immunosuppression through metabolic reprogramming and IDO1 upregulation in triple-negative breast cancer.
In Journal for Immunotherapy of Cancer on 1 July 2021 by Wei, J. L., Wu, S. Y., et al.
PubMed
Regulatory T cells (Tregs) heavily infiltrate triple-negative breast cancer (TNBC), and their accumulation is affected by the metabolic reprogramming in cancer cells. In the present study, we sought to identify cancer cell-intrinsic metabolic modulators correlating with Tregs infiltration in TNBC. Using the RNA-sequencing data from our institute (n=360) and the Molecular Taxonomy of Breast Cancer International Consortium TNBC cohort (n=320), we calculated the abundance of Tregs in each sample and evaluated the correlation between gene expression levels and Tregs infiltration. Then, in vivo and in vitro experiments were performed to verify the correlation and explore the underlying mechanism. We revealed that GTP cyclohydrolase 1 (GCH1) expression was positively correlated with Tregs infiltration and high GCH1 expression was associated with reduced overall survival in TNBC. In vivo and in vitro experiments showed that GCH1 increased Tregs infiltration, decreased apoptosis, and elevated the programmed cell death-1 (PD-1)-positive fraction. Metabolomics analysis indicated that GCH1 overexpression reprogrammed tryptophan metabolism, resulting in L-5-hydroxytryptophan (5-HTP) accumulation in the cytoplasm accompanied by kynurenine accumulation and tryptophan reduction in the supernatant. Subsequently, aryl hydrocarbon receptor, activated by 5-HTP, bound to the promoter of indoleamine 2,3-dioxygenase 1 (IDO1) and thus enhanced the transcription of IDO1. Furthermore, the inhibition of GCH1 by 2,4-diamino-6-hydroxypyrimidine (DAHP) decreased IDO1 expression, attenuated tumor growth, and enhanced the tumor response to PD-1 blockade immunotherapy. Tumor-cell-intrinsic GCH1 induced immunosuppression through metabolic reprogramming and IDO1 upregulation in TNBC. Inhibition of GCH1 by DAHP serves as a potential immunometabolic strategy in TNBC. © Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
- IHC,
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
The Interplay Between Regular T Cells and Immunotherapy in Cervical Cancer
Preprint on Research Square on 30 December 2020 by Xu, F., Zhang, F., et al.
PubMed
h4>Background: /h4> Immune checkpoint blockade inhibitors have aroused great expectation on many types of tumor eradication. However, the therapeutic effect of anti-PD-L1 treatment on cervical cancer is unsatisfactory and the potential antagonist is not very clear. Here, we investigated the therapeutic effect of anti-PD-L1 in cervical tumor mouse model and identified the potential threats for anti-PD-L1 therapeutic efficacy. h4>Results: /h4> we found that PD-L1 had a moderate expression in human and mouse cervical tumor cell lines and clinical samples compared to other tumor types and para-tumor tissue. Interestingly, our results showed that the anti-PD-L1 treated mice were dichotomously divided into responsive and unresponsive group even with the same genome background C57BL/6 syngeneic tumor model. The unresponsive tumors showed less immune cell infiltration and higher Tregs population induced immunosuppression activity than the responsive ones. Furthermore, we found that anti-PD-L1 autonomously upregulated Tregs proliferation and frequency in multiple immune organs, and, most importantly, Tregs depletion more significantly depressed the tumor growth rate and tumor weight than either anti-PD-L1 or anti-CD25 alone. Finally, we observed that the upregulating effector CD8+ T cell is associated with the better therapeutic effect of anti-PD-L1 therapy post Tregs depletion. h4>Conclusion: /h4> In conclusion, anti-PD-L1 therapy upregulates Tregs frequency and proliferation in tumor model, and the depletion of Tregs may be a useful adjuvant strategy for anti-PD-L1 therapy in the immunotherapy of cervical cancer.
- Immunology and Microbiology,
- Neuroscience
CD25-Targeted IL-2 Signals Promote Improved Outcomes of Influenza Infection and Boost Memory CD4 T Cell Formation.
In The Journal of Immunology on 15 June 2020 by Alam, F., Singh, A., et al.
PubMed
IL-2 is a pleotropic cytokine with potent pro- and anti-inflammatory effects. These divergent impacts can be directed in vivo by forming complexes of IL-2 and anti-IL-2 mAbs (IL-2C) to target IL-2 to distinct subsets of cells based on their expression of subunits of the IL-2R. In this study, we show that treatment of mice with a prototypical anti-inflammatory IL-2C, JES6-1-IL-2C, best known to induce CD25+ regulatory CD4 T cell expansion, surprisingly causes robust induction of a suite of inflammatory factors. However, treating mice infected with influenza A virus with this IL-2C reduces lung immunopathology. We compare the spectrum of inflammatory proteins upregulated by pro- and anti-inflammatory IL-2C treatment and uncover a pattern of expression that reveals potentially beneficial versus detrimental aspects of the influenza-associated cytokine storm. Moreover, we show that anti-inflammatory IL-2C can deliver survival signals to CD4 T cells responding to influenza A virus that improve their memory fitness, indicating a novel application of IL-2 to boost pathogen-specific T cell memory while simultaneously reducing immunopathology. Copyright © 2020 by The American Association of Immunologists, Inc.
Low-dose interleukin-2 reverses behavioral sensitization in multiple mouse models of headache disorders.
In Pain on 1 June 2020 by Zhang, J., Czerpaniak, K., et al.
PubMed
Headache disorders are highly prevalent and debilitating, with limited treatment options. Previous studies indicate that many proinflammatory immune cells contribute to headache pathophysiology. Given the well-recognized role of regulatory T (Treg) cells in maintaining immune homeostasis, we hypothesized that enhancing Treg function may be effective to treat multiple headache disorders. In a mouse model of chronic migraine, we observed that repeated nitroglycerin (NTG, a reliable trigger of migraine in patients) administration doubled the number of CD3 T cells in the trigeminal ganglia without altering the number of Treg cells, suggesting a deficiency in Treg-mediated immune homeostasis. We treated mice with low-dose interleukin-2 (ld-IL2) to preferentially expand and activate endogenous Treg cells. This not only prevented the development of NTG-induced persistent sensitization but also completely reversed the established facial skin hypersensitivity resulting from repeated NTG administration. The effect of ld-IL2 was independent of mouse sex and/or strain. Importantly, ld-IL2 treatment did not alter basal nociceptive responses, and repeated usage did not induce tolerance. The therapeutic effect of ld-IL2 was abolished by Treg depletion and was recapitulated by Treg adoptive transfer. Furthermore, treating mice with ld-IL2 1 to 7 days after mild traumatic brain injury effectively prevented as well as reversed the development of behaviors related to acute and chronic post-traumatic headache. In a model of medication overuse headache, Ld-IL2 completely reversed the cutaneous hypersensitivity induced by repeated administration of sumatriptan. Collectively, this study identifies ld-IL2 as a promising prophylactic for multiple headache disorders with a mechanism distinct from the existing treatment options.
- Immunology and Microbiology
A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult.
In Immunity on 21 May 2019 by Al Nabhani, Z., Dulauroy, S., et al.
PubMed
Microbes colonize all body surfaces at birth and participate in the development of the immune system. In newborn mammals, the intestinal microbiota is first shaped by the dietary and immunological components of milk and then changes upon the introduction of solid food during weaning. Here, we explored the reactivity of the mouse intestinal immune system during the first weeks after birth and into adulthood. At weaning, the intestinal microbiota induced a vigorous immune response-a "weaning reaction"-that was programmed in time. Inhibition of the weaning reaction led to pathological imprinting and increased susceptibility to colitis, allergic inflammation, and cancer later in life. Prevention of this pathological imprinting was associated with the generation of RORγt+ regulatory T cells, which required bacterial and dietary metabolites-short-chain fatty acids and retinoic acid. Thus, the weaning reaction to microbiota is required for immune ontogeny, the perturbation of which leads to increased susceptibility to immunopathologies later in life. Copyright © 2019 Elsevier Inc. All rights reserved.
- Mus musculus (House mouse),
- Stem Cells and Developmental Biology
A Subset of TREM2+ Dermal Macrophages Secretes Oncostatin M to Maintain Hair Follicle Stem Cell Quiescence and Inhibit Hair Growth.
In Cell Stem Cell on 4 April 2019 by Wang, E. C. E., Dai, Z., et al.
PubMed
Hair growth can be induced from resting mouse hair follicles by topical application of JAK inhibitors, suggesting that JAK-STAT signaling is required for maintaining hair follicle stem cells (HFSCs) in a quiescent state. Here, we show that Oncostatin M (OSM), an IL-6 family cytokine, negatively regulates hair growth by signaling through JAK-STAT5 to maintain HFSC quiescence. Genetic deletion of the OSM receptor or STAT5 can induce premature HFSC activation, suggesting that the resting telogen stage is actively maintained by the hair follicle niche. Single-cell RNA sequencing revealed that the OSM source is not intrinsic to the hair follicle itself and is instead a subset of TREM2+ macrophages that is enriched within the resting follicle and deceases immediately prior to HFSC activation. In vivo inhibition of macrophage function was sufficient to induce HFSC proliferation and hair cycle induction. Together these results clarify how JAK-STAT signaling actively inhibits hair growth.Copyright © 2019. Published by Elsevier Inc.
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
Age Correlates with Response to Anti-PD1, Reflecting Age-Related Differences in Intratumoral Effector and Regulatory T-Cell Populations.
In Clinical Cancer Research on 1 November 2018 by Kugel, C. H., Douglass, S. M., et al.
PubMed
Purpose: We have shown that the aged microenvironment increases melanoma metastasis, and decreases response to targeted therapy, and here we queried response to anti-PD1.Experimental Design: We analyzed the relationship between age, response to anti-PD1, and prior therapy in 538 patients. We used mouse models of melanoma, to analyze the intratumoral immune microenvironment in young versus aged mice and confirmed our findings in human melanoma biopsies.Results: Patients over the age of 60 responded more efficiently to anti-PD-1, and likelihood of response to anti-PD-1 increased with age, even when we controlled for prior MAPKi therapy. Placing genetically identical tumors in aged mice (52 weeks) significantly increased their response to anti-PD1 as compared with the same tumors in young mice (8 weeks). These data suggest that this increased response in aged patients occurs even in the absence of a more complex mutational landscape. Next, we found that young mice had a significantly higher population of regulatory T cells (Tregs), skewing the CD8+:Treg ratio. FOXP3 staining of human melanoma biopsies revealed similar increases in Tregs in young patients. Depletion of Tregs using anti-CD25 increased the response to anti-PD1 in young mice.Conclusions: While there are obvious limitations to our study, including our inability to conduct a meta-analysis due to a lack of available data, and our inability to control for mutational burden, there is a remarkable consistency in these data from over 500 patients across 8 different institutes worldwide. These results stress the importance of considering age as a factor for immunotherapy response. Clin Cancer Res; 24(21); 5347-56. ©2018 AACR See related commentary by Pawelec, p. 5193. ©2018 American Association for Cancer Research.